Identifying Predictors of Serious Adverse Events in Antidepressant Treatment from a Decade-Long Nationwide Pharmacovigilance Study: Impact of Dementia and Parkinson’s Disease Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. ADE Types and Risk of Reporting SAEs
3.3. Identification of Predictors
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADE | Adverse Drug Events |
| KIDS KAERS DB | Korean Adverse Drug Reporting System Database |
| MDD | Major Depressive Disorder |
| OR | Odds Ratio |
| PD | Pharmacodynamics |
| PK | Pharmacokinetics |
| ROR | Reporting Odds Ratio |
| RWD | Real World Data |
| SAE | Serious Adverse Events |
| SNRI | Serotonin Norepinephrine Reuptake Inhibitors |
| SOC | System Organ Class |
| SSRI | Selective Serotonin Reuptake Inhibitor |
| TCA | Tricyclic Antidepressants |
| WHO | World Health Organization |
References
- Campbell, J.E.; Gossell-Williams, M.; Lee, M.G. A review of pharmacovigilance. West Indian Med. J. 2014, 63, 771–774. [Google Scholar] [PubMed]
- Choi, Y.J.; Yang, S.W.; Kwack, W.G.; Lee, J.K.; Lee, T.H.; Jang, J.Y.; Chung, E.K. Comparative safety profiles of sedatives commonly used in clinical practice: A 10-year nationwide pharmacovigilance study in Korea. Pharmaceuticals 2021, 14, 783. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, T.; Koyama, T.; Hagiya, H.; Harada, K.; Linuma, S.; Ushio, S.; Zamami, Y.; Niimura, T.; Shinomiya, K.; Ishizawa, K.; et al. Population-based observational study of adverse drug event-related mortality in the super-aged society of Japan. Drug Saf. 2021, 44, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Iinuma, S.; Yamamoto, M.; Niimura, T.; Osaki, Y.; Nishimura, S.; Harada, K.; Zamami, Y.; Hagiya, H. International trends in adverse drug event-related mortality from 2001 to 2019: An analysis of the World Health Organization mortality database from 54 countries. Drug Saf. 2024, 47, 237–249. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, M.H.; Chung, E.K.; Lee, J.K.; Yoon, J.; Yug, J.S.; Jang, D.K. Prevalence and seriousness of analgesic-induced adverse events in Korea: A 10-yar nationwide surveillance. J. Patient Saf. 2020, 16, e215–e224. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Jackson, S.H. Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. Br. J. Clin. Pharmacol. 2004, 57, 6–14. [Google Scholar] [CrossRef]
- van Dam, C.S.; Labuschagne, H.A.; van Keulen, K.; Kramers, C.; Kleipool, E.E.; Hoogendijk, E.O.; Knol, W.; Nanayakkara, P.W.B.; Muller, M.; Trappenburg, M.C.; et al. Polypharmacy, comorbidity and frailty: A complex interplay in older patients at the emergency department. Eur. Geriatr. Med. 2022, 13, 849–857. [Google Scholar] [CrossRef]
- Kalin, N.H. The critical relationship between anxiety and depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, X.; Yang, Y.; Sun, N.; Shi, S.; Wang, W. Change in the global burden of depression from 1990–2019 and its prediction for 2030. J. Psychchiatr. Res. 2024, 178, 16–22. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- González de León, B.; Abt-Sacks, A.; Acosta Artiles, F.J.; Del Pino-Sedeño, T.; Ramos-García, V.; Rodríguez Álvarez, C.; Bejarano-Quisoboni, D.; Trujillo-Martín, M.M. Barriers and facilitating factors of adherence to antidepressant treatments: An exploratory qualitative study with patients and psychiatrists. Int. J. Environ. Res. Public Health 2022, 19, 16788. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.P.; Volerman, A.; Zhang, J.; Hua, J.; Conti, R.M. Antidepressant dispensing to US adolescents and young adults: 2016-2022. Pediatrics 2024, 153, e2023064245. [Google Scholar] [CrossRef] [PubMed]
- Parihar, H.S.; Yin, H.; Gooch, J.L.; Allen, S.; John, S.; Xuan, J. Trends in hospital admissions due to antidepressant-related adverse drug events from 2001 to 2011 in the U.S. BMC Health Serv. Res. 2017, 17, 51. [Google Scholar] [CrossRef]
- van Poelgeest, E.P.; Pronk, A.C.; Rhebergen, D.; van der Velde, N. Depression, antidepressants and fall risk: Therapeutic dilemmas-a clinical review. Eur. Geriatr. Med. 2021, 12, 585–596. [Google Scholar] [CrossRef]
- Man, K.K.C.; Chan, E.W.; Ip, P.; Coghill, D.; Simonoff, E.; Chan, P.K.L.; Lau, W.C.Y.; Schuemie, M.J.; Sturkenboom, M.; Wong, I.C.K. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: Population-based cohort study. BMJ 2017, 357, j2350. [Google Scholar] [CrossRef]
- Thaipisuttikul, P.; Ittasakul, P.; Waleeprakhon, P.; Wisajun, P.; Jullagate, S. Psychiatric comorbidities in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2014, 10, 2097–2103. [Google Scholar]
- Sisay, T.; Wami, R. Adverse drug reactions among major depressive disorders: Patterns by age and gender. Heliyon 2021, 7, e08655. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Shin, J.Y.; Jung, S.Y.; Ahn, S.H.; Lee, S.H.; Kim, S.J.; Seong, J.M.; Chung, S.Y.; Park, B.J. New initiatives for pharmacovigilance in South Korea: Introducing the Korea Institute of Drug Safety and Risk Management (KIDS). Pharmacoepidemiol. Drug Saf. 2014, 23, 1115–1122. [Google Scholar] [CrossRef]
- The Uppsala Monitoring Centre. The Use of the WHO-UMC System for Standarised Case Causality Assessment. Available online: https://www.who.int/docs/default-source/medicines/pharmacovigilance/whocausality-assessment.pdf (accessed on 25 October 2024).
- International Conference on Harmonisation. Post-Aproval Safety Data Management: Definitions and Standard for Expedites Reporting E2D. Available online: https://database.ich.org/sites/default/files/E2D_Guideline.pdf (accessed on 25 October 2024).
