Illuminating Bilateral Breast Cancer: A Multicenter Experience and Clinical Observations
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
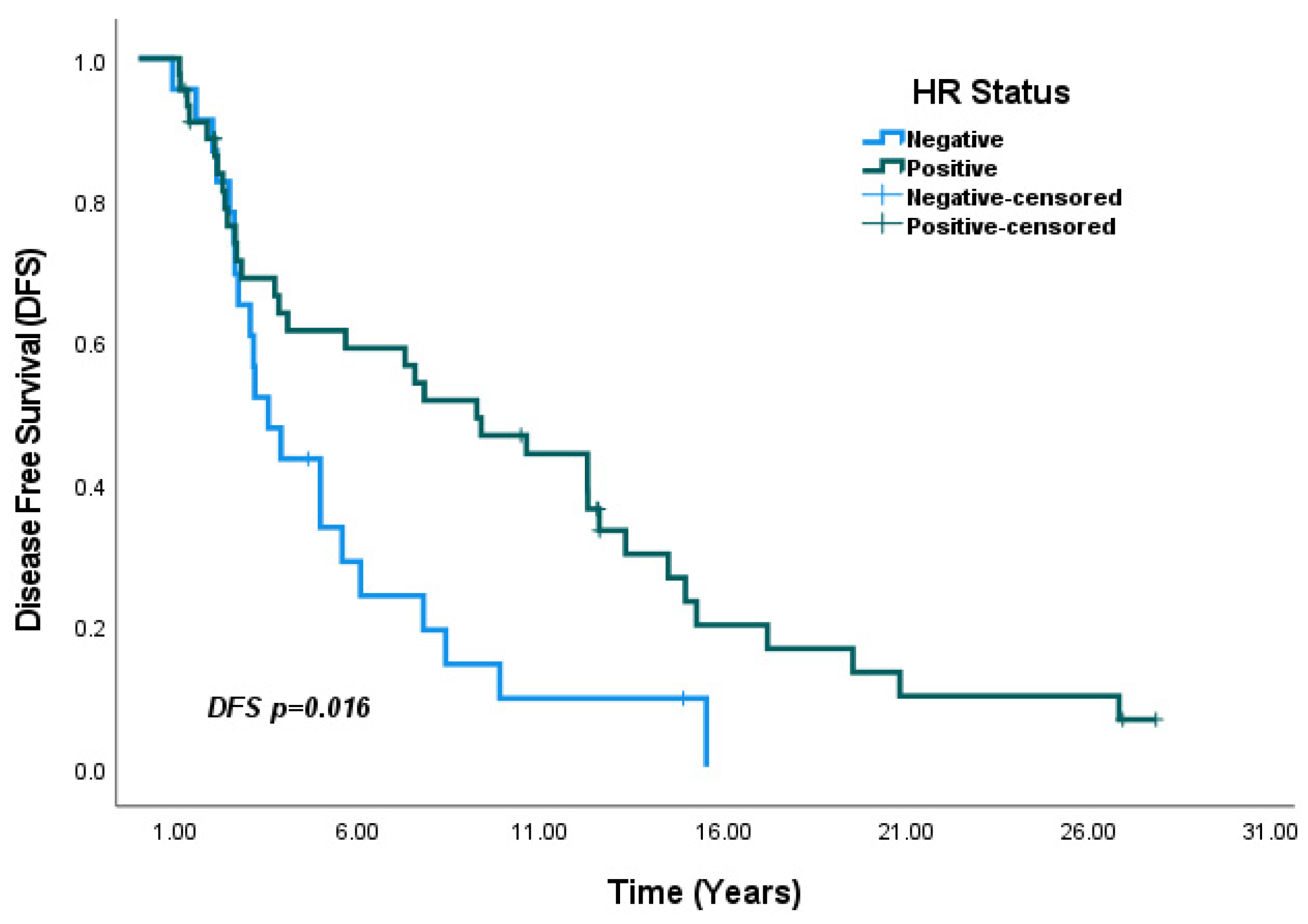
3. Results
4. Discussion
4.1. Clinicopathological and Histological Characteristics
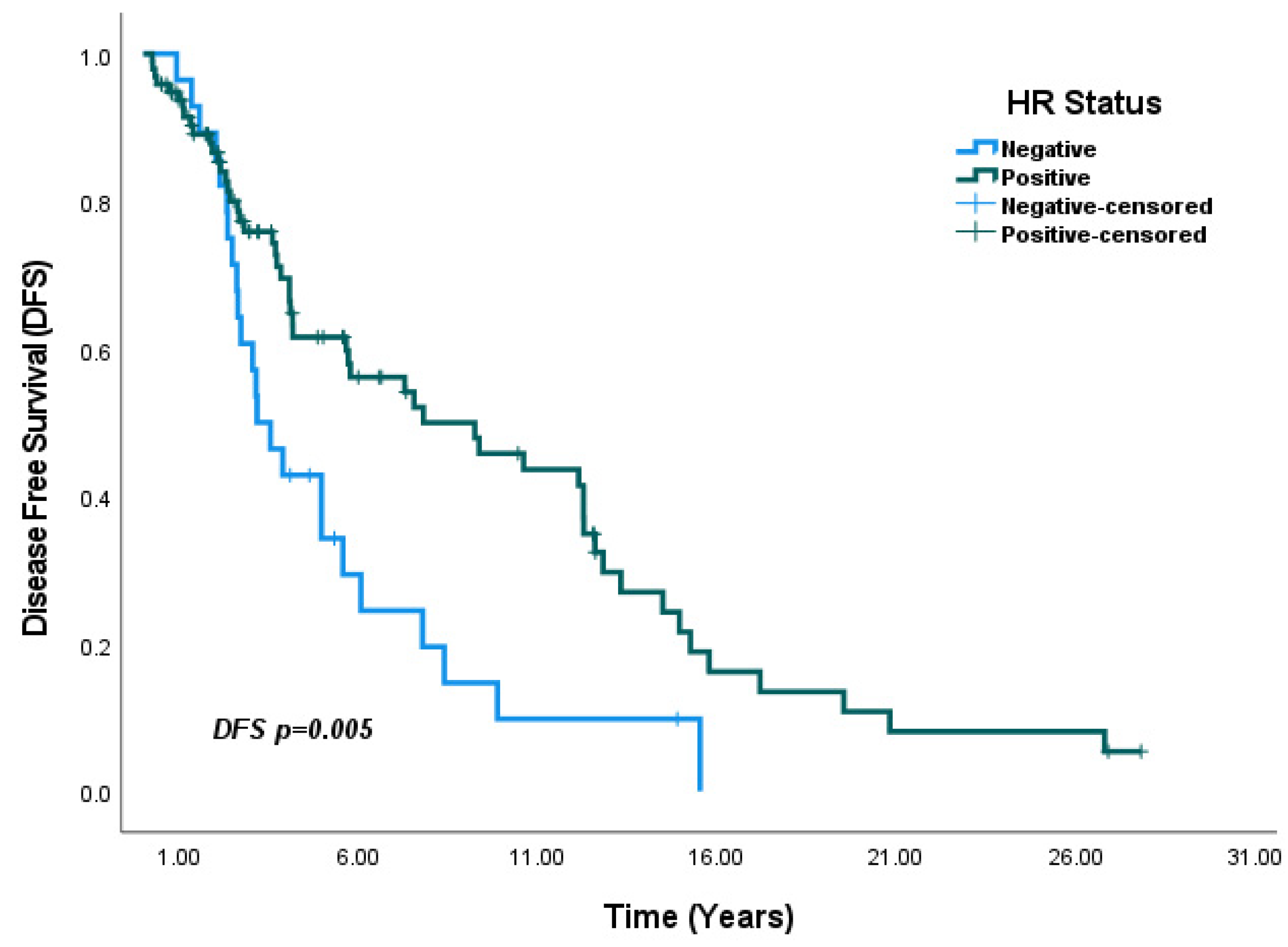
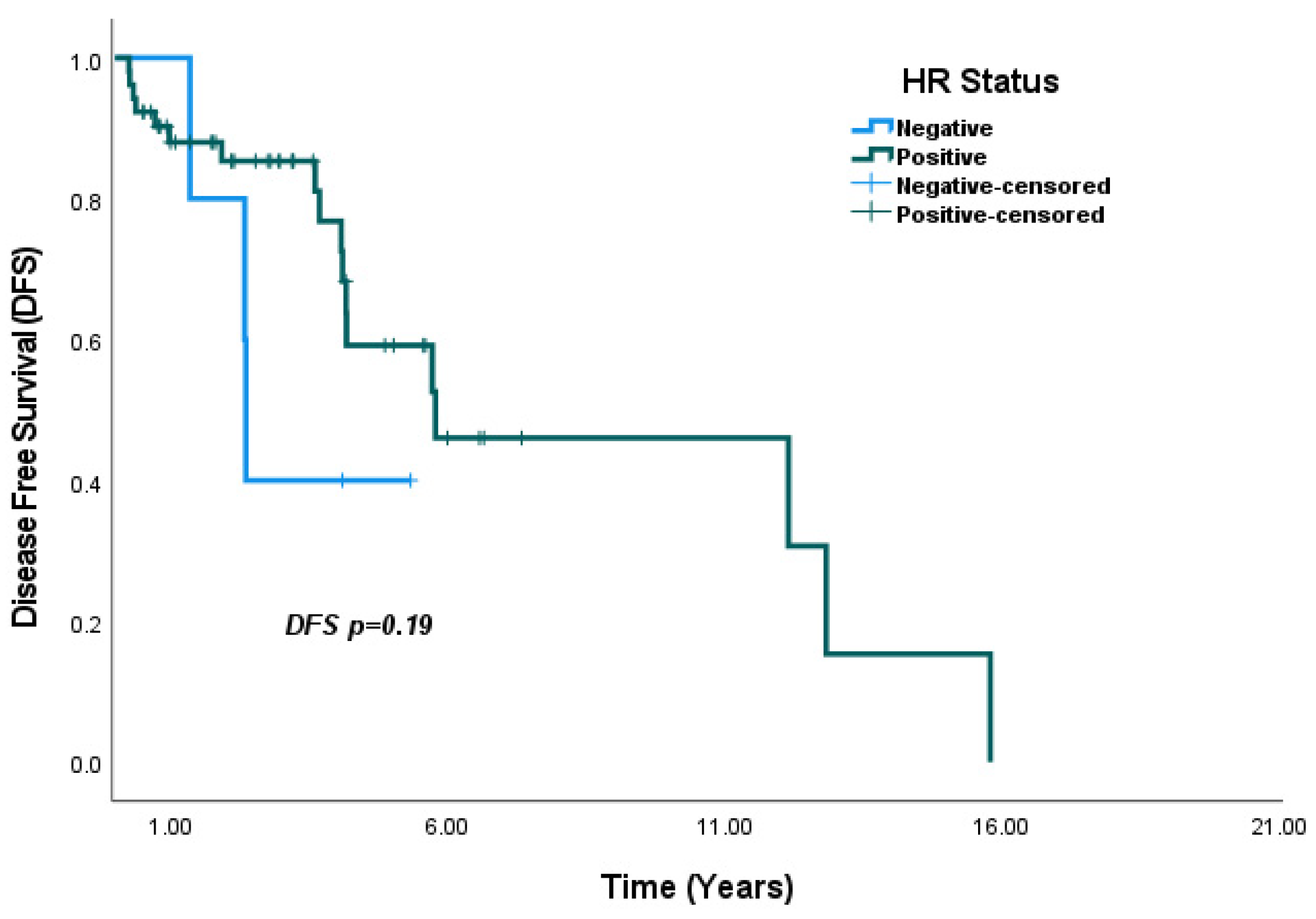
4.2. Hormone Receptor Status and IHC Subtype
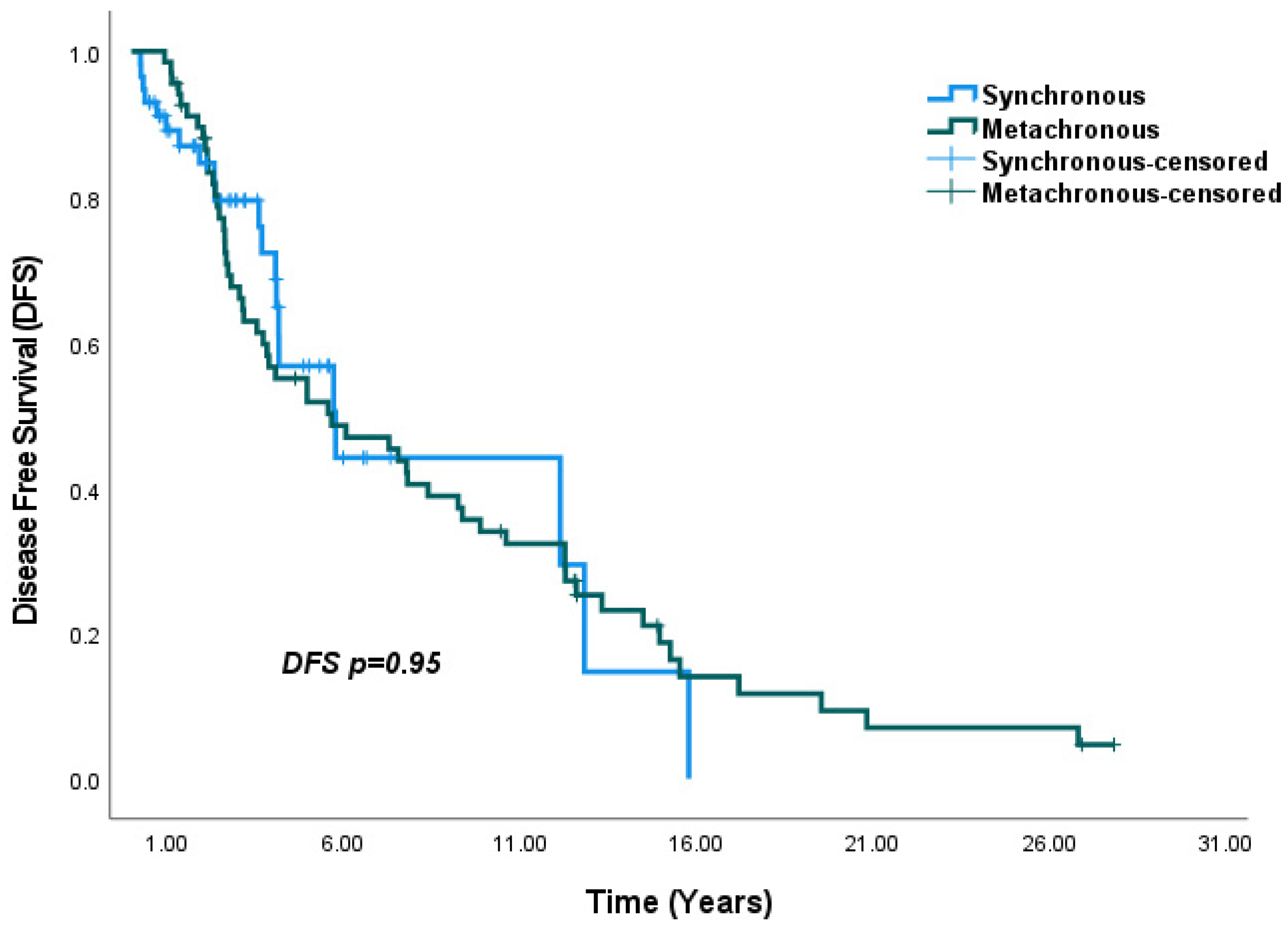
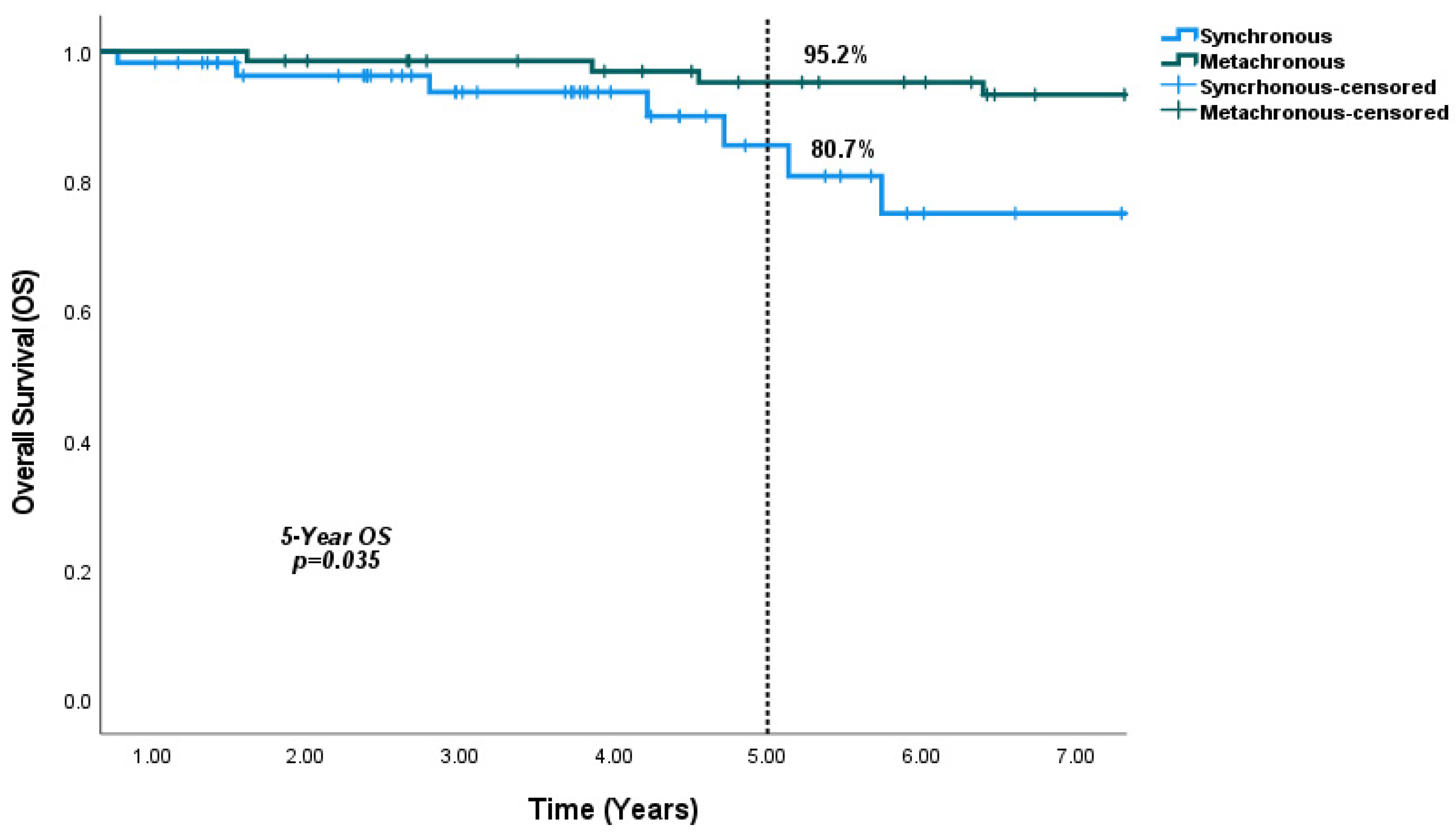
4.3. Survival Outcomes
4.4. Implications and Recommendations
4.5. Study Strengths and Novel Contributions
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBC | Bilateral Breast Cancer |
| SBBC | Synchronous Breast Cancer |
| MBBC | Metachronous Breast Cancer |
| DFS | Disease-Free Survival |
| OS | Overall Survival |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
| HR | Hormone Receptor |
| CHEK2 | Checkpoint Kinase 2 |
| FISH | Fluorescence In Situ Hybridization |
References
- Baykara, M.; Ozturk, S.C.; Buyukberber, S.; Helvaci, K.; Ozdemir, N.; Alkis, N.; Berk, V.; Koca, D.; Coskun, U.; Oksuzoglu, B.; et al. Clinicopathological features in bilateral breast cancer. Asian Pac. J. Cancer Prev. 2012, 13, 4571–4575. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, W.; Cao, J.; Guo, D.; Tao, Z.; Jin, J.; Hu, X. A study of clinical and molecular characteristics in bilateral primary breast cancer. Cancer Med. 2023, 12, 15881–15892. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.L.; Liu, Y.L.; Hsu, Y.Y.; Kuo, W.L. Retrospective Analysis of Clinicopathological Features and Familial Cancer History of Synchronous Bilateral Breast Cancer. Healthcare 