Helicobacter pylori Seroprevalence and Its Associations with Sociodemographic Characteristics, Environmental Factors, and Gastrointestinal Complaints: A Cross-Sectional Study in the Adult Population of Kaunas City, Lithuania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Ethics
2.2. Study Subjects
2.3. Data Collection and Variables
2.4. H. pylori IgG Antibodies Testing
2.5. Exclusion from Further Analysis
2.6. Statistical Analysis
3. Results
3.1. Demographic and Socioeconomic Data
3.2. Seroprevalence of H. pylori IgG Antibodies
3.3. Seroprevalence of H. pylori Antibodies in Relation to the Analyzed Independent Factors
3.4. History of Previous H. pylori Antibodies Testing and Eradication
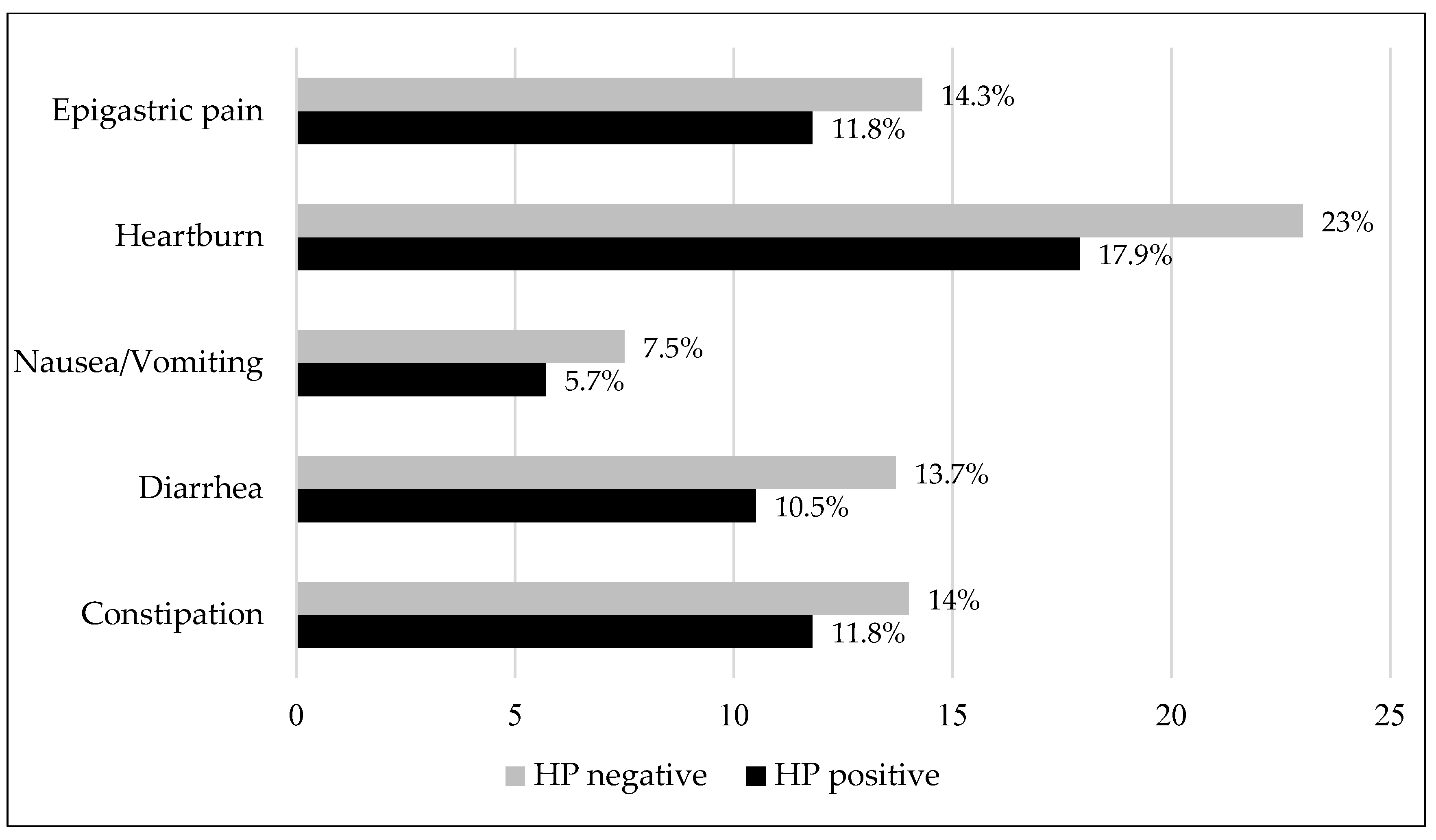
3.5. Dyspeptic Symptoms and Their Association with H. pylori Seroprevalence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| H. pylori | Helicobacter pylori |
| GC | Gastric cancer |
| PUD | Peptic ulcer disease |
| IgG | Immunoglobulin G |
| IARC | International Agency for Research on Cancer |
| GLOBOCAN | Global Cancer Observatory |
| 95% CI | 95% confidence interval |
| OR | Odds ratio |
References
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Howden, C.W.; Moss, S.F.; Morgan, D.R.; Greer, K.B.; Grover, S.; Shah, S.C. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2024, 119, 1730–1753. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.-M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Prim. 2023, 9, 19. [Google Scholar] [CrossRef]
- Ahn, H.J.; Lee, D.S. Helicobacter pylori in gastric carcinogenesis. World J. Gastrointest. Oncol. 2015, 7, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Malfertheiner, P.; Yu, H.-T.; Kuo, C.-L.; Chang, Y.-Y.; Meng, F.-T.; Wu, Y.-X.; Hsiao, J.-L.; Chen, M.-J.; Lin, K.-P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef]
- Brown, L.M. Helicobacter pylori: Epidemiology and routes of transmission. Epidemiol. Rev. 2000, 22, 283–297. [Google Scholar] [CrossRef]
- Leja, M.; Cine, E.; Rudzite, D.; Vilkoite, I.; Huttunen, T.; Daugule, I.; Rumba-Rozenfelde, I.; Pimanov, S.; Liepniece-Karele, I.; Pahomova, J.; et al. Prevalence of Helicobacter pylori infection and atrophic gastritis in Latvia. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1410–1417. [Google Scholar] [CrossRef]
- Thjodleifsson, B.; Asbjörnsdottir, H.; Sigurjonsdottir, R.B.; Gíslason, D.; Olafsson, I.; Cook, E.; Jogi, R.; Janson, C. Seroprevalence of Helicobacter pylori and cagA antibodies in Iceland, Estonia and Sweden. Scand. J. Infect. Dis. 2007, 39, 683–689. [Google Scholar] [CrossRef]
- Celiński, K.; Kurzeja-Mirosław, A.; Słomka, M.; Cichoz-Lach, H.; Madro, A.; Kasztelan-Szczerbińska, B. The effects of environmental factors on the prevalence of Helicobacter pylori infection in inhabitants of Lublin Province. Ann. Agric. Environ. Med. 2006, 13, 185–191. [Google Scholar] [PubMed]