- Choi, Y.J.; Choi, C.Y.; Kim, C.U.; Shin, S. A nationwide pharmacovigilance investigation on trends and seriousness of adverse events induced by anti-obesity medication. J. Glob. Health 2023, 13, 04095. [Google Scholar] [CrossRef] [PubMed]
- Fusaroli, M.; Salvo, F.; Begaud, B.; AlShammari, T.M.; Bate, A.; Battini, V.; Brueckner, A.; Candore, G.; Carnovale, C.; Crisafulli, S.; et al. The REporting of A Disproportionality Analysis for DrUg Safety Signal Detection Using Individual Case Safety Reports in PharmacoVigilance (READUS-PV): Explanation and Elaboration. Drug Saf. 2024, 47, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Lee, J.S.; Jang, S.; Lee, S.; Jeon, S.; Lee, S.; Kim, J.H.; Lee, K.H. Polypharmacy and elevated risk of severe adverse events in older adults based on the Korea Institute of Drug Safety and Risk Management-Korea Adverse Event Reporting System Database. J. Korean Med. Sci. 2024, 39, e205. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.; Laflamme, L.; Bültmann, U.; Möller, J. Number of medications and adverse drug events by unintentional poisoning among older adults in consideration of inappropriate drug use: A Swedish population-based matched case-control study. Eur. J. Clin. Pharmacol. 2017, 73, 743–749. [Google Scholar] [CrossRef]
- Gavazova, E.; Staynova, R.; Grekova-Kafalova, D. Managing polypharmacy through medication review tools—Pros and cons. Folia Medica 2024, 66, 161–170. [Google Scholar] [CrossRef]
- Gautam, S.; Jain, A.; Gautam, M.; Vahia, V.N.; Grover, S. Clinical practice guidelines for the management of depression. Indian J. Psychiatry 2017, 59 (Suppl. 1), S34–S50. [Google Scholar]
- 2023 American Geriatric Society Beers Criteria® Update Expert Panel. American Geratric Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 2023, 71, 2052–2081. [Google Scholar] [CrossRef]
- Costa, C.; Abeijon, P.; Rodrigues, D.A.; Figueiras, A.; Herdeiro, M.T.; Torre, C. Factors associated with underreporting of adverse drug reactions by patients: A systematic review. Int. J. Clin. Pharm. 2023, 45, 1349–1358. [Google Scholar] [CrossRef]
- Zakaraya, Z.; Abu Assab, M.; Tamimi, L.N.; Karameh, N.; Hailat, M.; Al-Omari, L.; Abu Dayyih, W.; Alasasfeh, O.; Awad, M.; Awad, R. Pharmacokinetics and pharmacodynamics: A Comprehensive analysis of the absorption, distribution, metabolism, and excretion of psychiatric drugs. Pharmaceuticals 2024, 17, 280. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Yabluchanskiy, A.; Csiszar, A. Potential adverse cardiovascular effects of treatment with fluoxetine and other selective serotonin reuptake inhibitors (SSRIs) in patients with geriatric depression. Implications for Atherogenesis and cerebromicrovascular dysregulation. Front. Genet. 2019, 10, 898. [Google Scholar] [CrossRef]