2021, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.C.; Lee, H.B.; Yoo, T.K.; Lee, E.S.; Kim, R.N.; Park, B.; Yoon, K.A.; Park, C.; Lee, E.S.; Moon, H.G.; et al. Detection of Germline Mutations in Breast Cancer Patients with Clinical Features of Hereditary Cancer Syndrome Using a Multi-Gene Panel Test. Cancer Res. Treat. 2020, 52, 697–713. [Google Scholar] [CrossRef]
- Nizic-Kos, T.; Krajc, M.; Blatnik, A.; Stegel, V.; Skerl, P.; Novakovic, S.; Gazic, B.; Besic, N. Bilateral Disease Common Among Slovenian CHEK2-Positive Breast Cancer Patients. Ann. Surg. Oncol. 2021, 28, 2561–2570. [Google Scholar] [CrossRef]
- Narod, S.A. Bilateral breast cancers. Nat. Rev. Clin. Oncol. 2014, 11, 157–166. [Google Scholar] [CrossRef]
- Jobsen, J.J.; van der Palen, J.; Ong, F.; Riemersma, S.; Struikmans, H. Bilateral breast cancer, synchronous and metachronous; differences and outcome. Breast Cancer Res. Treat. 2015, 153, 277–283. [Google Scholar] [CrossRef]
- Gao, X.; Fisher, S.G.; Emami, B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: A population-based study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1038–1045. [Google Scholar] [CrossRef]
- Pan, B.; Xu, Y.; Zhou, Y.D.; Yao, R.; Wu, H.W.; Zhu, Q.L.; Wang, C.J.; Mao, F.; Lin, Y.; Shen, S.J.; et al. The prognostic comparison among unilateral, bilateral, synchronous bilateral, and metachronous bilateral breast cancer: A meta-analysis of studies from recent decade (2008–2018). Cancer Med. 2019, 8, 2908–2918. [Google Scholar] [CrossRef]
- Holm, M.; Tjønneland, A.; Balslev, E.; Kroman, N. Prognosis of synchronous bilateral breast cancer: A review and meta-analysis of observational studies. Breast Cancer Res. Treat. 2014, 146, 461–475. