Mammalian PI-Phospholipase C Isozymes: Structural and Functional Insights and Roles in Health and Disease
Abstract
1. Introduction
2. Structural Motifs of PI-PLCs
2.1. PH Domain
2.2. EF-Hand Domain
2.3. XY Catalytic Domain
2.4. C2 Domain
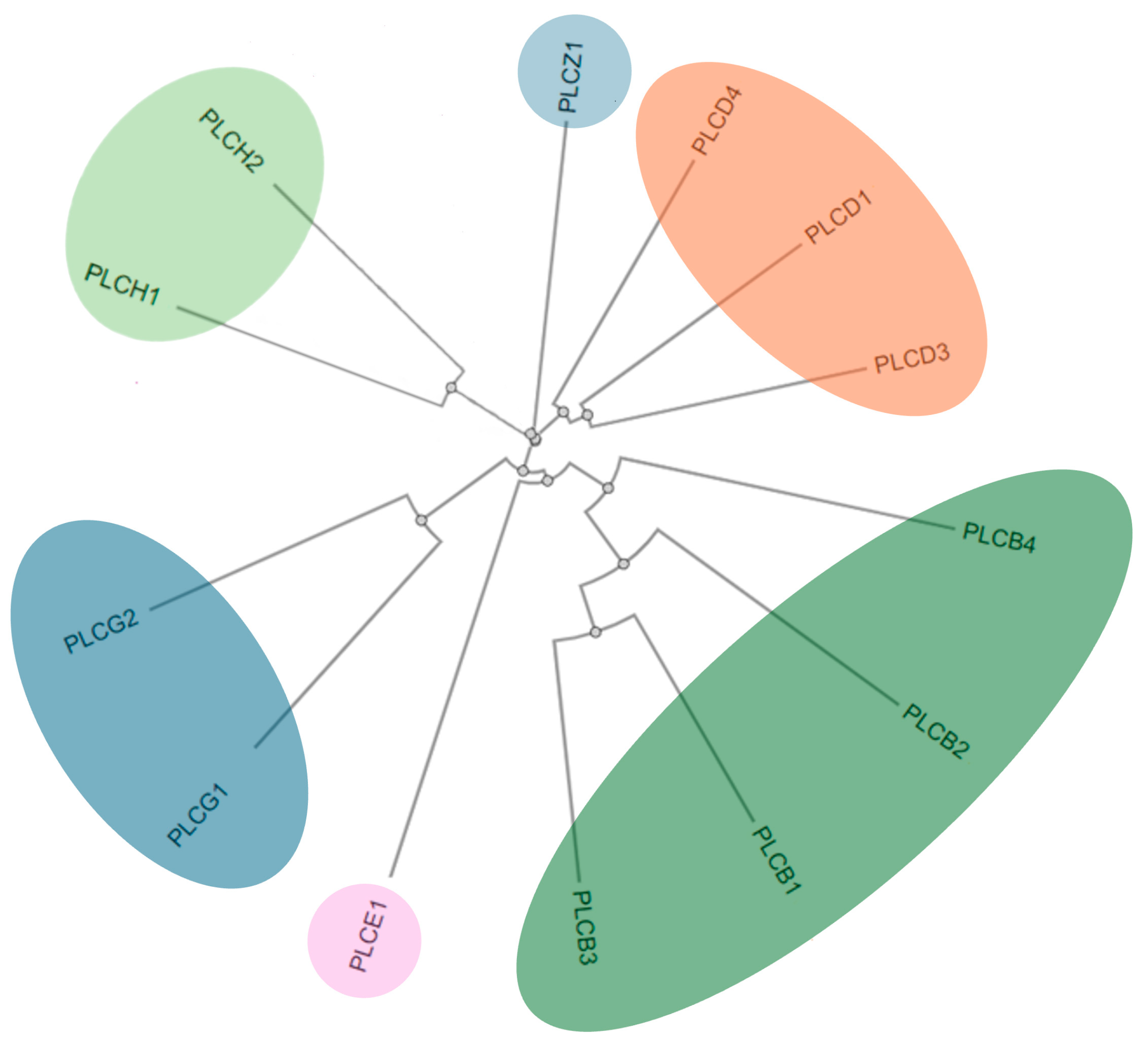
3. Structure, Localization, and Tissue Distribution of PI-PLC Isozymes
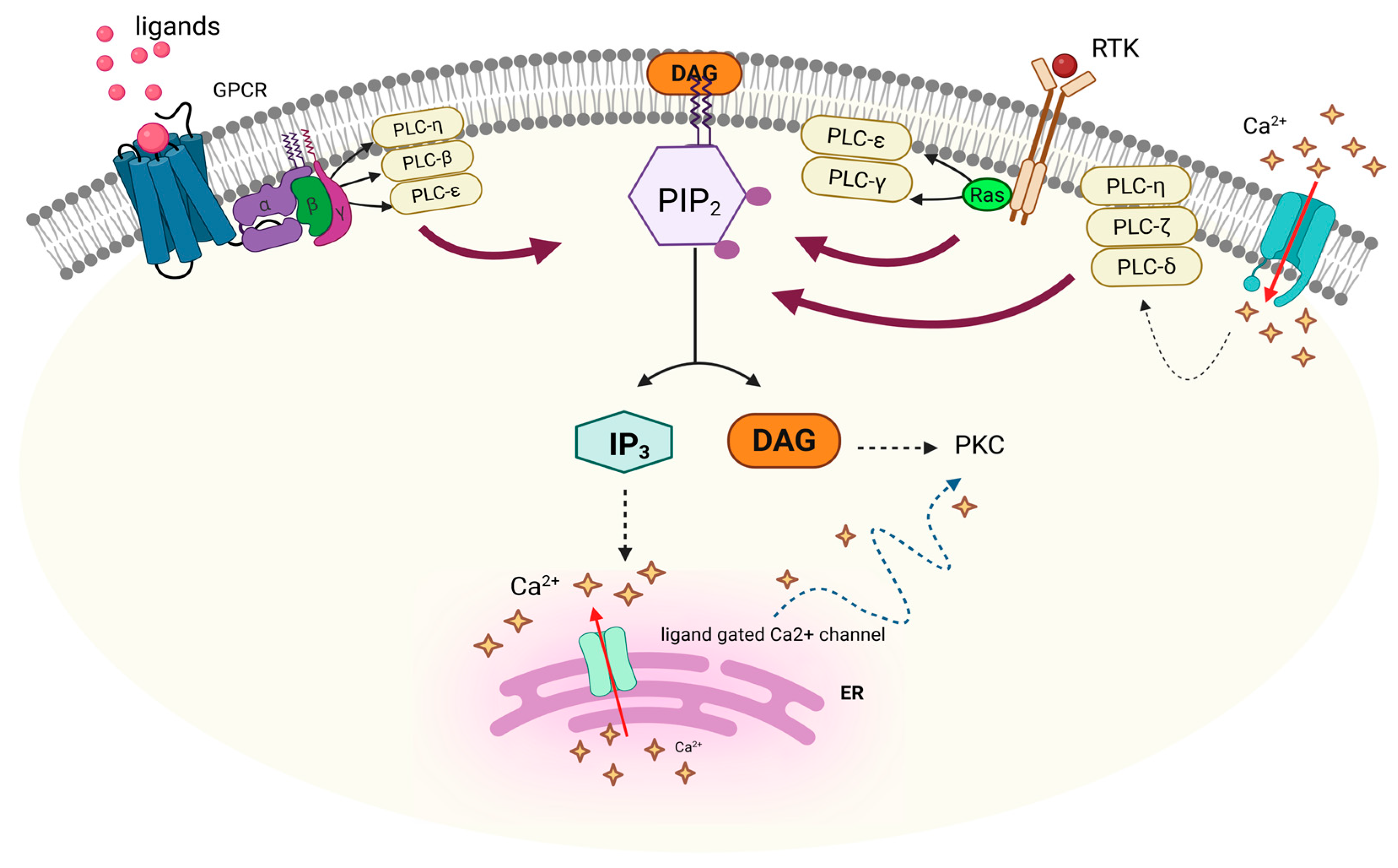
3.1. PI-PLCβ
3.2. PI-PLCγ
3.3. PI-PLCδ
3.4. PI-PLCε
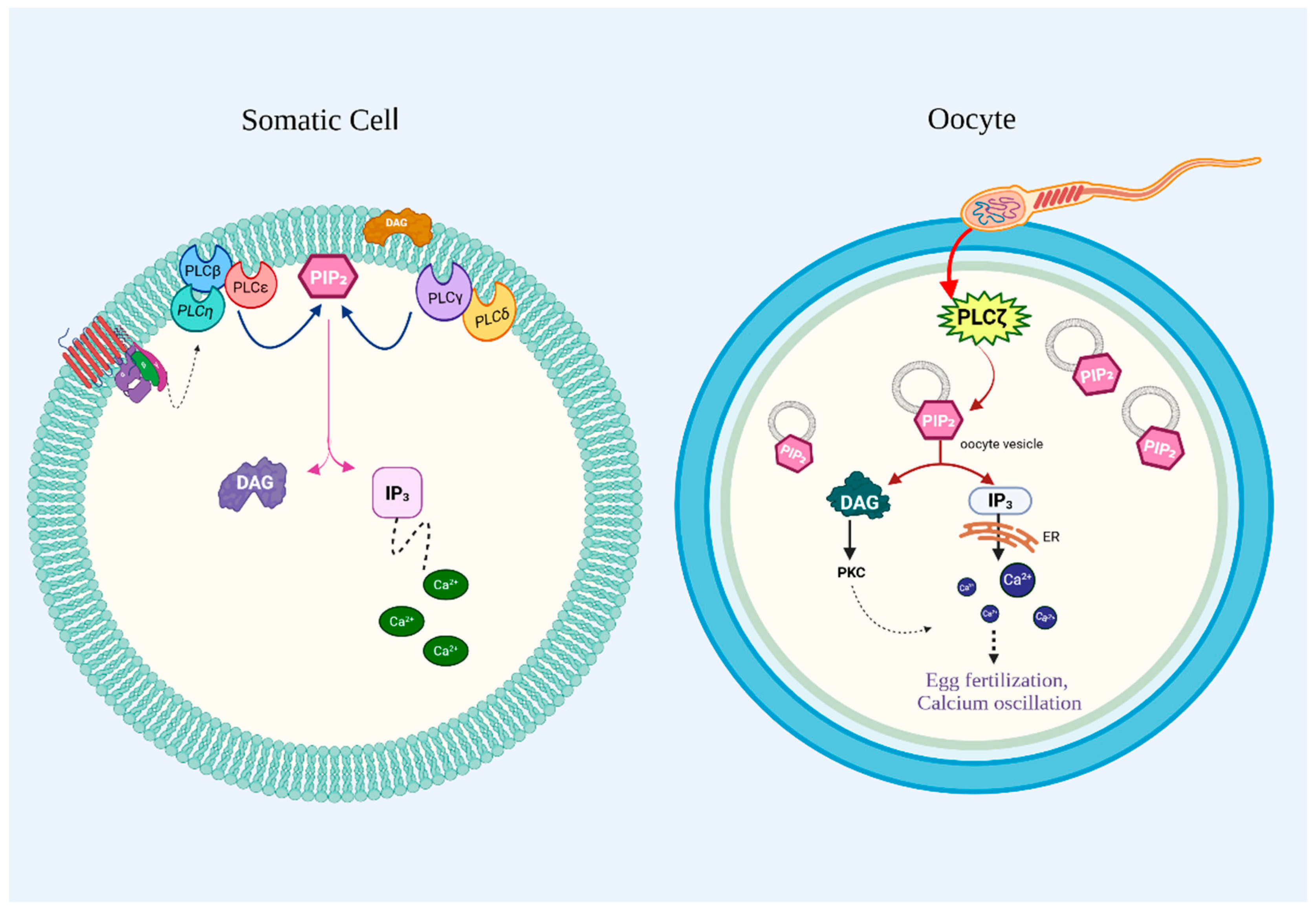
3.5. PI-PLCζ
3.6. PI-PLCη
4. Functions of PI-PLC Isozymes
5. Regulation of Mammalian PI-PLC Isozymes
5.1. Protein–Protein Interactions
5.2. Post-Translational Modifications
5.3. Lipid Modulation of PI-PLC Activity
5.4. Integration of Regulatory Mechanisms
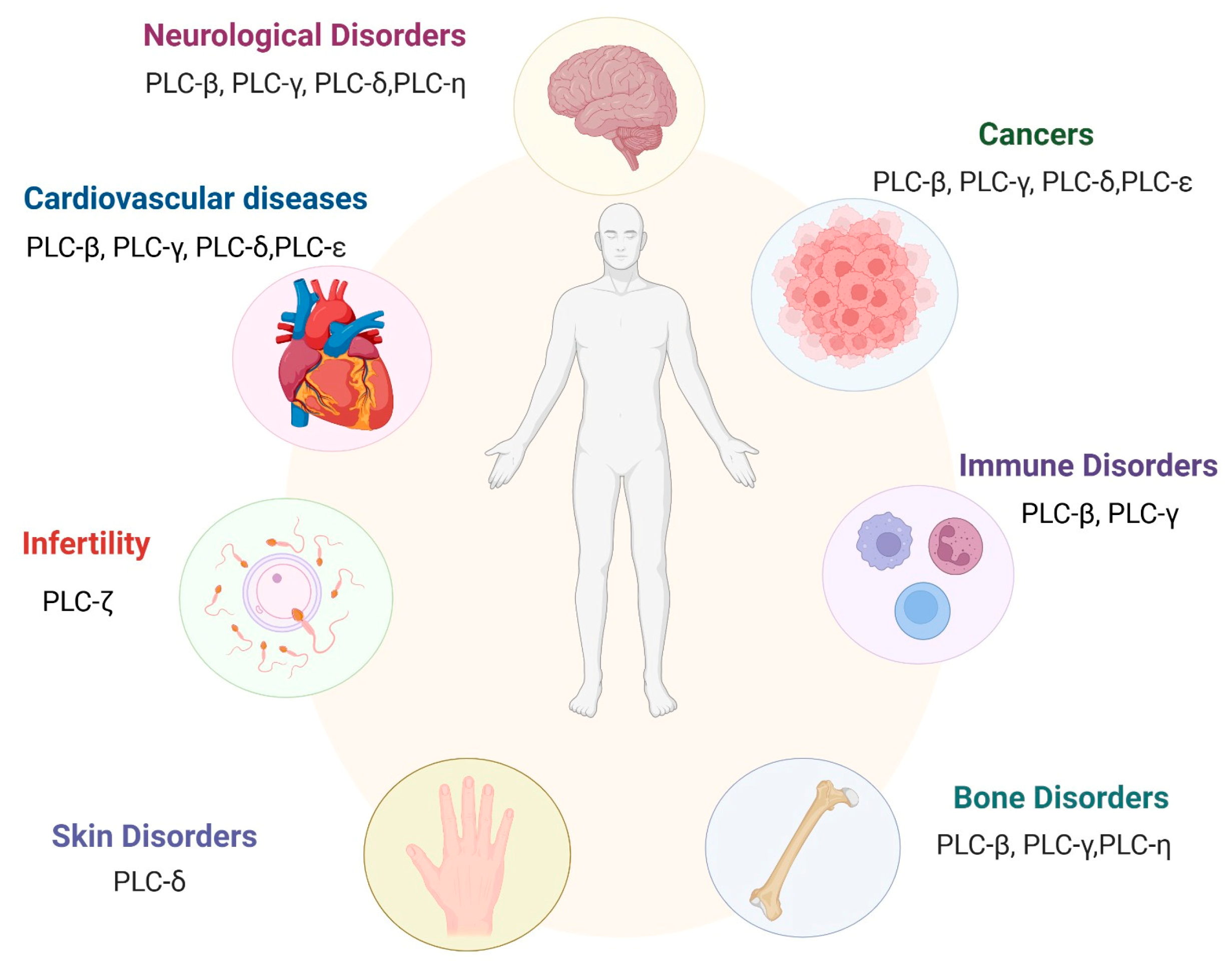
6. Implications of PI-PLCs in Health and Disease
6.1. PI-PLC and Cancer
6.1.1. PI-PLCβ and Cancer
6.1.2. PI-PLCγ and Cancer
6.1.3. PI-PLCδ and Cancer
6.1.4. PI-PLCε and Cancer
6.2. PI-PLC and Immune Disorders
6.2.1. PI-PLCβ and Immune Disorders
6.2.2. PI-PLCγ and Immune Disorders
6.3. PI-PLC and Cardiac Disorders
6.3.1. PI-PLCβ and Cardiac Disorders
6.3.2. PI-PLCε and Cardiac Disorders
6.4. PI-PLC and Neurological Disorders
6.4.1. PI-PLCβ and Neurological Disorders
6.4.2. PI-PLCγ and Neurological Disorders
6.5. PI-PLCζ and Infertility
6.6. PI-PLCβ and Bone Diseases
6.7. I-PLCδ and Skin Disorders
7. Therapeutic Potential of Targeting PI-PLCs
7.1. U73122 in Management of Inflammatory Disorders
7.2. U73122 in Management of Cancer
7.3. NSC768313 in Management of Cancer
7.4. Recombinant PI-PLCζ in the Treatment of Male Infertility
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Lamari, F.; Rossignol, F.; Mitchell, G.A. Glycerophospholipids: Roles in Cell Trafficking and Associated Inborn Errors. J. Inherit. Metab. Dis. 2025, 48, e70019. [Google Scholar] [CrossRef] [PubMed]
- Pabst, G.; Keller, S. Exploring membrane asymmetry and its effects on membrane proteins. Trends Biochem. Sci. 2024, 49, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Tang, H.; Pang, S. The Crucial Roles of Phospholipids in Aging and Lifespan Regulation. Front. Physiol. 2021, 12, 775648. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A. Introduction to Thematic Review Series: Phospholipases: Central Role in Lipid Signaling and Disease. J. Lipid Res. 2015, 56, 1245–1247. [Google Scholar] [CrossRef]
- Aloulou, A.; Rahier, R.; Arhab, Y.; Noiriel, A.; Abousalham, A. Phospholipases: An Overview. Methods Mol. Biol. 2018, 1835, 69–105. [Google Scholar]
- Yaginuma, S.; Kawana, H.; Aoki, J. Current Knowledge on Mammalian Phospholipase A(1), Brief History, Structures, Biochemical and Pathophysiological Roles. Molecules 2022, 27, 2487. [Google Scholar] [CrossRef]
- Khan, S.A.; Ilies, M.A. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. Int. J. Mol. Sci. 2023, 24, 1353. [Google Scholar] [CrossRef]
- Chap, H. Forty five years with membrane phospholipids, phospholipases and lipid mediators: A historical perspective. Biochimie 2016, 125, 234–249. [Google Scholar] [CrossRef]
- Bowling, F.Z.; Frohman, M.A.; Airola, M.V. Structure and regulation of human phospholipase D. Adv. Biol. Regul. 2021, 79, 100783. [Google Scholar] [CrossRef]
- Murakami, C.; Dilimulati, K.; Atsuta-Tsunoda, K.; Kawai, T.; Inomata, S.; Hijikata, Y.; Sakai, H.; Sakane, F. Multiple activities of sphingomyelin synthase 2 generate saturated fatty acid- and/or monounsaturated fatty acid-containing diacylglycerol. J. Biol. Chem. 2024, 300, 107960. [Google Scholar] [CrossRef]
- Katan, M.; Cockcroft, S. Phospholipase C families: Common themes and versatility in physiology and pathology. Prog. Lipid Res. 2020, 80, 101065. [Google Scholar] [CrossRef]