- Clark, A.; Tate, B.; Urban, B.; Schroeder, R.; Gennuso, S.; Ahmadzadeh, S.; McGregor, D.; Girma, B.; Shekoohi, S.; Kaye, A.D. Bupropion mediated effects on depression, attention deficit hyperactivity disorder, and smoking cessation. Health Psychol. Res. 2023, 11, 81043. [Google Scholar] [CrossRef] [PubMed]
- Frisina, P.G.; Borod, J.C.; Foldi, N.S.; Tenenbaum, H.R. Depression in Parkinson’s disease: Health risks, etiology, and treatment options. Neuropsychiatr. Dis. Treat. 2008, 4, 81–91. [Google Scholar] [PubMed]
- Marsh, L. Depression and Parkinson’s disease: Current knowledge. Curr. Neurol. Neurosci. Rep. 2013, 13, 409. [Google Scholar] [CrossRef]
- Byers, A.L.; Yaffe, K. Depression and risk of developing dementia. Nat. Rev. Neurol. 2011, 7, 323–331. [Google Scholar] [CrossRef]
- Reeve, E.; Trenaman, S.C.; Rockwood, K.; Hilmer, S.N. Pharmacokinetic and pharmacodynamic alterations in older people with dementia. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 651–668. [Google Scholar] [CrossRef]
- Tan, E.Y.L.; Köhler, S.; Hamel, R.E.G.; Muñoz-Sánchez, J.L.; Verhey, F.R.J.; Ramakers, I. Depressive symptoms in mild cognitive impairment and the risk of dementia: A systematic review and comparative meta-analysis of clinical and community-based studies. J. Alzheimers Dis. 2019, 67, 1319–1329. [Google Scholar] [CrossRef]
- Pagonabarraga, J.; Álamo, C.; Castellanos, M.; Díaz, S.; Manzano, S. Depression in major neurodegenerative diseases and strokes: A critical review of similarities and differences among neurological disorders. Brain Sci. 2023, 13, 318. [Google Scholar] [CrossRef]
- Mo, S.H.; Lee, S.H.; Choi, C.Y.; Sunwoo, Y.; Shin, S.; Choi, Y.J. A comprehensive 10-year nationwide pharmacovigilance surveillance on antibacterial agents in Korea: Data mining for signal detection of trends and seriousness of adverse events. Microorganisms 2025, 13, 136. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Scandlyn, M.J.; Stuart, E.C.; Rosengren, R.J. Sex-specific differences in CYP450 isoforms in humans. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 413–424. [Google Scholar] [CrossRef]
- Eid, R.S.; Gobinath, A.R.; Galea, L.A.M. Sex differences in depression: Insights from clinical and preclinical studies. Prog. Neurobiol. 2019, 176, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, K.P.; Mazure, C.M.; Staley, J.K. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry 2007, 62, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Sagar-Ouriaghli, I.; Godfrey, E.; Bridge, L.; Meade, L.; Brown, J.S.L. Improving mental health service utilization among men: A systematic review and synthesis of behavior change techniques within interventions targeting help-seeking. Am. J. Mens. Health 2019, 13, 1557988319857009. [Google Scholar] [CrossRef] [PubMed]
- Sophie, H.B.; Sanne, A.E.P.; Mark, W. Sex differences in coronary heart disease and stroke mortality: A global assessment of the effect of ageing between 1980 and 2010. BMJ Glob. Health 2017, 2, e000298. [Google Scholar]
