Improving Outcomes in Pancreatic Adenocarcinoma: A Systematic Review of Immunotherapy in Multimodal Treatment
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Selection and Data Collection Process
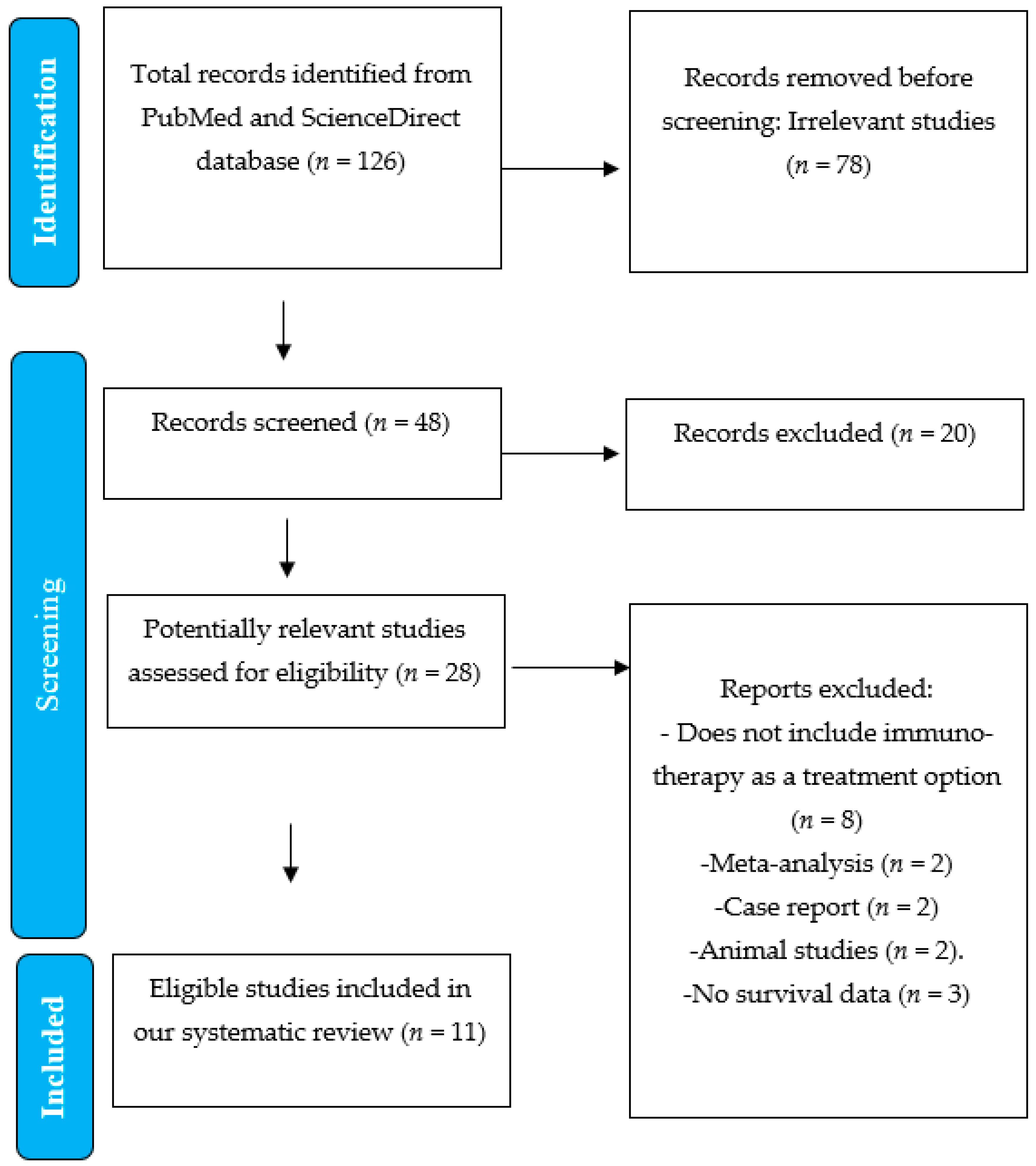
3. Results
Study Selection
- 1 retrospective study
- 6 randomized controlled trials
- 4 non-randomized controlled trials
- Two were Phase 1 trials, assessing safety and initial efficacy.
- Two were Phase 1/2 trials, evaluating safety, dosing, and preliminary therapeutic outcomes.