- Sekiya, F.; Kim, Y.J.; Rhee, S.G. Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-γ1. Proc. Natl. Acad. Sci. USA 2005, 102, 4276–4281. [Google Scholar]
- Bill, C.A.; Vines, C.M. Phospholipase C. Adv. Exp. Med. Biol. 2020, 1131, 215–242. [Google Scholar] [PubMed]
- Charnock-Jones, D.S.; Day, K.; Smith, S.K. Cloning, expression and genomic organization of human placental protein disulfide isomerase (previously identified as phospholipase C alpha). Int. J. Biochem. Cell Biol. 1996, 28, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Gellatly, S.A.; Kalujnaia, S.; Cramb, G. Cloning, tissue distribution and sub-cellular localisation of phospholipase C X-domain containing protein (PLCXD) isoforms. Biochem. Biophys. Res. Commun. 2012, 424, 651–656. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Q.; Wang, H.; Yang, X.; Mu, H. Alternative splicing and related RNA binding proteins in human health and disease. Signal Transduct. Target. Ther. 2024, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001, 70, 281–312. [Google Scholar] [CrossRef]
- Cocco, L.; Follo, M.Y.; Manzoli, L.; Suh, P.-G. Phosphoinositide-specific phospholipase C in health and disease. J. Lipid Res. 2015, 56, 1853–1860. [Google Scholar] [CrossRef]
- Gresset, A.; Sondek, J.; Harden, T.K. The phospholipase C isozymes and their regulation. In Phosphoinositides I: Enzymes of Synthesis and Degradation; Springer: Berlin/Heidelberg, Germany, 2012; pp. 61–94. [Google Scholar]
- Philip, F.; Guo, Y.; Scarlata, S. Multiple roles of pleckstrin homology domains in phospholipase Cbeta function. FEBS Lett. 2002, 531, 28–32. [Google Scholar] [CrossRef]
- Powis, G.; Meuillet, E.J.; Indarte, M.; Booher, G.; Kirkpatrick, L. Pleckstrin Homology [PH] domain, structure, mechanism, and contribution to human disease. Biomed. Pharmacother. 2023, 165, 115024. [Google Scholar] [CrossRef] [PubMed]
- Drin, G.; Douguet, D.; Scarlata, S. The pleckstrin homology domain of phospholipase Cbeta transmits enzymatic activation through modulation of the membrane-domain orientation. Biochemistry 2006, 45, 5712–5724. [Google Scholar] [CrossRef] [PubMed]
- Bunney, T.D.; Esposito, D.; Mas-Droux, C.; Lamber, E.; Baxendale, R.W.; Martins, M.; Cole, A.; Svergun, D.; Driscoll, P.C.; Katan, M. Structural and functional integration of the PLCgamma interaction domains critical for regulatory mechanisms and signaling deregulation. Structure 2012, 20, 2062–2075. [Google Scholar] [CrossRef]
- Lemmon, M.A. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 2008, 9, 99–111. [Google Scholar] [CrossRef]
- Nomikos, M.; Sanders, J.R.; Parthimos, D.; Buntwal, L.; Calver, B.L.; Stamatiadis, P.; Smith, A.; Clue, M.; Sideratou, Z.; Swann, K.; et al. Essential Role of the EF-hand Domain in Targeting Sperm Phospholipase Czeta to Membrane Phosphatidylinositol 4,5-Bisphosphate (PIP2). J. Biol. Chem. 2015, 290, 29519–29530. [Google Scholar] [CrossRef]
- Kouchi, Z.; Shikano, T.; Nakamura, Y.; Shirakawa, H.; Fukami, K.; Miyazaki, S. The role of EF-hand domains and C2 domain in regulation of enzymatic activity of phospholipase Czeta. J. Biol. Chem. 2005, 280, 21015–21021. [Google Scholar] [CrossRef]
- Lewit-Bentley, A.; Rety, S. EF-hand calcium-binding proteins. Curr. Opin. Struct. Biol. 2000, 10, 637–643. [Google Scholar] [CrossRef]
- Ellis, M.V.; James, S.R.; Perisic, O.; Downes, C.P.; Williams, R.L.; Katan, M. Catalytic domain of phosphoinositide-specific phospholipase C (PLC). Mutational analysis of residues within the active site and hydrophobic ridge of plcdelta1. J. Biol. Chem. 1998, 273, 11650–11659. [Google Scholar] [CrossRef] [PubMed]