- Lee, K.M.N.; Rushovich, T.; Gompers, A.; Boulicault, M.; Worthington, S.; Lockhart, J.W.; Richardson, S.S. A gender hypothesis of sex disparities in adverse drug events. Soc. Sci. Med. 2023, 339, 116385. [Google Scholar] [CrossRef]
| Characteristics | No. of Cases (n) | Percentage |
|---|---|---|
| Sex a | ||
| Men | 6305 | 29.88% |
| Women | 13,862 | 65.70% |
| Age (56.5 ± 18.1) b | ||
| 0~9 | 110 | 0.52% |
| 10~19 | 371 | 1.78% |
| 20~29 | 1177 | 5.58% |
| 30~39 | 1213 | 5.75% |
| 40~49 | 1787 | 8.47% |
| 50~59 | 2917 | 13.82% |
| 60~69 | 3544 | 16.79% |
| 70~79 | 3013 | 14.28% |
| 80~89 | 1061 | 5.03% |
| 90~99 | 50 | 0.24% |
| Causality | ||
| Certain | 292 | 1.38% |
| Probable/Likely | 3605 | 17.08% |
| Possible | 17,206 | 81.53% |
| Seriousness | ||
| Non-serious ADE | 20,902 | 99.05% |
| Serious ADE | 201 | 0.95% |
| Reporter Types | ||
| Doctors | 4230 | 20.04% |
| Pharmacists | 8764 | 41.53% |
| Other Healthcare Professionals | 5399 | 25.58% |
| General Public | 2057 | 9.75% |
| Unknown | 651 | 3.08% |
| Number of Concomitant Medications | ||
| 1 | 11,427 | 54.15% |
| 2 | 2903 | 13.76% |
| 3 | 2168 | 10.27% |
| 4 | 1594 | 7.55% |
| ≥5 | 3011 | 14.27% |
| SAE (n = 201) | Non-SAE (n = 20,902) | Total (n = 21,103) | |
|---|---|---|---|
| SSRI | 77 (38.31%) | 8170 (39.09%) | 8247 (39.08%) |
| citalopram | 0 (0.00%) | 1 (0.00%) | 1 (0.00%) |
| escitalopram | 33 (16.42%) | 3616 (17.30%) | 3649 (17.29%) |
| fluoxetine | 19 (9.45%) | 1152 (5.51%) | 1171 (5.55%) |
| paroxetine | 10 (4.98%) | 1050 (5.02%) | 1060 (5.02%) |
| sertraline | 3 (1.49) | 785 (3.76%) | 788 (3.73%) |
| vortioxetine | 12 (5.97%) | 1566 (7.49%) | 1578 (7.48%) |
| SNRI | 72 (35.82%) | 6729 (32.19%) | 6801 (32.22%) |
| duloxetine | 65 (32.34%) | 6115 (29.26%) | 6180 (29.28%) |
| venlafaxine | 7 (3.48%) | 614 (2.94%) | 621 (2.94%) |
| TCA | 33 (16.42%) | 4443 (21.26%) | 4476 (21.21%) |
| amitriptyline | 31 (0.1%) | 4080 (19.52%) | 4111 (19.48%) |
| clomipramine | 0 (0.00%) | 23 (0.11%) | 23 (0.11%) |
| imipramine | 2 (1.00%) | 340 (1.63%) | 342 (1.62%) |
| Others | 19 (9.45%) | 1560 (7.46%) | 1579 (7.48%) |
| bupropion | 12 (5.97%) | 758 (3.63%) | 770 (3.65%) |
| mirtazapine | 3 (1.49%) | 243 (1.16%) | 246 (1.17%) |
| tianeptine | 2 (1.00%) | 73 (0.35%) | 75 (0.36%) |
| trazodone | 2 (1.00%) | 486 (2.33%) | 488 (2.31%) |
| SSRI | SNRI | TCA | Others | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Citalopram (n = 1) | Escitalopram (n = 3649) | Fluoxetine (n = 1171) | Paroxetine (n = 1060) | Sertraline (n = 788) | Vortioxetine (n = 1578) | Duloxetine (n = 6180) | Venlafaxine (n = 621) | Amitriptyline (n = 4111) | Clomipramine (n = 23) | Imipramine (n = 342) | Bupropion (n = 770) | Mirtazapine (n = 246) | Tianeptine (n = 75) | Trazodone (n = 488) | |
| Skin and appendage disorders | 0 (0.00%) | 249 (6.82%) | 92 (7.86%) | 47 (4.43%) | 40 (5.08%) | 123 (7.79%) | 350 (5.66%) | 31 (4.99%) | 216 (5.25%) | 1 (4.35%) | 14 (4.09%) | 60 (7.79%) | 13 (5.28%) | 2 (2.67%) | 15 (3.07%) |