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Pan, B.; Li, M.; Gao, J.; Zhao, Y.; Zhao, Z.; Chinese Society of Breast Surgery. Clinical characteristics and clinicopathological correlations of bilateral breast cancer in China: A multicenter study from Chinese Society of Breast Surgery (CSBrS-006). Chin. J. Cancer Res. 2021, 33, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Leis, H.P., Jr.; Mersheimer, W.L.; Black, M.M.; De Chabon, A. The second breast. N. Y. State J. Med. 1965, 65, 2460–2468. [Google Scholar] [CrossRef]
- Heron, D.E.; Komarnicky, L.T.; Hyslop, T.; Schwartz, G.F.; Mansfield, C.M. Bilateral breast carcinoma: Risk factors and outcomes for patients with synchronous and metachronous disease. Cancer 2000, 88, 2739–2750. [Google Scholar] [CrossRef]
- Vuoto, H.D.; García, A.M.; Candás, G.B.; Zimmermann, A.G.; Uriburu, J.L.; Isetta, J.A.; Cogorno, L.; Khoury, M.; Bernabó, O.L. Bilateral breast carcinoma: Clinical characteristics and its impact on survival. Breast J. 2010, 16, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.; Czene, K.; Reilly, M.; Adolfsson, J.; Bergh, J.; Adami, H.O.; Dickman, P.W.; Hall, P. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J. Clin. Oncol. 2007, 25, 4210–4216. [Google Scholar] [CrossRef]
- Londero, A.P.; Bernardi, S.; Bertozzi, S.; Angione, V.; Gentile, G.; Dri, C.; Minucci, A.; Caponnetto, F.; Petri, R. Synchronous and metachronous breast malignancies: A cross-sectional retrospective study and review of the literature. BioMed Res. Int. 2014, 2014, 250727. [Google Scholar] [CrossRef]