- Rebecchi, M.J.; Pentyala, S.N. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol. Rev. 2000, 80, 1291–1335. [Google Scholar] [CrossRef]
- Nomikos, M.; Elgmati, K.; Theodoridou, M.; Calver, B.L.; Nounesis, G.; Swann, K.; Lai, F.A. Phospholipase Czeta binding to PtdIns(4,5)P2 requires the XY-linker region. J. Cell Sci. 2011, 124, 2582–2590. [Google Scholar] [CrossRef]
- Nomikos, M.; Elgmati, K.; Theodoridou, M.; Georgilis, A.; Gonzalez-Garcia, J.R.; Nounesis, G.; Swann, K.; Lai, F.A. Novel regulation of PLCzeta activity via its XY-linker. Biochem. J. 2011, 438, 427–432. [Google Scholar] [CrossRef]
- Corbin, J.A.; Evans, J.H.; Landgraf, K.E.; Falke, J.J. Mechanism of specific membrane targeting by C2 domains: Localized pools of target lipids enhance Ca2+ affinity. Biochemistry 2007, 46, 4322–4336. [Google Scholar] [CrossRef] [PubMed]
- Ananthanarayanan, B.; Das, S.; Rhee, S.G.; Murray, D.; Cho, W. Membrane targeting of C2 domains of phospholipase C-delta isoforms. J. Biol. Chem. 2002, 277, 3568–3575. [Google Scholar] [CrossRef] [PubMed]
- Corbalan-Garcia, S.; Gomez-Fernandez, J.C. Signaling through C2 domains: More than one lipid target. Biochim. Biophys. Acta 2014, 1838, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Peruzzi, D.; Aluigi, M.; Manzoli, L.; Billi, A.M.; Di Giorgio, F.P.; Morleo, M.; Martelli, A.M.; Cocco, L. Molecular characterization of the human PLC β1 gene. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2002, 1584, 46–54. [Google Scholar] [CrossRef]
- Bahk, Y.Y.; Lee, Y.H.; Lee, T.G.; Seo, J.; Ryu, S.H.; Suh, P.G. Two forms of phospholipase C-beta 1 generated by alternative splicing. J. Biol. Chem. 1994, 269, 8240–8245. [Google Scholar] [CrossRef]
- Gonzalez-Burguera, I.; Lin, G.; Lopez de Jesus, M.; Saumell-Esnaola, M.; Barrondo, S.; Garcia Del Cano, G.; Salles, J.; Scarlata, S. PLCbeta1 by-passes early growth response -1 to induce the differentiation of neuronal cells. Cell Death Discov. 2024, 10, 250. [Google Scholar] [CrossRef]
- Grubb, D.R.; Crook, B.; Ma, Y.; Luo, J.; Qian, H.W.; Gao, X.M.; Kiriazis, H.; Du, X.J.; Gregorevic, P.; Woodcock, E.A. The atypical ‘b’ splice variant of phospholipase Cbeta1 promotes cardiac contractile dysfunction. J. Mol. Cell Cardiol. 2015, 84, 95–103. [Google Scholar] [CrossRef]
- Sun, L.; Mao, G.; Kunapuli, S.P.; Dhanasekaran, D.N.; Rao, A.K. Alternative splice variants of phospholipase C-beta2 are expressed in platelets: Effect on Galphaq-dependent activation and localization. Platelets 2007, 18, 217–223. [Google Scholar] [CrossRef]
- Adamski, F.M.; Timms, K.M.; Shieh, B.-H. A unique isoform of phospholipase Cβ4 highly expressed in the cerebellum and eye. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1999, 1444, 55–60. [Google Scholar] [CrossRef]
- Fisher, I.J.; Jenkins, M.L.; Tall, G.G.; Burke, J.E.; Smrcka, A.V. Activation of Phospholipase C beta by Gbetagamma and Galpha(q) Involves C-Terminal Rearrangement to Release Autoinhibition. Structure 2020, 28, 810–819 e5. [Google Scholar] [CrossRef]
- Hajicek, N.; Charpentier, T.H.; Rush, J.R.; Harden, T.K.; Sondek, J. Autoinhibition and phosphorylation-induced activation of phospholipase C-gamma isozymes. Biochemistry 2013, 52, 4810–4819. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Rai, R.; Mathew, B.J.; Chourasia, R.; Singh, A.K.; Kumar, A.; Chaurasiya, S.K. Phospholipase C: Underrated players in microbial infections. Front. Cell. Infect. Microbiol. 2023, 13, 1089374. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fukami, K. Regulation and physiological functions of mammalian phospholipase C. J. Biochem. 2017, 161, 315–321. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kanemarum, K.; Fukami, K. Physiological functions of phospholipase Cdelta1 and phospholipase Cdelta3. Adv. Biol. Regul. 2013, 53, 356–362. [Google Scholar] [CrossRef]