| Musculo-skeletal system disorders | 0 (0.00%) | 46 (1.26%) | 41 (3.50%) | 7 (0.66%) | 4 (0.51%) | 8 (0.51%) | 59 (0.95%) | 10 (1.61%) | 28 (0.68%) | 0 (0.00%) | 1 (0.29%) | 22 (2.86%) | 2 (0.81%) | 1 (1.33%) | 8 (1.64%) |
| Collagen disorders | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.02%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Central and peripheral nervous system disorders | 0 (0.00%) | 739 (20.25%) | 193 (16.48%) | 202 (19.06%) | 153 (19.42%) | 284 (18.00%) | 1370 (22.17%) | 118 (19.00%) | 849 (20.65%) | 0 (0.00%) | 54 (15.79%) | 175 (22.73%) | 46 (18.70%) | 16 (21.33%) | 103 (21.11%) |
| Vision disorders | 0 (0.00%) | 30 (0.82%) | 21 (1.79%) | 17 (1.60%) | 15 (1.90%) | 18 (1.14%) | 46 (0.74%) | 7 (1.13%) | 46 (1.12%) | 0 (0.00%) | 4 (1.17%) | 7 (0.91%) | 1 (0.41%) | 1 (1.33%) | 3 (0.61%) |
| Hearing and vestibular disorders | 0 (0.00%) | 10 (0.27%) | 2 (0.17%) | 2 (0.19%) | 1 (0.13%) | 5 (0.32%) | 3 (0.05%) | 0 (0.00%) | 9 (0.22%) | 0 (0.00%) | 0 (0.00%) | 4 (0.52%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Special sense, other disorders | 0 (0.00%) | 14 (0.38%) | 3 (0.26%) | 0 (0.00%) | 0 (0.00%) | 1 (0.06%) | 16 (0.26%) | 4 (0.64%) | 21 (0.51%) | 0 (0.00%) | 2 (0.58%) | 7 (0.91%) | 0 (0.00%) | 0 (0.00%) | 5 (1.02%) |
| Psychiatric disorders | 0 (0.00%) | 932 (25.54%) | 347 (29.63%) | 263 (24.81%) | 208 (26.40%) | 317 (20.09%) | 936 (15.15%) | 127 (20.45%) | 1077 (26.20%) | 7 (30.43%) | 46 (13.45%) | 200 (25.97%) | 78 (31.71%) | 18 (24.00%) | 101 (20.70%) |
| Gastro-intestinal system disorders | 1 (100%) | 828 (22.69%) | 226 (19.30%) | 295 (27.83%) | 212 (26.90%) | 593 (37.58%) | 2438 (39.45%) | 190 (30.60%) | 1056 (25.69%) | 9 (39.13%) | 156 (45.61%) | 166 (21.56%) | 42 (17.07%) | 19 (25.33%) | 123 (25.20%) |
| Liver and biliary system disorders | 0 (0.00%) | 25 (0.69%) | 8 (0.68%) | 4 (0.38%) | 8 (1.02%) | 3 (0.19%) | 22 (0.36%) | 6 (0.97%) | 25 (0.61%) | 0 (0.00%) | 2 (0.58%) | 7 (0.91%) | 6 (2.44%) | 0 (0.00%) | 2 (0.41%) |
| Metabolic and nutritional disorders | 0 (0.00%) | 170 (4.66%) | 27 (2.31%) | 45 (4.25%) | 42 (5.33%) | 46 (2.92%) | 113 (1.83%) | 25 (4.03%) | 130 (3.16%) | 0 (0.00%) | 12 (3.51%) | 14 (1.82%) | 22 (8.94%) | 3 (4.00%) | 38 (7.79%) |
| Endocrine disorders | 0 (0.00%) | 3 (0.08%) | 0 (0.00%) | 0 (0.00%) | 2 (0.25%) | 1 (0.06%) | 2 (0.03%) | 1 (0.16%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.41%) | 0 (0.00%) | 0 (0.00%) |
| Cardiovascular disorders, general | 0 (0.00%) | 40 (1.10%) | 26 (2.22%) | 29 (2.74%) | 2 (0.25%) | 28 (1.77%) | 36 (0.58%) | 25 (4.03%) | 37 (0.90%) | 0 (0.00%) | 7 (2.05%) | 25 (3.25%) | 5 (2.03%) | 0 (0.00%) | 35 (7.17%) |
| Myo-, endo-, pericardial, and valve disorders | 0 (0.00%) | 2 (0.05%) | 1 (0.09%) | 0 (0.00%) | 1 (0.13%) | 1 (0.06%) | 0 (0.00%) | 1 (0.16%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (1.33%) | 0 (0.00%) |