- Five were Phase 2 trials, focusing on treatment efficacy and adverse event profiles.
- One was a Phase 2/3 trial, designed to assess both efficacy and long-term clinical benefits in a larger patient cohort.
- One was a retrospective study, analyzing real-world treatment outcomes.
4. Discussion
Limitations
| Stage of Cancer | Reference | Status of Clinical Trial | Study Phase | Immunotherapy + Chemotherapy Group | Chemotherapy Group | Number of Patients | Mean Age of Patients | Median Overall Survival, Months (95% CI); p-Value | Median Progression-Free Survival (95% CI); p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Metastatic | Ma J. et al. (2020) [8] | Completed | Retrospective | A = Pembrolizumab + Chemotherapy (n = 4); Nivolumab + Chemotherapy (n = 17); Atezolizumab + Chemotherapy (n = 1) | B = Chemotherapy Group (n = 36) | 58 | A = 56 B = 54 | A = 18.1 months B = 6.1 months p = 0.021 | A = 3.24 months B = 2.14 months p = 0.041 |
| 2. | Borderline Resectable and Metastatic | Padron L. J. et al. (2022) [18] | Abandoned | 2 | A = Nivolumab + Chemotherapy (n = 37) C = Sotigalimab + Chemotherapy + Nivolumab (n= 31) | B = Sotigalimab + Chemotherapy (n = 31) | 105 (Stage I-III: n = 25 Stage IV: n = 80) | N/A | A = 16.7 months B = 11.4 months C = 10.1 months p = N/A | A = 8.1 months B = 7.3 months C = 6.7 months p = N/A |
| 3. | Borderline Resectable PDAC | Katz M. H. G. et al. (2023) [12] | Abandoned | 1b/2 | A = Pembrolizumab + Chemotherapy (n = 24) | B = Chemotherapy (n = 13) | 37 (Stage IA: n = 4 Stage IB: n = 12 Stage IIA: n = 8 Stage IIB: n = 6 Stage III: n = 7) | A = 66 B = 64 | A = 27.8 months B = 24.3 months p = N/A | A = 18.2 months B = 14.1 months p = N/A |
| 4. | Metastatic | Ko A. H. et al. (2023) [29] | Abandoned | 1b/2 | A = Atezolizumab +PEGPH20 (n = 71) | B = Chemotherapy (n = 46) | 117 | A = 60 B = 62 | A = 7.1 months B = 6.8 months p = N/A | A = 1.5 months B = 2.3 months p = N/A |
| 5. | Metastatic | O’Hara M. H. et al. (2021) [20] | Moved onto next phase | 1b | A1 = Chemotherapy + Sotigalimab 0.1 mg/kg + Nivolumab (n = 6) A2 = Chemotherapy + Sotigalimab 0.3 mg/kg + Nivolumab (n = 6) | B1 = Chemotherapy + Sotigalimab 0.1 mg/kg (n = 6) B2 = Chemotherapy +Sotigalimab 0.3 mg/kg (n = 6) | 24 | A1 = 68 A2 = 63 B1 = 68 B2 = 61 | A1 = 12.7 months A2 = 20.1 months B1 = 15.6 months B2 = Not estimable | A1 = 12.5 months A2 = 10.4 months B1 = 10.8 months B2 = 12.4 months p = N/A |
| 6. | Metastatic | Renouf D. J. et al. (2022) [13] | Abandoned | 2 | A = Chemotherapy + Durvalumab + Tremelimumab (n = 119) | B = Chemotherapy (n = 61) | 180 | N/A | A = 9.8 months B = 8.8 months p = 0.72 | A = 5.5 months B = 5.4 months p = 0.91 |
| 7. | Borderline to Metastatic | Michael O. et al. (2020) [22] | Abandoned | 2 | A = Acalabrutinib + Pembrolizumab (n = 38) | B = Acalabrutinib (n = 35) | 73 (Stage I-III: n = 13 Stage IV: n = 58 No data: n = 2) | 64 | A = 3.6 months B = 3.8 months p = N/A | A = 1.4 months B = 1.4 months p = N/A |
| 8. | Metastatic | Devalingam M. et al. (2020) [28] | Moved onto next phase | 1b | A = Chemotherapy + Pelareorep + Pembrolizumab (n = 11) | 11 | 64 | A = 3.1 months | A = 2 months | |
| 9. | Metastatic | Bruno B. et al. (2021) [24] | Moved onto next phase | 2 | A = Chemotherapy + Motixafortide + Pembrolizumab | 39 | N/A | A = 6.6 months | A = 3.8 months | |