- Ruibys, G.; Denapiene, G.; Wright, R.A.; Irnius, A. Prevalence of Helicobacter pylori Infection in Lithuanian Children. Am. J. Gastroenterol. 2004, 99, S32. Available online: https://journals.lww.com/ajg/Fulltext/2004/10001/PREVALENCE_OF_HELICOBACTER_PYLORI_INFECTION_IN.95.aspx (accessed on 17 March 2025). [CrossRef]
- Kupcinskas, J.; Leja, M. Management of Helicobacter pylori-Related Diseases in the Baltic States. Dig. Dis. 2014, 32, 295–301. Available online: https://www.karger.com/DOI/10.1159/000357862 (accessed on 16 March 2025). [CrossRef] [PubMed]
- Jonaitis, L.; Ivanauskas, A.; Janciauskas, D.; Funka, K.; Sudraba, A.; Tolmanis, I.; Krams, A.; Stirna, D.; Vanags, A.; Kupcinskas, L.; et al. Precancerous gastric conditions in high Helicobacter pylori prevalence areas: Comparison between Eastern European (Lithuanian, Latvian) and Asian (Taiwanese) patients. Medicina 2007, 43, 623–629. [Google Scholar] [CrossRef]
- Jonaityte, I.R.; Ciupkeviciene, E.; Jonaitis, P.; Kupcinskas, J.; Petkeviciene, J.; Jonaitis, L. Changes in the Seroprevalence of Helicobacter pylori among the Lithuanian Medical Students over the Last 25 Years and Its Relation to Dyspeptic Symptoms. Medicina 2021, 57, 254. [Google Scholar] [CrossRef]
- Leja, M. Where are we with gastric cancer screening in Europe in 2024? Gut 2024, 73, 2074–2082. [Google Scholar] [CrossRef]
- Jonaitis, P.; Kupčinskas, J.; Nyssen, O.P.; Puig, I.; Gisbert, J.P.; Jonaitis, L.V. Evaluation of the Effectiveness of Helicobacter Pylori Eradication Regimens in Lithuania During the Years 2013–2020: Data from the European Registry on Helicobacter pylori Management (Hp-EuReg). Medicina 2021, 57, 642. [Google Scholar] [CrossRef]
- Laszewicz, W.; Iwańczak, F.; Iwańczak, B. Seroprevalence of Helicobacter pylori infection in Polish children and adults depending on socioeconomic status and living conditions. Adv. Med. Sci. 2014, 59, 147–150. [Google Scholar] [CrossRef]
- Kondratiuk, N.; Paliy, I.; Zaika, S. Analysis of the prevalence of Helicobacter pylori infection and the effectiveness of eradication schemes in patients with the upper gastrointestinal tract disorders (according to the results of 13C-urea breath tests for 2006–2019). Prz. Gastroenterol. 2021, 16, 229–234. [Google Scholar] [CrossRef]
- Razuka-Ebela, D.; Polaka, I.; Parshutin, S.; Santare, D.; Ebela, I.; Murillo, R.; Tzivian, L.; Park, J.Y.; Leja, M. Sociodemographic, Lifestyle and Medical Factors Associated with Helicobacter pylori Infection. J. Gastrointest. Liver Dis. 2020, 29, 319–327. [Google Scholar] [CrossRef]
- Taylor, C.S.; McMahon, M.V.; Ward, Z.J.; Alarid-Escudero, F.; Camargo, M.C.; Laszkowska, M.; Roa, J.; Yeh, J.M. Birth cohort and age-specific trends in global Helicobacter pylori seroprevalence: A scoping review. Lancet Reg. Health-Am. 2025, 41, 100877. Available online: https://www.sciencedirect.com/science/article/pii/S2667193X24002047 (accessed on 17 March 2025). [CrossRef] [PubMed]