| Heart rate and rhythm disorders | 0 (0.00%) | 64 (1.75%) | 44 (3.76%) | 10 (0.94%) | 11 (1.40%) | 15 (0.95%) | 88 (1.42%) | 6 (0.97%) | 60 (1.46%) | 0 (0.00%) | 3 (0.88%) | 19 (2.47%) | 2 (0.81%) | 0 (0.00%) | 2 (0.41%) |
| Vascular (extracardiac) disorders | 0 (0.00%) | 1 (0.03%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 5 (0.32%) | 5 (0.08%) | 0 (0.00%) | 2 (0.05%) | 0 (0.00%) | 1 (0.29%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Respiratory system disorders | 0 (0.00%) | 45 (1.23%) | 15 (1.28%) | 5 (0.47%) | 6 (0.76%) | 16 (1.01%) | 58 (0.94%) | 5 (0.81%) | 36 (0.88%) | 0 (0.00%) | 2 (0.58%) | 9 (1.17%) | 2 (0.81%) | 0 (0.00%) | 1 (0.20%) |
| Red blood cell disorders | 0 (0.00%) | 4 (0.11%) | 2 (0.17%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.02%) | 0 (0.00%) | 3 (0.07%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| White cell and RES | 0 (0.00%) | 6 (0.16%) | 0 (0.00%) | 3 (0.28%) | 2 (0.25%) | 0 (0.00%) | 11 (0.18%) | 1 (0.16%) | 15 (0.36%) | 0 (0.00%) | 0 (0.00%) | 1 (0.13%) | 0 (0.00%) | 0 (0.00%) | 1 (0.20%) |
| Platelet, bleeding, and clotting disorders | 0 (0.00%) | 15 (0.41%) | 8 (0.68%) | 2 (0.19%) | 2 (0.25%) | 5 (0.32%) | 12 (0.19%) | 0 (0.00%) | 9 (0.22%) | 0 (0.00%) | 0 (0.00%) | 1 (0.13%) | 0 (0.00%) | 0 (0.00%) | 3 (0.61%) |
| Urinary system disorders | 0 (0.00%) | 44 (1.21%) | 10 (0.85%) | 30 (2.83%) | 17 (2.16%) | 13 (0.82%) | 146 (2.36%) | 8 (1.29%) | 163 (3.96%) | 1 (4.35%) | 21 (6.14%) | 5 (0.65%) | 9 (3.66%) | 1 (1.33%) | 3 (0.61%) |
| Reproductive disorders (male) | 0 (0.00%) | 16 (0.44%) | 3 (0.26%) | 12 (1.13%) | 1 (0.13%) | 0 (0.00%) | 6 (0.10%) | 3 (0.48%) | 3 (0.07%) | 0 (0.00%) | 1 (0.29%) | 2 (0.26%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Reproductive disorders (female) | 0 (0.00%) | 12 (0.33%) | 18 (1.54%) | 5 (0.47%) | 5 (0.63%) | 7 (0.44%) | 7 (0.11%) | 2 (0.32%) | 13 (0.32%) | 0 (0.00%) | 1 (0.29%) | 0 (0.00%) | 1 (0.41%) | 1 (1.33%) | 0 (0.00%) |
| Neoplasms | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.02%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Body-as-a-whole general disorders | 0 (0.00%) | 317 (8.69%) | 81 (6.92%) | 78 (7.36%) | 56 (7.11%) | 69 (4.37%) | 422 (6.83%) | 43 (6.92%) | 306 (7.44%) | 5 (21.74%) | 15 (4.39%) | 45 (5.84%) | 16 (6.50%) | 12 (16.00%) | 44 (9.02%) |
| Application site disorders | 0 (0.00%) | 1 (0.03%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.06%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 00 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Resistance mechanism disorders | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 2 (0.03%) | 0 (0.00%) | 2 (0.05%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Secondary terms—events | 0 (0.00%) | 36 (0.99%) | 3 (0.26%) | 4 (0.38%) | 0 (0.00%) | 19 (1.20%) | 29 (0.47%) | 7 (1.13%) | 5 (0.12%) | 0 (0.00%) | 0 (0.00%) | 1 (0.13%) | 0 (0.00%) | 0 (0.00%) | 1 (0.20%) |