| 10. | Metastatic | O’Reilly E. M. et al. (2019) [16] | Abandoned | 2 | A = Durvalumab + Tremelimumab (n = 32) B = Durvalumab (n = 32) | 64 | 65 | A = 3.1 months B = 3.6 months p = N/A | A = 1.5 months B = 1.5 months p = N/A | |
| 11. | Metastatic | Bruno B. et al. (2020) [26] | Abandoned | 2a | A = Bl-8040 + Pembrolizumab (n = 37) B = BL-8040 + Pembrolizumab + Chemotherapy (n = 22) | 59 | 68 | A1 = 3.3 months (third line therapy or beyond) A2 = 7.5 months (second line therapy B = 7.8 months p = N/A | N/A |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| The following abbreviations are used in this manuscript: |
| PDAC | Pancreatic ductal adenocarcinoma |
| ctDNA | Circulating tumor deoxyribonucleic acid |
| SPARC | Secreted Protein Acidic and Rich in Cysteine |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene Homolog |
| RAS | Rat sarcoma |
| RCTs | Randomized controlled trials |
| ECM | Extracellular matrix |
| EMT | Epithelial-to-mesenchymal transition |
| PD-1 | Checkpoint inhibitors targeting the programmed cell death protein 1 |
| OS | Overall survival |
| PFS | Progression-free survival |
| PD-L1 | Monoclonal antibody that targets programmed cell death ligand 1 |
| CRT | Chemoradiotherapy |
| mOS | Median overall survival |
| BTK | Bruton tyrosine kinase |
| CTLA-4 | Monoclonal antibody targeting cytotoxic T-Lymphocyte-associated protein-3 |
| CXCR4 | Chemokine receptor type 4 |
References
- Trifan, A.; Gheorghe, C.; Dumitrașcu, D.; Diculescu, M.; Gheorghe, L.; Sporea, I.; Tantau, M.; Ciurea, T. Gastroenterologie și Hepatologie Clinica; Editura Medicală: Bucureşti, Romania, 2018; pp. 472–481. [Google Scholar]
- Fabiana, P.; Ingrid, G. Focus on Pancreatic cancer microenvironment. Curr. Oncol. 2024, 31, 4241–4260. [Google Scholar]
- Crişan, A.; Semenescu, L.E.; Mireştean, C.C.; Mitrea, A.; Iancu, I.R.; Iancu, D.P.T. FOLFIRINOX in adjuvant and metastatic settings for pancreatic cancer in the era of precision oncology. Oncol.-Hematol. 2021, 10, 457. [Google Scholar] [CrossRef]
- Khoschy, S.; Matia, A.; Jonathan, N.; Koenraad, J. Pancreatic ductal Adenocarcinoma and its Variants: Pearls and Perils. RadioGraphics 2020, 40, 5. [Google Scholar]
- Karamitopoulou, E. Molecular Pathology of Pancreatic Cancer. Cancers 2022, 14, 16. [Google Scholar] [CrossRef]
- Brown, B.A.; Myers, P.J.; Adair, S.J.; Pitarresi, J.R.; Sah-Teli, S.K.; Campbell, L.A.; Hart, W.S.; Barbeau, M.C.; Leong, K.; Seyler, N.; et al. A histone Metylation-MAPK signaling axis drives durable epithelial-mesenchymal transition in hypoxic pancreatic cancer. Cancer Res. 2024, 84, 1764–1780. [Google Scholar] [CrossRef]
- Hong, E.; Barczak, W.; Park, S.; Heo, J.S.; Ooshima, A.; Munro, S.; Hong, C.P.; Park, J.; An, H.; Park, J.O.; et al. Combination treatment of T1-44, a PRMT5 inhibitor with Vactoserib, an inhibitor of TGF-β signaling, inhibits invasion and prolongs survival in a mouse model of pancreatic tumors. Cell Death Dis. 2023, 14, 93. [Google Scholar] [CrossRef]
- Ma, J.; Sun, D.; Wang, J.; Han, C.; Qian, Y.; Chen, G.; Li, X.; Zhang, J.; Cui, P.; Du, W.; et al. Immune checkpoint inhibitors combined with chemotherapy for the treatment of advanced pancreatic cancer patients. Cancer Immunol. Immunother. 2020, 69, 365–372. [Google Scholar] [CrossRef]
- Cowzer, D.; Zameer, M.; Conroy, M.; Kolch, W.; Duffy, A.G. Targeting KRAS in Pancreatic Cancer. J. Pers. Med. 2022, 12, 1870. [Google Scholar] [CrossRef]