- Mruthyunjayappa, S.; Zhang, K.; Zhang, L.; Eltoum, I.A.; Siegal, G.P.; Wei, S. Synchronous and metachronous bilateral breast cancer: Clinicopathologic characteristics and prognostic outcomes. Hum. Pathol. 2019, 92, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Zheng, Y.; Geng, R.; Hu, H.; Zhong, Y.; Guan, Q.; Zhang, Y.; Li, X. Clinicopathological features and prognosis of bilateral breast cancer: A single-center cohort study based on Chinese data. Ann. Transl. Med. 2022, 10, 742. [Google Scholar] [CrossRef]
- Verkooijen, H.M.; Chatelain, V.; Fioretta, G.; Vlastos, G.; Rapiti, E.; Sappino, A.P.; Bouchardy, C.; Chappuis, P.O. Survival after bilateral breast cancer: Results from a population-based study. Breast Cancer Res. Treat. 2007, 105, 347–357. [Google Scholar] [CrossRef]
- Mejdahl, M.K.; Wohlfahrt, J.; Holm, M.; Knoop, A.S.; Tjønneland, A.; Melbye, M.; Kroman, N.; Balslev, E. Synchronous bilateral breast cancer: A nationwide study on histopathology and etiology. Breast Cancer Res. Treat. 2020, 182, 229–238. [Google Scholar] [CrossRef]
- Shi, Y.X.; Xia, Q.; Peng, R.J.; Yuan, Z.Y.; Wang, S.S.; An, X.; Cao, Y.; Tan, Y.T.; Jin, Y.; Cai, X.Y.; et al. Comparison of clinicopathological characteristics and prognoses between bilateral and unilateral breast cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 705–714. [Google Scholar] [CrossRef]
- McCart Reed, A.E.; Kutasovic, J.R.; Lakhani, S.R.; Simpson, P.T. Invasive lobular carcinoma of the breast: Morphology, biomarkers and ’omics. Breast Cancer Res. 2015, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Pascual, T.; Martin, M.; Fernández-Martínez, A.; Paré, L.; Alba, E.; Rodríguez-Lescure, Á.; Perrone, G.; Cortés, J.; Morales, S.; Lluch, A.; et al. A Pathology-Based Combined Model to Identify PAM50 Non-luminal Intrinsic Disease in Hormone Receptor-Positive HER2-Negative Breast Cancer. Front. Oncol. 2019, 9, 303. [Google Scholar] [CrossRef]