| Poison-specific terms | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (0.16%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
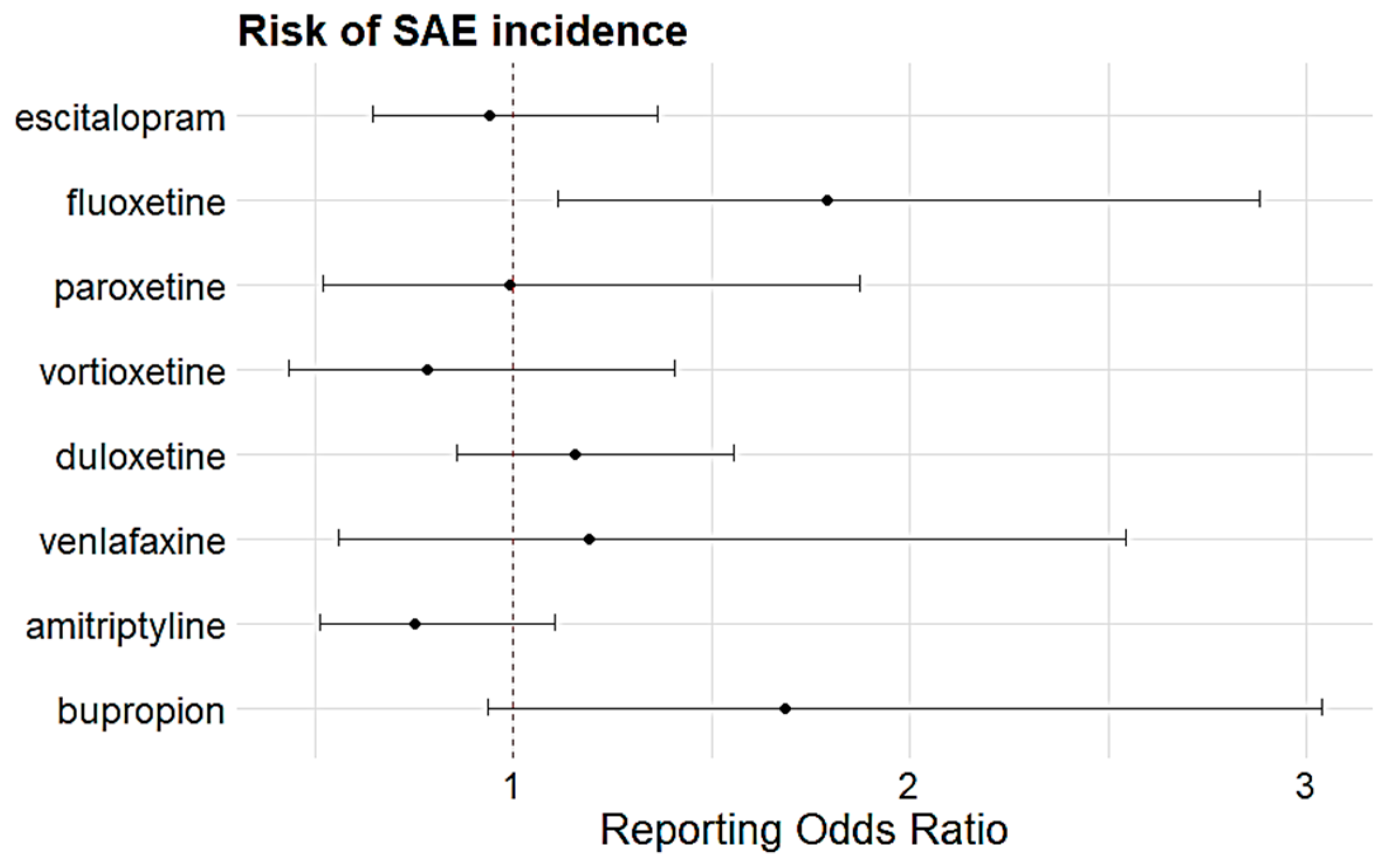
| Antidepressants | Sensitivity | ROR (95% CI) | p-Value |
|---|---|---|---|
| SSRIs | |||
| escitalopram | Age & Sex | 0.96 (0.64–1.44) | 0.85 |
| Age ≥ 60 years | 1.34 (0.81–2.24) | 0.259 | |
| Causality | 2.42 (1.24–4.73) | 0.01 | |
| fluoxetine | Age and Sex | 2.62 (1.58–4.36) | <0.001 |
| paroxetine | Age and Sex | 0.88 (0.45–1.72) | 0.705 |
| vortioxetine | Age and Sex | 1.05 (0.55–1.99) | 0.886 |
| Age ≥ 60 years | 1.63 (0.71–3.76) | 0.254 | |
| SNRIs | |||
| duloxetine | Age and Sex | 0.87 (0.62–1.22) | 0.416 |
| Age ≥ 60 years | 1.17 (0.76–1.81) | 0.478 | |
| Causality | 0.60 (0.31–1.13) | 0.11 | |
| venlafaxine | Age and Sex | 1.32 (0.62–2.83) | 0.476 |
| TCA | |||
| amitriptyline | Age and Sex | 0.76 (0.52–1.13) | 0.175 |
| Age ≥ 60 years | 0.51 (0.28–0.92) | 0.026 | |
| Others | |||
| bupropion | Age and Sex | 2.35 (1.26–4.37) | 0.007 |
| Age ≥ 60 years | 5.08 (2.17–11.89) | <0.001 | |
| Causality | 3.92 (1.52–10.13) | 0.005 | |
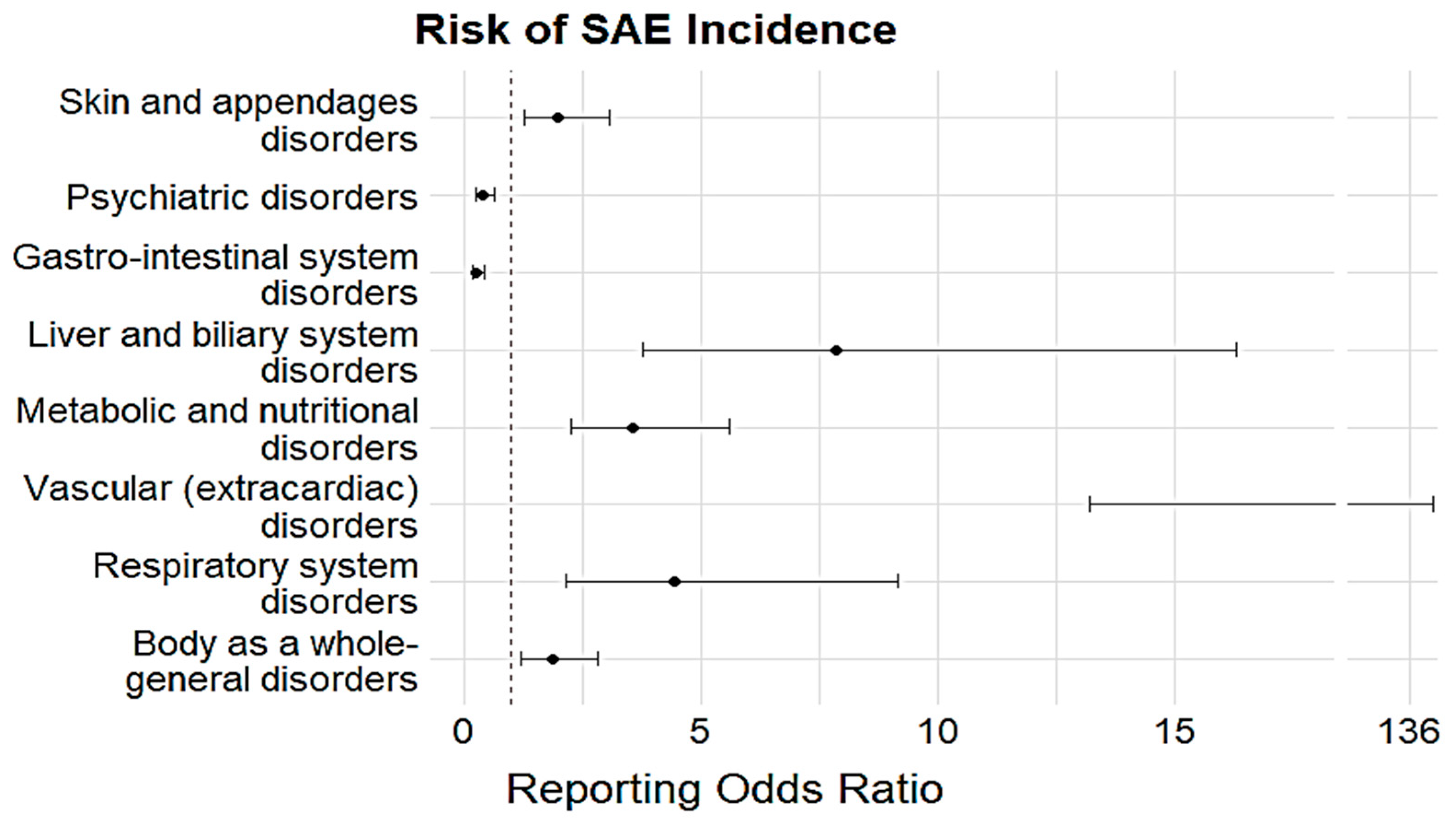
| SOC | Sensitivity | ROR | p-Value |
|---|---|---|---|
| Skin and appendage disorders | Age and Sex | 2.07 (1.29–3.31) | 0.003 |
| Age ≥ 60 years | 2.81 (1.52–5.21) | 0.001 | |
| Central and peripheral nervous system disorders | Age and Sex | 1.23 (0.87–1.74) | 0.243 |
| Age ≥ 60 years | 1.14 (0.70–1.87) | 0.602 | |
| Causality | 3.08 (1.67–5.61) | <0.001 | |
| Psychiatric disorders | Age and Sex | 0.42 (0.26–0.69) | <0.001 |
| Gastro-intestinal system disorders | Age and Sex | 0.30 (0.19–0.47) | <0.001 |
| Age ≥ 60 years | 0.33 (0.18–0.62) | <0.001 | |
| Causality | 0.37 (0.17–0.83) | 0.016 | |
| Liver and biliary system disorders | Age and Sex | 10.66 (5.04–22.53) | <0.001 |
| Metabolic and nutritional disorders | Age and Sex | 4.12 (2.53–6.69) | <0.001 |
| Age ≥ 60 years | 8.53 (4.93–14.75) | <0.001 | |
| Causality | 12.19 (5.47–27.20) | <0.001 | |
| Respiratory system disorders | Age and Sex | 5.26 (2.54–10.92) | <0.001 |
| Age ≥ 60 years | 8.76 (3.69–20.82) | <0.001 | |
| Body-as-a-whole general disorders | Age and Sex | 1.82 (1.14–2.92) | 0.012 |
| Age ≥ 60 years | 2.00 (1.05–3.78) | 0.034 | |
| Causality | 1.41 (0.50–3.98) | 0.514 |
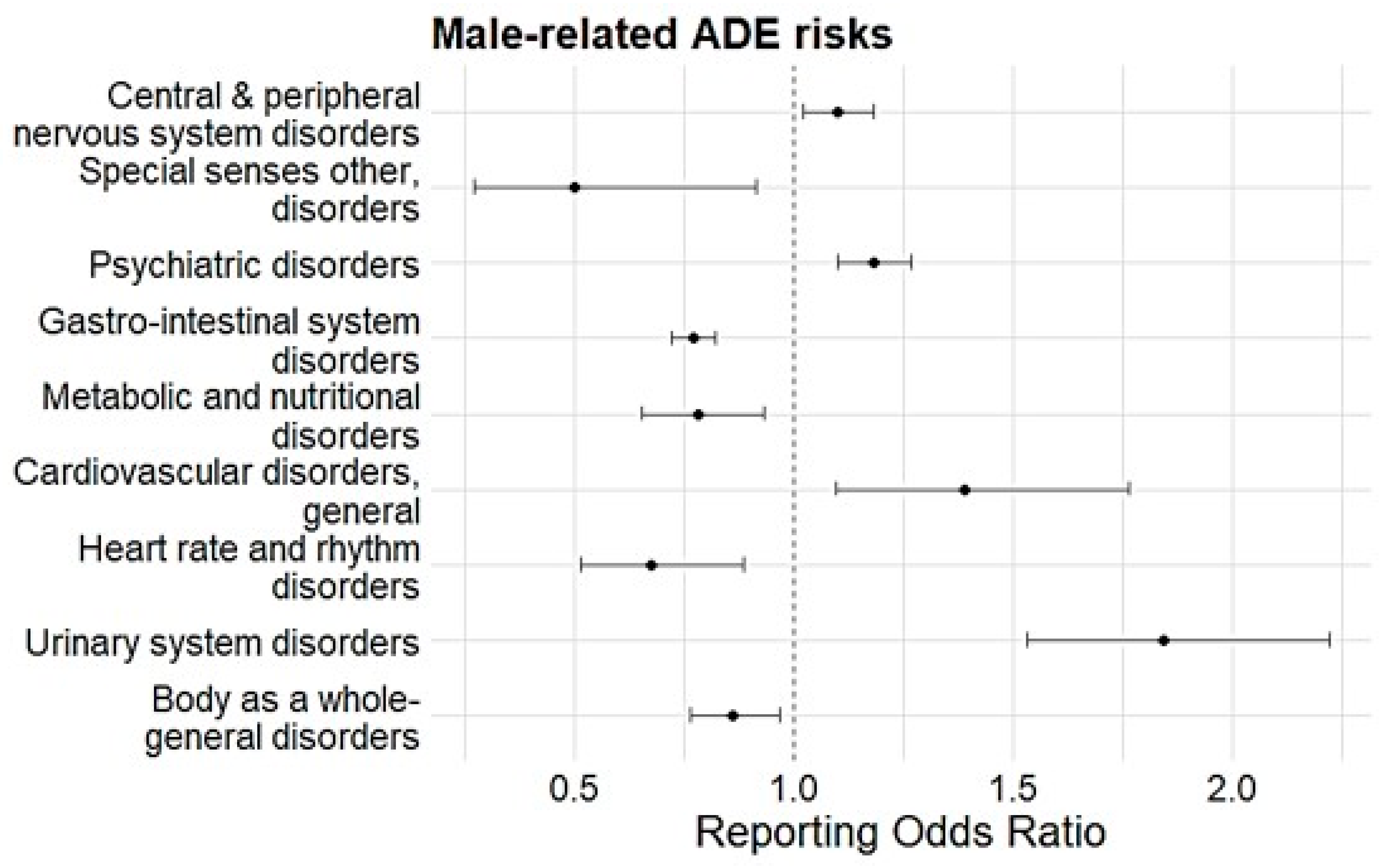
| Male-Related SOC | Sensitivity | ROR | p-Value |
|---|---|---|---|
| Vision disorders | Age and Sex | 0.05 (0.04–0.07) | <0.001 |
| Special sense, other disorders | Age and Sex | 0.47 (0.23–0.97) | 0.04 |
| Psychiatric disorders | Age and Sex | 1.19 (1.10–1.30) | <0.001 |
| Age ≥ 60 years | 1.14 (1.01–1.28) | <0.001 | |
| Causality | 0.82 (0.70–0.97) | 0.021 | |
| Gastro-intestinal system disorders | Age and Sex | 0.75 (0.70–0.81) | <0.001 |
| Age ≥ 60 years | 0.74 (0.66–0.82) | <0.001 | |