- Sorli, S.C.; Bunney, T.D.; Sugden, P.H.; Paterson, H.F.; Katan, M. Signaling properties and expression in normal and tumor tissues of two phospholipase C epsilon splice variants. Oncogene 2005, 24, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, K.; Van Camp, M.M.; Lyon, A.M. Structure and regulation of phospholipase Cbeta and epsilon at the membrane. Chem. Phys. Lipids 2021, 235, 105050. [Google Scholar] [CrossRef]
- Saunders, C.M.; Larman, M.G.; Parrington, J.; Cox, L.J.; Royse, J.; Blayney, L.M.; Swann, K.; Lai, F.A. PLC zeta: A sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 2002, 129, 3533–3544. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Yoon, S.Y.; Alfandari, D.; Fukami, K.; Sato, K.; Fissore, R.A. Proteolytic processing of phospholipase Czeta and [Ca2+]i oscillations during mammalian fertilization. Dev. Biol. 2007, 312, 407–418. [Google Scholar] [CrossRef]
- Nomikos, M. Novel signalling mechanism and clinical applications of sperm-specific PLCzeta. Biochem. Soc. Trans. 2015, 43, 371–376. [Google Scholar] [CrossRef]
- Phillips, S.V.; Yu, Y.; Rossbach, A.; Nomikos, M.; Vassilakopoulou, V.; Livaniou, E.; Cumbes, B.; Lai, F.A.; George, C.H.; Swann, K. Divergent effect of mammalian PLCzeta in generating Ca(2)(+) oscillations in somatic cells compared with eggs. Biochem. J. 2011, 438, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Thanassoulas, A.; Swann, K.; Lai, F.A.; Nomikos, M. Sperm Factors and Egg Activation: The structure and function relationship of sperm PLCZ1. Reproduction 2022, 164, F1–F8. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Mohanty, P.; Bhatnagar, S. Modeling, dynamics and phosphoinositide binding of the pleckstrin homology domain of two novel PLCs: eta1 and eta2. J. Mol. Graph. Model. 2018, 85, 130–144. [Google Scholar] [CrossRef]
- Popovics, P.; Lu, J.; Nadia Kamil, L.; Morgan, K.; Millar, R.P.; Schmid, R.; Blindauer, C.A.; Stewart, A.J. A canonical EF-loop directs Ca(2+) -sensitivity in phospholipase C-eta2. J. Cell Biochem. 2014, 115, 557–565. [Google Scholar] [CrossRef]
- Owusu Obeng, E.; Rusciano, I.; Marvi, M.V.; Fazio, A.; Ratti, S.; Follo, M.Y.; Xian, J.; Manzoli, L.; Billi, A.M.; Mongiorgi, S. Phosphoinositide-dependent signaling in cancer: A focus on phospholipase C isozymes. Int. J. Mol. Sci. 2020, 21, 2581. [Google Scholar] [CrossRef]
- Lim, P.S.; Sutton, C.R.; Rao, S. Protein kinase C in the immune system: From signalling to chromatin regulation. Immunology 2015, 146, 508–522. [Google Scholar] [CrossRef]
- Litalien, C.; Beaulieu, P. Molecular mechanisms of drug actions. Pediatr. Crit. Care 2011, 1553–1568. [Google Scholar] [CrossRef]
- Cheng, H.-F.; Jiang, M.-J.; Chen, C.-L.; Liu, S.-M.; Wong, L.-P.; Lomasney, J.W.; King, K. Cloning and Identification of Amino Acid Residues of Human Phospholipase Cδ1 Essential for Catalysis (∗). J. Biol. Chem. 1995, 270, 5495–5505. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, T.O.; Lass, A. DAG tales: The multiple faces of diacylglycerol—Tereochemistry, metabolism, and signaling. Cell Mol. Life Sci. 2015, 72, 3931–3952. [Google Scholar] [CrossRef]
- Harden, T.K.; Hicks, S.N.; Sondek, J. Phospholipase C isozymes as effectors of Ras superfamily GTPases. J. Lipid Res. 2009, 50, S243–S248. [Google Scholar] [CrossRef]