- Kheirelseid, E.A.; Jumustafa, H.; Miller, N.; Curran, C.; Sweeney, K.; Malone, C.; McLaughlin, R.; Newell, J.; Kerin, M.J. Bilateral breast cancer: Analysis of incidence, outcome, survival and disease characteristics. Breast Cancer Res. Treat. 2011, 126, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, K.R.; Buckingham, J.; Craft, P.; Dahlstrom, J.E.; Zhang, Y.; Roder, D.; Stuart-Harris, R. Clinical characteristics and outcomes of bilateral breast cancer in an Australian cohort. Breast 2011, 20, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Kollias, J.; Ellis, I.O.; Elston, C.W.; Blamey, R.W. Prognostic significance of synchronous and metachronous bilateral breast cancer. World J. Surg. 2001, 25, 1117–1124. [Google Scholar] [CrossRef]
- Takahashi, H.; Watanabe, K.; Takahashi, M.; Taguchi, K.; Sasaki, F.; Todo, S. The impact of bilateral breast cancer on the prognosis of breast cancer: A comparative study with unilateral breast cancer. Breast Cancer 2005, 12, 196–202. [Google Scholar] [CrossRef]
- He, X.M.; Zou, D.H. The association of young age with local recurrence in women with early-stage breast cancer after breast-conserving therapy: A meta-analysis. Sci. Rep. 2017, 7, 11058. [Google Scholar] [CrossRef]
- Bertozzi, S.; Londero, A.P.; Diaz Nanez, J.A.; Di Vora, R.; Baita, B.; La Verghetta, L.; Prada, S.; Seriau, L.; Mariuzzi, L.; Cedolini, C. Breast cancer care for the aging population: A focus on age-related disparities in breast cancer treatment. BMC Cancer 2025, 25, 492. [Google Scholar] [CrossRef]
- Goyal, R.K.; Chen, H.; Abughosh, S.M.; Holmes, H.M.; Candrilli, S.D.; Johnson, M.L. Overall survival associated with CDK4/6 inhibitors in patients with HR+/HER2- metastatic breast cancer in the United States: A SEER-Medicare population-based study. Cancer 2023, 129, 1051–1063. [Google Scholar] [CrossRef]