- Wernly, S.; Semmler, G.; Rezar, R.; Schaffler-Schaden, D.; Flamm, M.; Aigner, E.; Datz, C.; Wernly, B. Assessing the association between H. pylori infection and educational status: Implications for screening strategies? Minerva Gastroenterol. 2024, 70, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Jun, J.S.; Seo, J.-H.; Youn, H.-S.; Rhee, K.-H. Changing prevalence of Helicobacter pylori infection in children and adolescents. Clin. Exp. Pediatr. 2021, 64, 21–25. [Google Scholar] [CrossRef]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef]
- Borka Balas, R.; Meliț, L.E.; Mărginean, C.O. Worldwide Prevalence and Risk Factors of Helicobacter pylori Infection in Children. Children 2022, 9, 1359. [Google Scholar] [CrossRef]
- Amaral, O.; Fernandes, I.; Veiga, N.; Pereira, C.; Chaves, C.; Nelas, P.; Silva, D. Living Conditions and Helicobacter pylori in Adults. Biomed Res. Int. 2017, 2017, 9082716. [Google Scholar] [CrossRef]
- Contreras, M.; Fernández-Delgado, M.; Reyes, N.; García-Amado, M.A.; Rojas, H.; Michelangeli, F. Helicobacter pylori Infection in Rural and Urban Dyspeptic Patients from Venezuela. Am. J. Trop. Med. Hyg. 2015, 93, 730–732. [Google Scholar] [CrossRef]
- Rosenstock, S.; Kay, L.; Rosenstock, C.; Andersen, L.P.; Bonnevie, O.; Jørgensen, T. Relation between Helicobacter pylori infection and gastrointestinal symptoms and syndromes. Gut 1997, 41, 169–176. [Google Scholar] [CrossRef]
- Marzio, L.; Cappello, G.; Ballone, E. Evaluation of dyspeptic symptoms in patients with and without Helicobacter pylori infection and normal upper gastrointestinal endoscopy. Dig. Liver Dis. 2003, 35, 138–142. [Google Scholar] [CrossRef]
- O’Connor, A.; O’Morain, C.A.; Ford, A.C. Population screening and treatment of Helicobacter pylori infection. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 230–240. [Google Scholar] [CrossRef]
- Januszewicz, W.; Turkot, M.H.; Malfertheiner, P.; Regula, J. A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When? Cancers 2023, 15, 664. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.-H.; Cheng, H.-C.; Chuang, S.-L.; Chen, Y.-R.; Hsu, Y.-H.; Hsu, T.-H.; Lin, L.-J.; Lin, Y.-W.; Chu, C.-H.; Wu, M.-S.; et al. Mass screening and eradication of Helicobacter pylori as the policy recommendations for gastric cancer prevention. J. Formos. Med. Assoc. 2022, 121, 2378–2392. Available online: https://www.sciencedirect.com/science/article/pii/S0929664622003229 (accessed on 14 March 2025). [CrossRef] [PubMed]
| Characteristics | Males | Females | Total | p-Value Between Males and Females | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age group (years) | |||||||
| 25–34 | 101 | 19.2 | 78 | 15.0 | 179 | 17.1 | 0.062 |
| 35–44 | 149 | 28.3 | 128 | 24.6 | 277 | 26.5 | |