- Simcock, R.; Wright, J. Beyond performance status. Clin. Oncol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Weiss, G.J.; Blaydorn, L.; Beck, J.; Bornemann-Kolatzki, K.; Urnovitz, H.; Schütz, E.; Khemka, V. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Investig. New Drugs 2018, 36, 96–102. [Google Scholar] [CrossRef]
- Katz, M.H.; Petroni, G.R.; Bauer, T.; Reilley, M.J.; Wolpin, B.M.; Stucky, C.C.; Bekaii-Saab, T.S.; Elias, R.; Merchant, N.; Costa, A.D.; et al. Multicenter randomized controlled trial of neoadjuvant chemoradiotherapy alone or in combination with pembrolizumab in patients with resectable or borderline resectable pancreatic adenocarcinoma. J. Immunother. Cancer 2023, 11, e007586. [Google Scholar] [CrossRef] [PubMed]
- Renouf, D.J.; Loree, J.M.; Knox, J.J.; Topham, J.T.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; et al. The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat. Commun. 2022, 13, 5020. [Google Scholar] [CrossRef] [PubMed]
- Dougan, S.K. The pancreatic cancer microenvironment. Cancer J. Sudbury Mass 2017, 23, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Windon, A.L.; Loaiza-Bonilla, A.; Jensen, C.E.; Randall, M.; Morrissette, J.J.D.; Shroff, S.G. A KRAS wild type mutational status confers a survival advantage in pancreatic ductal adenocarcinoma. J. Gastrointest. Oncol. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Oh, D.Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.C.; Vlahovic, G.; et al. Durvalumab with or Without Tremelimumab for Patients with Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef]
- Di Marco, M.; Grassi, E.; Durante, S.; Vecchiarelli, S.; Palloni, A.; Macchini, M.; Casadei, R.; Ricci, C.; Panzacchi, R.; Santini, D.; et al. State of the art biological therapies in pancreatic cancer. World J. Gastrointest. Oncol. 2016, 8, 55–66. [Google Scholar] [CrossRef]
- Padrón, L.J.; Maurer, D.M.; O’hara, M.H.; O’reilly, E.M.; Wolff, R.A.; Wainberg, Z.A.; Ko, A.H.; Fisher, G.; Rahma, O.; Lyman, J.P.; et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: Clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat. Med. 2022, 28, 1167–1177. [Google Scholar] [CrossRef]
- Patwardhan, A.; Harris, J.; Leng, N.; Bartha, G.; Church, D.M.; Luo, S.; Haudenschild, C.; Pratt, M.; Zook, J.; Salit, M.; et al. Achieving high-sensitivity for clinical applications using augmented exome sequencing. Genome Med. 2015, 7, 71. [Google Scholar] [CrossRef]
- O’Hara, M.H.; O’Reilly, E.M.; Varadhachary, G.; Wolff, R.A.; Wainberg, Z.A.; Ko, A.H.; Fisher, G.; Rahma, O.; Lyman, J.P.; Cabanski, C.R.; et al. CD40 agonistic monoclonal antibody APX005M (sotigalimab) and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma: An open-label, multicentre, phase 1b study. Lancet Oncol. 2021, 22, 118–131. [Google Scholar] [CrossRef]
- Morrison, A.H.; Diamond, M.S.; Hay, C.A.; Byrne, K.T.; Vonderheide, R.H. Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity. Proc. Natl. Acad. Sci. USA 2020, 117, 8022–8031. [Google Scholar] [CrossRef]
- Overman, M.; Javle, M.; Davis, R.E.; Vats, P.; Kumar-Sinha, C.; Xiao, L.; Mettu, N.B.; Parra, E.R.; Benson, A.B.; Lopez, C.D.; et al. Randomized phase II study of the Bruton tyrosine kinase inhibitor acalabrutinib, alone or with pembrolizumab in patients with advanced pancreatic cancer. J. Immunother. Cancer 2020, 8, e000587. [Google Scholar] [CrossRef] [PubMed]