- Swain, S.M.; Kim, S.B.; Cortés, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Knott, A.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013, 14, 461–471. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Hurvitz, S.A.; Im, S.A.; Iwata, H.; Curigliano, G.; Kim, S.B.; Chiu, J.W.Y.; Pedrini, J.L.; Li, W.; Yonemori, K.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: Long-term survival analysis of the DESTINY-Breast03 trial. Nat. Med. 2024, 30, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Welch, H.G.; Black, W.C. Overdiagnosis in cancer. J. Natl. Cancer Inst. 2010, 102, 605–613. [Google Scholar] [CrossRef]
- Giobbie-Hurder, A.; Gelber, R.D.; Regan, M.M. Challenges of guarantee-time bias. J. Clin. Oncol. 2013, 31, 2963–2969. [Google Scholar] [CrossRef]
| SBBC n: 57 (%) | MBBC n: 68 (%) | p-Value | |
|---|---|---|---|
| Age | |||
| <50 | 29 (50.9) | 38 (55.9) | 0.576 1 |
| ≥50 | 28 (49.1) | 30 (44.1) | |
| Menapousal Status | |||
| Premenapousal | 25 (43.9) | 33 (48.5) | 0.602 1 |
| Postmenapousal | 32 (56.1) | 35 (51.5) | |
| T Stage | |||
| T1–2 | 43 (75.4) | 51 (75) | 0.955 1 |
| T3–4 | 14 (24.6) | 17 (25) | |
| Nodal Status at Diagnosis | |||
| Negative | 17 (29.8) | 29 (42.6) | 0.139 1 |
| Positive | 40 (70.2) | 39 (57.4) | |
| ER Status at Diagnosis | |||
| ER (-) | 5 (8.8) | 24 (35.3) | <0.001 *1 |
| ER (+) | 52 (91.2) | 44 (64.7) | |
| PR Status at Diagnosis | |||
| PR (-) | 7 (12.3) | 23 (33.8) | 0.005 *1 |
| PR (+) | 50 (87.7) | 45 (66.2) | |
| HER2 Status at Diagnosis | |||
| HER2 (-) | 39 (68.4) | 44 (64.7) | 0.66 1 1 |