- Williams, R.L. Mammalian phosphoinositide-specific phospholipase C. Biochim. Biophys. Acta 1999, 1441, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Schoeters, F.; Van Dijck, P. Protein-Protein Interactions in Candida albicans. Front. Microbiol. 2019, 10, 1792. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhao, J.; Guo, L.; Kim, S.C.; Deng, X.; Wang, G.; Zhang, G.; Li, M.; Wang, X. Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res. 2016, 62, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Wang, Z. Akt binds to and phosphorylates phospholipase C-gamma1 in response to epidermal growth factor. Mol. Biol. Cell 2006, 17, 2267–2277. [Google Scholar] [CrossRef]
- Posor, Y.; Jang, W.; Haucke, V. Phosphoinositides as membrane organizers. Nat. Rev. Mol. Cell Biol. 2022, 23, 797–816. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, J.G.; Kim, Y.J.; Balla, T. Integrated regulation of the phosphatidylinositol cycle and phosphoinositide-driven lipid transport at ER-PM contact sites. Traffic 2020, 21, 200–219. [Google Scholar] [CrossRef]
- Balla, T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef]
- Follo, M.Y.; Finelli, C.; Clissa, C.; Mongiorgi, S.; Bosi, C.; Martinelli, G.; Baccarani, M.; Manzoli, L.; Martelli, A.M.; Cocco, L. Phosphoinositide-phospholipase C beta1 mono-allelic deletion is associated with myelodysplastic syndromes evolution into acute myeloid leukemia. J. Clin. Oncol. 2009, 27, 782–790. [Google Scholar] [CrossRef]
- Fili, C.; Malagola, M.; Follo, M.Y.; Finelli, C.; Iacobucci, I.; Martinelli, G.; Cattina, F.; Clissa, C.; Candoni, A.; Fanin, R.; et al. Prospective phase II Study on 5-days azacitidine for treatment of symptomatic and/or erythropoietin unresponsive patients with low/INT-1-risk myelodysplastic syndromes. Clin. Cancer Res. 2013, 19, 3297–3308. [Google Scholar] [CrossRef]
- Xiao, W.; Kawakami, Y.; Kawakami, T. Immune regulation by phospholipase C-beta isoforms. Immunol. Res. 2013, 56, 9–19. [Google Scholar] [CrossRef]
- Ceneri, E.; De Stefano, A.; Casalin, I.; Finelli, C.; Curti, A.; Paolini, S.; Parisi, S.; Ardizzoia, F.; Cristiano, G.; Boultwood, J.; et al. Signaling pathways and bone marrow microenvironment in myelodysplastic neoplasms. Adv. Biol. Regul. 2025, 95, 101071. [Google Scholar] [CrossRef]
- Jang, H.J.; Suh, P.G.; Lee, Y.J.; Shin, K.J.; Cocco, L.; Chae, Y.C. PLCgamma1: Potential arbitrator of cancer progression. Adv. Biol. Regul. 2018, 67, 179–189. [Google Scholar] [CrossRef]
- Bunney, T.D.; Kampyli, C.; Gregory, A.; Katan, M. Characterisation of molecular mechanisms for PLCgamma2 disease-linked variants. Adv. Biol. Regul. 2024, 94, 101053. [Google Scholar] [CrossRef]
- Jiang, Y.; Liao, L.; Shrestha, C.; Ji, S.; Chen, Y.; Peng, J.; Wang, L.; Liao, E.; Xie, Z. Reduced expression of E-cadherin and p120-catenin and elevated expression of PLC-gamma1 and PIKE are associated with aggressiveness of oral squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 9042–9051. [Google Scholar] [PubMed]
- Cai, S.; Sun, P.H.; Resaul, J.; Shi, L.; Jiang, A.; Satherley, L.K.; Davies, E.L.; Ruge, F.; Douglas-Jones, A.; Jiang, W.G.; et al. Expression of phospholipase C isozymes in human breast cancer and their clinical significance. Oncol. Rep. 2017, 37, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, W.; Zhang, T.; Ji, J.; Qian, Q.; Lu, L.; Fu, H.; Jin, W.; Cui, D. Differential expression of phospholipase C epsilon 1 is associated with chronic atrophic gastritis and gastric cancer. PLoS ONE 2012, 7, e47563. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, S.A.; Cekaite, L.; Agesen, T.H.; Sveen, A.; Nesbakken, A.; Thiis-Evensen, E.; Skotheim, R.I.; Lind, G.E.; Lothe, R.A. Phospholipase C isozymes are deregulated in colorectal cancer--insights gained from gene set enrichment analysis of the transcriptome. PLoS ONE 2011, 6, e24419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Ou, L.; Yang, X.; Wang, X.; Tang, M.; Chen, E.; Luo, C. PLCepsilon knockdown inhibits prostate cancer cell proliferation via suppression of Notch signalling and nuclear translocation of the androgen receptor. Cancer Lett. 2015, 362, 61–69. [Google Scholar] [CrossRef]