| 45–54 | 124 | 23.6 | 149 | 28.7 | 273 | 26.1 | |
| 55–69 | 152 | 28.9 | 165 | 31.7 | 317 | 30.3 | |
| Level of education | |||||||
| Basic | 194 | 37.0 | 130 | 25.1 | 334 | 31.1 | <0.001 |
| Intermediate | 40 | 7.6 | 63 | 12.2 | 103 | 9.9 | |
| Advanced | 291 | 55.4 | 325 | 62.7 | 616 | 59.0 | |
| Place of residence in childhood | |||||||
| Urban | 405 | 77.1 | 386 | 74.5 | 791 | 75.8 | 0.322 |
| Rural | 120 | 22.9 | 132 | 25.5 | 252 | 24.2 | |
| Drinking water from municipal supply in childhood | |||||||
| Yes | 395 | 75.2 | 378 | 73.0 | 773 | 74.1 | 0.404 |
| No | 130 | 24.8 | 140 | 27.0 | 270 | 25.9 | |
| Hot tap water in childhood | |||||||
| Yes | 398 | 75.8 | 370 | 71.4 | 768 | 73.6 | 0.108 |
| No | 127 | 24.2 | 148 | 28.6 | 275 | 26.4 | |
| Number of household members in childhood | |||||||
| 2–3 | 112 | 21.3 | 101 | 19.5 | 213 | 20.4 | 0.044 |
| 4 | 266 | 50.6 | 234 | 45.2 | 500 | 47.9 | |
| 5 and more | 148 | 28.1 | 183 | 35.3 | 331 | 31.7 | |
| Age Groups | Males (n = 472) | Females (n = 442) | Total (n = 914) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HP-Negative | HP-Positive | HP-Negative | HP-Positive | HP-Negative | HP-Positive | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| 25–34 | 48 | 52.2 a | 44 | 47.8 a | 37 | 52.1 c | 34 | 47.9 c | 85 | 52.1 c | 78 | 47.9 c |
| 35–44 | 50 | 37.9 b | 82 | 62.1 b | 39 | 35.1 | 72 | 64.9 | 89 | 36.6 | 154 | 63.4 |
| 45–54 | 26 | 23.4 b | 85 | 76.6 b | 39 | 32.8 | 80 | 67.2 | 65 | 28.3 | 165 | 71.7 |
| 55–69 | 27 | 19.7 * | 110 | 80.3 * | 56 | 39.7 | 85 | 60.3 | 83 | 29.9 | 195 | 70.1 |
| Total non-standardized | 151 | 32.0 * | 321 | 68.0 * | 171 | 38.7 | 271 | 61.3 | 322 | 35.2 | 592 | 64.8 |
| Total age-standardized, % (95% CI) | 33.7 (27.8–36.2) | 66.3 (62.3–70.8) | 39.8 (34.2–43.2) | 60.2 (55.8–65.0) | 36.9 (32.1–38.3) | 63.1 (60.4–66.7) | ||||||
| Characteristics | HP+ | HP− | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Education | |||||
| Basic | 216 | 73.2 a | 79 | 26.8 | <0.001 |
| Intermediate | 49 | 57.6 | 36 | 42.4 | |
| Advanced | 325 | 61.1 | 207 | 38.9 | |
| Childhood living environment | |||||
| Urban | 442 | 63.5 | 254 | 36.5 | 0.178 |
| Rural | 148 | 68.5 | 68 | 31.5 | |
| Drinking water sourced from a municipal supply | |||||
| Yes | 419 | 63.3 | 254 | 37.7 | 0.010 |
| No | 171 | 71.5 | 68 | 28.5 | |
| Childhood access to hot water | |||||
| Yes | 418 | 62.4 | 252 | 37.6 | 0.015 |
| No | 172 | 71.1 | 70 | 28.9 | |
| Number of household members in childhood | |||||
| 2–3 | 112 | 60.9 | 72 | 39.1 | 0.353 |
| 4 | 283 | 64.6 | 155 | 35.4 | |
| 5 and more | 196 | 67.4 | 95 | 32.6 | |
| Factors | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Sex | ||||||
| Females | 1 | 1 | ||||
| Males | 1.34 | 1.02–1.76 | 0.034 | 1.32 | 1.0–1.75 | 0.049 |
| Age * | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.01–1.04 | <0.001 |
| Education | ||||||
| Basic | 1 | 1 | ||||