| HER2 (+) | 18 (31.6) | 24 (35.3) | |
| Immunohistochemical (IHC) Subtype Discordance (Between the Two Breast Cancers) | |||
| None | 40 (70.2) | 29 (42.6) | 0.006 *2 |
| Yes | 15 (26.3) | 35 (51.5) | |
| Unknown | 2 (3.5) | 4 (5.9) | |
| Presence of Invasive Lobular Carcinoma Component at Diagnosis | |||
| Negative | 36 (63.2) | 54 (79.4) | 0.001 *2 |
| Positive | 20 (35.0) | 7 (10.3) | |
| Unknown | 1 (1.8) | 7 (10.3) | |
| Recurrens/Metastasis Status | |||
| None | 36 (63.2) | 12 (17.6) | <0.001 *1 |
| Yes | 21 (36.8) | 56 (82.4) | |
| Grade at Diagnosis | |||
| Grade 1 | 9 (16.1) | 3 (4.4) | 0.087 1 |
| Grade 2 | 29 (51.8) | 38 (55.9) | |
| Grade 3 | 18 (32.1) | 27 (39.7) | |
| SBBC n: 57 (%) | MBBC n: 68 (%) | |
|---|---|---|
| First Primer Tumor Histology | ||
| Invasive Ductal Carcionma | 34 (59.6) | 51 (75.0) |
| Invasive Lobular Carcinoma | 14 (24.6) | 6 (8.8) |
| Mix Type | 2 (3.5) | 2 (2.9) |
| Other | 7 (12.3) | 9 (13.2) |
| Second Primer Tumor Histology | ||
| Invasive Ductal Carcinoma | 33 (57.9) | 41 (60.3) |
| Invasive Lobular Carcinoma | 8 (14.0) | 6 (8.8) |
| Mix Type | 2 (3.5) | 0 (0.0) |
| Other | 14 (24.6) | 21 (30.9) |
| First Primer Tumor Subtype (IHC) | ||
| HR (+)/HER2 (+) | 13 (22.8) | 12 (17.6) |
| HR (+)/HER2 (-) | 37 (64.9) | 37 (54.4) |
| HR (-)/HER2 (+) | 5 (8.8) | 8 (11.8) |
| TNBC | 2 (3.5) | 11 (16.2) |
| Unknown | 0 (0.0) | 0 (0.0) |
| Second Primer Tumor Subtype (IHC) | ||
| HR (+)/HER2 (+) | 9 (15.8) | 7 (10.3) |
| HR (+)/HER2 (-) | 41 (71.9) | 34 (50.0) |
| HR (-)/HER2 (+) | 4 (7.0) | 9 (13.2) |
| TNBC | 1 (1.8) | 14 (20.6) |
| Unknown | 2 (3.5) | 4 (5.9) |
| Univariate Cox Regression | Multivariate Cox Regression | |||
|---|---|---|---|---|