| Causality | 1.42 (1.22–1.65) | <0.001 | |
| Urinary system disorders | Age and Sex | 1.78 (1.44–2.21) | <0.001 |
| Age ≥ 60 years | 2.15 (1.66–2.80) | <0.001 | |
| Causality | 0.49 (0.31–0.76) | 0.002 | |
| Cardiovascular disorders, general | Age and Sex | 1.32 (1.02–1.70) | 0.032 |
| Heart rate and rhythm disorders | Age and Sex | 0.66 (0.48–0.92) | 0.013 |
| Causality | 2.93 (1.45–5.93) | 0.003 | |
| Body-as-a-whole general disorders | Age and Sex | 0.87 (0.75–1.00) | 0.044 |
| Age ≥ 60 years | 0.80 (0.65–0.97) | 0.026 | |
| Sin and appendage disorders | Age ≥ 60 years | 1.24 (1.01–1.53) | <0.001 |
| Central and peripheral nervous system | Causality | 0.85 (0.72–1.00) | 0.046 |
| Metabolic and nutritional disorders | Age ≥ 60 years | 8.53 (4.93–14.75) | <0.001 |
| Liver and biliary system disorders | Causality | 0.20 (0.08–0.52) | 0.001 |
| Platelet, bleeding, and clotting disorders | Age ≥ 60 years | 2.91 (1.27–6.65) | 0.011 |
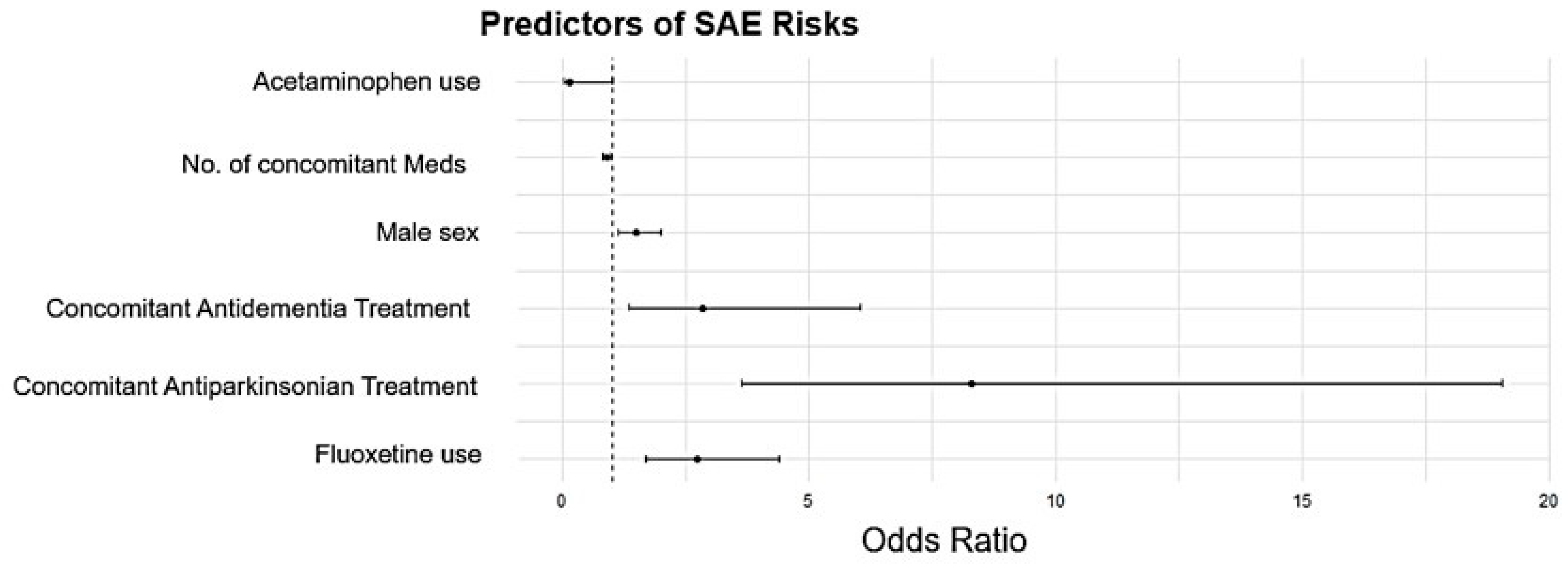
| Predictors | Sensitivity | OR | p-Value |
|---|---|---|---|
| Fluoxetine use | Age and Sex | 3.13 (1.88–5.22) | <0.001 |
| Antiparkinsonian treatment | Age and Sex | 8.86 (3.76–20.86) | <0.001 |
| Age ≥ 60 years | 5.67 (2.22–14.50) | <0.001 | |
| Causality | 46.97 (10.81–204.14) | <0.001 | |
| Antidementia treatment | Age and Sex | 2.95 (1.38–6.33) | 0.005 |
| Age ≥ 60 years | 1.56 (1.19–2.04) | 0.001 | |
| No. of concomitant Meds | Age and Sex | 0.87 (0.79–0.96) | 0.004 |
| Causality | 1.12 (1.01–1.25) | 0.036 | |
| Aging | Age ≥ 60 years | 11.16 (4.28–29.09) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Kim, M.; Kim, Y.; Lee, S.H.; Shin, S.; Choi, Y.J. Identifying Predictors of Serious Adverse Events in Antidepressant Treatment from a Decade-Long Nationwide Pharmacovigilance Study: Impact of Dementia and Parkinson’s Disease Treatment. Medicina 2025, 61, 1103. https://doi.org/10.3390/medicina61061103
Han J, Kim M, Kim Y, Lee SH, Shin S, Choi YJ. Identifying Predictors of Serious Adverse Events in Antidepressant Treatment from a Decade-Long Nationwide Pharmacovigilance Study: Impact of Dementia and Parkinson’s Disease Treatment. Medicina. 2025; 61(6):1103. https://doi.org/10.3390/medicina61061103
Chicago/Turabian StyleHan, Jungmin, Minsung Kim, Yujin Kim, Soo Hyeon Lee, Sooyoung Shin, and Yeo Jin Choi. 2025. "Identifying Predictors of Serious Adverse Events in Antidepressant Treatment from a Decade-Long Nationwide Pharmacovigilance Study: Impact of Dementia and Parkinson’s Disease Treatment" Medicina 61, no. 6: 1103. https://doi.org/10.3390/medicina61061103
APA StyleHan, J., Kim, M., Kim, Y., Lee, S. H., Shin, S., & Choi, Y. J. (2025). Identifying Predictors of Serious Adverse Events in Antidepressant Treatment from a Decade-Long Nationwide Pharmacovigilance Study: Impact of Dementia and Parkinson’s Disease Treatment. Medicina, 61(6), 1103. https://doi.org/10.3390/medicina61061103