- Kawakami, T.; Xiao, W. Phospholipase C-beta in immune cells. Adv. Biol. Regul. 2013, 53, 249–257. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, B.; Wang, P.; Dong, X.; Fernandez-Hernando, C.; Li, Z.; Hla, T.; Li, Z.; Claffey, K.; Smith, J.D.; et al. Phospholipase C beta3 deficiency leads to macrophage hypersensitivity to apoptotic induction and reduction of atherosclerosis in mice. J. Clin. Invest. 2008, 118, 195–204. [Google Scholar] [CrossRef]
- Smit, M.J.; Verdijk, P.; van der Raaij-Helmer, E.M.; Navis, M.; Hensbergen, P.J.; Leurs, R.; Tensen, C.P. CXCR3-mediated chemotaxis of human T cells is regulated by a Gi- and phospholipase C-dependent pathway and not via activation of MEK/p44/p42 MAPK nor Akt/PI-3 kinase. Blood 2003, 102, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Kamatani, Y.; Takahashi, A.; Matsuda, K.; Hosono, N.; Ohmiya, H.; Daigo, Y.; Yamamoto, K.; Kubo, M.; Nakamura, Y.; et al. Common variations in PSMD3-CSF3 and PLCB4 are associated with neutrophil count. Hum. Mol. Genet. 2010, 19, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Ombrello, M.J.; Remmers, E.F.; Sun, G.; Freeman, A.F.; Datta, S.; Torabi-Parizi, P.; Subramanian, N.; Bunney, T.D.; Baxendale, R.W.; Martins, M.S.; et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N. Engl. J. Med. 2012, 366, 330–338. [Google Scholar] [CrossRef]
- Koss, H.; Bunney, T.D.; Behjati, S.; Katan, M. Dysfunction of phospholipase Cgamma in immune disorders and cancer. Trends Biochem. Sci. 2014, 39, 603–611. [Google Scholar] [CrossRef]
- Zhou, Q.; Lee, G.S.; Brady, J.; Datta, S.; Katan, M.; Sheikh, A.; Martins, M.S.; Bunney, T.D.; Santich, B.H.; Moir, S.; et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cgamma2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am. J. Hum. Genet. 2012, 91, 713–720. [Google Scholar] [CrossRef]
- Fazio, A.; Evangelisti, C.; Cappellini, A.; Mongiorgi, S.; Koufi, F.D.; Neri, I.; Marvi, M.V.; Russo, M.; Ghigo, A.; Manzoli, L.; et al. Emerging Roles of Phospholipase C Beta Isozymes as Potential Biomarkers in Cardiac Disorders. Int. J. Mol. Sci. 2023, 24, 13096. [Google Scholar] [CrossRef]
- Filtz, T.M.; Grubb, D.R.; McLeod-Dryden, T.J.; Luo, J.; Woodcock, E.A. Gq-initiated cardiomyocyte hypertrophy is mediated by phospholipase Cbeta1b. FASEB J. 2009, 23, 3564–3570. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gareri, C.; Rockman, H.A. G-Protein-Coupled Receptors in Heart Disease. Circ. Res. 2018, 123, 716–735. [Google Scholar] [CrossRef]
- Dorn, G.W.; Force, T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Invest. 2005, 115, 527–537. [Google Scholar] [CrossRef]
- Rebolledo, B.; Lai, N.C.; Gao, M.H.; Takahashi, T.; Roth, D.M.; Baird, S.M.; Hammond, H.K. Adenylylcyclase gene transfer increases function of the failing heart. Hum. Gene Ther. 2006, 17, 1043–1048. [Google Scholar] [CrossRef]
- Heyliger, C.E.; Pierce, G.N.; Singal, P.K.; Beamish, R.E.; Dhalla, N.S. Cardiac alpha- and beta-adrenergic receptor alterations in diabetic cardiomyopathy. Basic. Res. Cardiol. 1982, 77, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, T.; Zhang, D.; Tang, Y.; Gu, J. Phospholipase C epsilon 1 as a therapeutic target in cardiovascular diseases. J. Adv. Res. 2025, 1, 32. [Google Scholar] [CrossRef]