| HR (CI 95%) | p-Value | HR (CI 95%) | p-Value | |
| <50 age vs. ≥50 age | 1.23 (0.78–1.93) | 0.363 | ||
| Premenopausal vs. Postmenopausal | 0.69 (0.44–1.09) | 0.11 | ||
| SBBC vs. MBBC | 1.01 (0.59–1.71) | 0.95 | ||
| HR negative vs. HR positive | 0.49 (0.30–0.82) | <0.01 | 0.57 (0.33–0.98) | 0.04 * |
| CerbB2 negative vs. CerbB2 positive | 1.73 (1.07–2.79) | 0.02 | 1.43 (0.85–2.40) | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabuğa, B.; Büyükkör, M.; Karabuğa, E.K.; Yıldız, S.; Mehtiyev, M.; Çınkır, H.Y.; Koçoğlu, S.S.; Demir, H.; Yazıcı, O.; Uncu, D.; et al. Illuminating Bilateral Breast Cancer: A Multicenter Experience and Clinical Observations. Medicina 2025, 61, 1029. https://doi.org/10.3390/medicina61061029
Karabuğa B, Büyükkör M, Karabuğa EK, Yıldız S, Mehtiyev M, Çınkır HY, Koçoğlu SS, Demir H, Yazıcı O, Uncu D, et al. Illuminating Bilateral Breast Cancer: A Multicenter Experience and Clinical Observations. Medicina. 2025; 61(6):1029. https://doi.org/10.3390/medicina61061029
Chicago/Turabian StyleKarabuğa, Berkan, Mustafa Büyükkör, Ekin Konca Karabuğa, Sedat Yıldız, Mirmehdi Mehtiyev, Havva Yeşil Çınkır, Sıla Soylu Koçoğlu, Hacer Demir, Ozan Yazıcı, Doğan Uncu, and et al. 2025. "Illuminating Bilateral Breast Cancer: A Multicenter Experience and Clinical Observations" Medicina 61, no. 6: 1029. https://doi.org/10.3390/medicina61061029
APA StyleKarabuğa, B., Büyükkör, M., Karabuğa, E. K., Yıldız, S., Mehtiyev, M., Çınkır, H. Y., Koçoğlu, S. S., Demir, H., Yazıcı, O., Uncu, D., Öksüzoğlu, Ö. B., & Yalçıntaş Arslan, Ü. (2025). Illuminating Bilateral Breast Cancer: A Multicenter Experience and Clinical Observations. Medicina, 61(6), 1029. https://doi.org/10.3390/medicina61061029