| Intermediate | 0.50 | 0.30–0.82 | 0.006 | 0.45 | 0.27–0.75 | 0.002 |
| Advanced | 0.57 | 0.42–0.78 | <0.001 | 0.65 | 0.47–0.89 | 0.008 |
| Childhood living environment | ||||||
| Urban | 1 | 1 | ||||
| Rural | 1.25 | 0.90–1.73 | 0.179 | 1.03 | 0.72–1.48 | 0.876 |
| Drinking water sourced from a municipal supply | ||||||
| Yes | 1 | 1 | ||||
| No | 1.48 | 1.08–2.04 | 0.016 | 1.20 | 0.64–1.57 | 0.399 |
| Childhood access to hot water | ||||||
| Yes | 1 | 1 | ||||
| No | 1.52 | 1.11–2.10 | 0.010 | 0.98 | 0.64–1.51 | 0.931 |
| History of H. pylori Serologic Testing | Males | Females | Total | p-Value Between Males and Females | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Not tested | 395 | 75.2 | 342 | 65.8 | 737 | 70.5 | <0.001 |
| Tested—negative | 47 | 9.0 | 74 | 14.2 | 121 | 11.6 | |
| Tested—positive | 61 | 11.6 | 96 | 18.5 | 157 | 15.0 | |
| Unknown | 22 | 4.2 | 8 | 1.5 | 30 | 2.9 | |
| Total | 525 | 100 | 520 | 100 | 1046 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonaitis, P.; Petkeviciene, J.; Salteniene, V.; Ciupkeviciene, E.; Jonaitis, L.; Kriukas, M.; Luksiene, D.; Lesauskaite, V.; Kupcinskas, J.; Kupcinskas, L. Helicobacter pylori Seroprevalence and Its Associations with Sociodemographic Characteristics, Environmental Factors, and Gastrointestinal Complaints: A Cross-Sectional Study in the Adult Population of Kaunas City, Lithuania. Medicina 2025, 61, 1049. https://doi.org/10.3390/medicina61061049
Jonaitis P, Petkeviciene J, Salteniene V, Ciupkeviciene E, Jonaitis L, Kriukas M, Luksiene D, Lesauskaite V, Kupcinskas J, Kupcinskas L. Helicobacter pylori Seroprevalence and Its Associations with Sociodemographic Characteristics, Environmental Factors, and Gastrointestinal Complaints: A Cross-Sectional Study in the Adult Population of Kaunas City, Lithuania. Medicina. 2025; 61(6):1049. https://doi.org/10.3390/medicina61061049
Chicago/Turabian StyleJonaitis, Paulius, Janina Petkeviciene, Violeta Salteniene, Egle Ciupkeviciene, Laimas Jonaitis, Mantas Kriukas, Dalia Luksiene, Vaiva Lesauskaite, Juozas Kupcinskas, and Limas Kupcinskas. 2025. "Helicobacter pylori Seroprevalence and Its Associations with Sociodemographic Characteristics, Environmental Factors, and Gastrointestinal Complaints: A Cross-Sectional Study in the Adult Population of Kaunas City, Lithuania" Medicina 61, no. 6: 1049. https://doi.org/10.3390/medicina61061049
APA StyleJonaitis, P., Petkeviciene, J., Salteniene, V., Ciupkeviciene, E., Jonaitis, L., Kriukas, M., Luksiene, D., Lesauskaite, V., Kupcinskas, J., & Kupcinskas, L. (2025). Helicobacter pylori Seroprevalence and Its Associations with Sociodemographic Characteristics, Environmental Factors, and Gastrointestinal Complaints: A Cross-Sectional Study in the Adult Population of Kaunas City, Lithuania. Medicina, 61(6), 1049. https://doi.org/10.3390/medicina61061049