- Yang, Y.R.; Jung, J.H.; Kim, S.J.; Hamada, K.; Suzuki, A.; Kim, H.J.; Lee, J.H.; Kwon, O.B.; Lee, Y.K.; Kim, J.; et al. Forebrain-specific ablation of phospholipase Cgamma1 causes manic-like behavior. Mol. Psych. 2017, 22, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.T.; Mulazzani, E.; Nutt, S.L.; Masters, S.L. The role of PLCgamma2 in immunological disorders, cancer, and neurodegeneration. J. Biol. Chem. 2021, 297, 100905. [Google Scholar] [CrossRef]
- Andreone, B.J.; Przybyla, L.; Llapashtica, C.; Rana, A.; Davis, S.S.; van Lengerich, B.; Lin, K.; Shi, J.; Mei, Y.; Astarita, G.; et al. Alzheimer’s-associated PLCgamma2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat. Neurosci. 2020, 23, 927–938. [Google Scholar] [CrossRef]
- Yang, Y.R.; Kang, D.S.; Lee, C.; Seok, H.; Follo, M.Y.; Cocco, L.; Suh, P.G. Primary phospholipase C and brain disorders. Adv. Biol. Regul. 2016, 61, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Yang, S.; Kim, S.H.; Ko, E.; Kang, M.; Joo, J.Y. Cryptic mutations of PLC family members in brain disorders: Recent discoveries and a deep-learning-based approach. Brain 2023, 146, 1267–1280. [Google Scholar] [CrossRef]
- Amdani, S.N.; Jones, C.; Coward, K. Phospholipase C zeta (PLCzeta): Oocyte activation and clinical links to male factor infertility. Adv. Biol. Regul. 2013, 53, 292–308. [Google Scholar] [CrossRef]
- Winata, C.L.; Korzh, V. The translational regulation of maternal mRNAs in time and space. FEBS Lett. 2018, 592, 3007–3023. [Google Scholar] [CrossRef]
- Nomikos, M.; Elgmati, K.; Theodoridou, M.; Calver, B.L.; Cumbes, B.; Nounesis, G.; Swann, K.; Lai, F.A. Male infertility-linked point mutation disrupts the Ca2+ oscillation-inducing and PIP(2) hydrolysis activity of sperm PLCzeta. Biochem. J. 2011, 434, 211–217. [Google Scholar] [CrossRef]
- Saleh, A.; Kashir, J.; Thanassoulas, A.; Safieh-Garabedian, B.; Lai, F.A.; Nomikos, M. Essential Role of Sperm-Specific PLC-Zeta in Egg Activation and Male Factor Infertility: An Update. Front. Cell Dev. Biol. 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Jin, X.; Choi, P.R.; Cui, Y.; Che, X.; Lee, S.; Hur, K.; Kim, H.J.; Choi, J.Y. Phospholipase C beta4 promotes RANKL-dependent osteoclastogenesis by interacting with MKK3 and p38 MAPK. Exp. Mol. Med. 2025, 57, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Urciuoli, E.; Leopizzi, M.; Di Maio, V.; Petrini, S.; D’Oria, V.; Giorda, E.; Scarsella, M.; Della Rocca, C.; Lo Vasco, V.R.; Peruzzi, B. Phosphoinositide-specific phospholipase C isoforms are conveyed by osteosarcoma-derived extracellular vesicles. J. Cell Commun. Signal 2020, 14, 417–426. [Google Scholar] [CrossRef]
- Nomikos, M.; Thanassoulas, A.; Beck, K.; Theodoridou, M.; Kew, J.; Kashir, J.; Calver, B.L.; Matthews, E.; Rizkallah, P.; Sideratou, Z.; et al. Mutations in PLCdelta1 associated with hereditary leukonychia display divergent PIP2 hydrolytic function. FEBS J. 2016, 283, 4502–4514. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yuan, C.; Ma, Y.; Ding, X.; Zhu, G.; Zhu, Q. Anti-inflammatory activities of phospholipase C inhibitor U73122: Inhibition of monocyte-to-macrophage transformation and LPS-induced pro-inflammatory cytokine expression. Int. Immunopharmacol. 2015, 29, 622–627. [Google Scholar] [CrossRef]
- Roh, T.H.; Chae, M.K.; Ko, J.S.; Kikkawa, D.O.; Jang, S.Y.; Yoon, J.S. Phospholipase C-gamma as a Potential Therapeutic Target for Graves’ Orbitopathy. Endocrinol. Metab. 2023, 38, 739–749. [Google Scholar] [CrossRef]
- Maheshwari, R.; Weis, E. Thyroid associated orbitopathy. Indian. J. Ophthalmol. 2012, 60, 87–93. [Google Scholar] [CrossRef]
- Lo Vasco, V.R.; Leopizzi, M.; Di Maio, V.; Della Rocca, C. U-73122 reduces the cell growth in cultured MG-63 ostesarcoma cell line involving Phosphoinositide-specific Phospholipases C. SpringerPlus 2016, 5, 156. [Google Scholar] [CrossRef]