- Massó-Vallés, D.; Jauset, T.; Serrano, E.; Sodir, N.M.; Pedersen, K.; Affara, N.I.; Whitfield, J.R.; Beaulieu, M.E.; Evan, G.I.; Elias, L.; et al. Ibrutinib exerts potent antifibrotic and intitumor activities in mouse models of pancreatic adenocarcinoma. Cancer Res. 2015, 75, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Bockorny, B.; Macarulla, T.; Semenisty, V.; Borazanci, E.; Feliu, J.; Ponz-Sarvise, M.; Abad, D.G.; Oberstein, P.; Alistar, A.; Munoz, A.; et al. Motixafortide and pembrolizumab combined to nanoliposomal irinotecan, fluorouracil, and folinic acid in metastatic pancreatic cancer: The COMBAT/KEYNOTE-202 trial. Clin. Cancer Res. 2021, 27, 5020–5027. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Pereg, Y.; Bulvik, B.; Klein, S.; Mishalian, I.; Wald, H.; Eizenberg, O.; Beider, K.; Nagler, A.; Golan, R.; et al. Single dose of the CXCR4 antagonist BL-8040 induces rapid mobilization for the collection of human CD34(þ) cells in healthy volunteers. Clin. Cancer Res. 2017, 23, 6790–6801. [Google Scholar] [CrossRef]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: The COMBAT trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.-T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 2018, 108, 78–87. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilkinson, G.A.; Eng, K.H.; Fields, P.; Raber, P.; Moseley, J.L.; Cheetham, K.; Coffey, M.; Nuovo, G.; Kalinski, P.; et al. Pembrolizumab in combination with the oncolytic virus pelareorep and chemotherapy in patients with advanced pancreatic adenocarcinoma: A phase Ib study a C. Clin. Cancer Res. 2020, 26, 71–81. [Google Scholar] [CrossRef]
- Ko, A.H.; Kim, K.-P.; Siveke, J.T.; Lopez, C.D.; Lacy, J.; O’reilly, E.M.; Macarulla, T.; Manji, G.A.; Lee, J.; Ajani, J.; et al. Atezolizumab Plus PEGPH20 Versus Chemotherapy in Advanced Pancreatic Ductal Adenocarcinoma and Gastric Cancer: MORPHEUS Phase Ib/II Umbrella Randomized Study Platform. Oncologist 2023, 28, 553-e472. [Google Scholar] [CrossRef]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS (G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef]
- Nusrat, F.; Gorgov, E.; Khanna, A.; Isesele, O.; Bowne, W.; Lavu, H.; Yeo, C.J.; Jiang, W.; Jain, A.; Nevler, A. Prognostic properties of KRAS gene mutation subtypes in resected pancreatic ductal adenocarcinoma. Pancreas 2025, 54, e449–e454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borz, P.-C.; Borz, M.B.; Borz, O.-C.; Zaharie, T.; Hagiu, C.; Munteanu, L.; Gurzu, S. Improving Outcomes in Pancreatic Adenocarcinoma: A Systematic Review of Immunotherapy in Multimodal Treatment. Medicina 2025, 61, 1076. https://doi.org/10.3390/medicina61061076
Borz P-C, Borz MB, Borz O-C, Zaharie T, Hagiu C, Munteanu L, Gurzu S. Improving Outcomes in Pancreatic Adenocarcinoma: A Systematic Review of Immunotherapy in Multimodal Treatment. Medicina. 2025; 61(6):1076. https://doi.org/10.3390/medicina61061076
Chicago/Turabian StyleBorz, Paul-Cristian, Mihnea Bogdan Borz, Oliviu-Cristian Borz, Toader Zaharie, Claudia Hagiu, Lidia Munteanu, and Simona Gurzu. 2025. "Improving Outcomes in Pancreatic Adenocarcinoma: A Systematic Review of Immunotherapy in Multimodal Treatment" Medicina 61, no. 6: 1076. https://doi.org/10.3390/medicina61061076
APA StyleBorz, P.-C., Borz, M. B., Borz, O.-C., Zaharie, T., Hagiu, C., Munteanu, L., & Gurzu, S. (2025). Improving Outcomes in Pancreatic Adenocarcinoma: A Systematic Review of Immunotherapy in Multimodal Treatment. Medicina, 61(6), 1076. https://doi.org/10.3390/medicina61061076