- Li, T.; Yang, Z.; Li, H.; Zhu, J.; Wang, Y.; Tang, Q.; Shi, Z. Phospholipase Cgamma1 (PLCG1) overexpression is associated with tumor growth and poor survival in IDH wild-type lower-grade gliomas in adult patients. Lab. Invest. 2022, 102, 143–153. [Google Scholar] [CrossRef]
- Schwebe, M.; Ameling, S.; Hammer, E.; Monzel, J.V.; Bonitz, K.; Budde, S.; Schult, K.; Oswald, S.; Scheuch, E.; Grube, M.; et al. Protective effects of endothelin receptor A and B inhibitors against doxorubicin-induced cardiomyopathy. Biochem. Pharmacol. 2015, 94, 109–129. [Google Scholar] [CrossRef]
- Singal, T.; Dhalla, N.S.; Tappia, P.S. Regulation of c-Fos and c-Jun gene expression by phospholipase C activity in adult cardiomyocytes. Mol. Cell Biochem. 2009, 327, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Reynisson, J.; Jaiswal, J.K.; Barker, D.; D’Mello, S.A.; Denny, W.A.; Baguley, B.C.; Leung, E.Y. Evidence that phospholipase C is involved in the antitumour action of NSC768313, a new thieno[2,3-b]pyridine derivative. Cancer Cell. Int. 2016, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Haverkate, N.A.; Leung, E.; Pilkington, L.I.; Barker, D. Tethered Aryl Groups Increase the Activity of Anti-Proliferative Thieno[2,3-b]Pyridines by Targeting a Lipophilic Region in the Active Site of PI-PLC. Pharmaceutics 2021, 13, 2020. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Russo, G.I.; Kandil, H.; Boitrelle, F.; Saleh, R.; Chung, E.; Kavoussi, P.; Mostafa, T.; Shah, R.; Agarwal, A. Male Infertility: New Developments, Current Challenges, and Future Directions. World J. Mens. Health 2024, 42, 502–517. [Google Scholar] [CrossRef]
- Saleh, A.; Thanassoulas, A.; Aliyev, E.; Swann, K.; Naija, A.; Yalcin, H.C.; Lai, F.A.; Nomikos, M. Development of Recombinant PLC-Zeta Protein as a Therapeutic Intervention for the Clinical Treatment of Oocyte Activation Failure. Biomedicines 2024, 12, 1083. [Google Scholar] [CrossRef]

| Isoform | Main Tissue Distribution | Activation Triggers/Mechanisms |
|---|---|---|
| PLCβ | Ubiquitous (brain, heart, etc.) | GPCRs via Gαq and Gαi (directly by Gαq; indirectly via βγ subunits) |
| PLCγ | Widespread (immune cells, etc.) | Receptor tyrosine kinases (RTKs; e.g., EGFR, PDGFR) |
| PLCδ | Ubiquitous | GPCRs (indirectly via Ca2+), increased cytosolic Ca2+ |
| PLCε | Heart, brain, others | Ras, Rap GTPases, Gα12/13, Gβγ, RTKs |
| PLCζ | Sperm | Intracellular Ca2+ |
| PLCη | Brain, others | GPCRs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdi, M.; Al-Matwi, M.; Elghoul, N.; Al-Kuwari, H.; Sayed, T.S.; Riguene, E.; Nomikos, M. Mammalian PI-Phospholipase C Isozymes: Structural and Functional Insights and Roles in Health and Disease. Medicina 2025, 61, 1054. https://doi.org/10.3390/medicina61061054
Hamdi M, Al-Matwi M, Elghoul N, Al-Kuwari H, Sayed TS, Riguene E, Nomikos M. Mammalian PI-Phospholipase C Isozymes: Structural and Functional Insights and Roles in Health and Disease. Medicina. 2025; 61(6):1054. https://doi.org/10.3390/medicina61061054
Chicago/Turabian StyleHamdi, May, Mohammed Al-Matwi, Nour Elghoul, Hissa Al-Kuwari, Tahseen S. Sayed, Emna Riguene, and Michail Nomikos. 2025. "Mammalian PI-Phospholipase C Isozymes: Structural and Functional Insights and Roles in Health and Disease" Medicina 61, no. 6: 1054. https://doi.org/10.3390/medicina61061054
APA StyleHamdi, M., Al-Matwi, M., Elghoul, N., Al-Kuwari, H., Sayed, T. S., Riguene, E., & Nomikos, M. (2025). Mammalian PI-Phospholipase C Isozymes: Structural and Functional Insights and Roles in Health and Disease. Medicina, 61(6), 1054. https://doi.org/10.3390/medicina61061054





