Cardiac Clues in Major Depressive Disorder: Evaluating Electrical Risk Score as a Predictive Electrocardiography Biomarker
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data
2.2. Electrocardiography
2.3. Hamilton Depression Rating Scale (HAM-D)
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDD | Major depressive disorder |
| CVD | Cardiovascular disease |
| SCD | Sudden cardiac death |
| ERS | Electrical risk score |
| ECG | Electrocardiography |
| HAM-D | Hamilton Depression Rating Scale |
| ROC | Receiver operating characteristic |
| ANS | Autonomic nervous system |
| CHD | Coronary heart disease |
References
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public. Health. 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D. Measuring the global burden of disease. N. Engl. J. Med. 2013, 369, 448–457. [Google Scholar] [CrossRef]
- Rajan, S.; McKee, M.; Rangarajan, S.; Bangdiwala, S.; Rosengren, A.; Gupta, R.; Kutty, V.R.; Wielgosz, A.; Lear, S.; AlHabib, K.F.; et al. Prospective Urban Rural Epidemiology (PURE) Study Investigators. Association of Symptoms of Depression with Cardiovascular Disease and Mortality in Low-, Middle-, and High-Income Countries. JAMA Psychiatry 2020, 77, 1052–1063. [Google Scholar] [CrossRef]
- McFarlane, A.; Kamath, M.V.; Fallen, E.L.; Malcolm, V.; Cherian, F.; Norman, G. Effect of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction. Am. Heart J. 2001, 142, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Wilhelm, M.; Salzmann, S.; Rief, W.; Euteneuer, F. A meta-analysis of heart rate variability in major depression. Psychol. Med. 2019, 49, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5166 patients and 5083 controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef]
- Pitharouli, M.C.; Hagenaars, S.P.; Glanville, K.P.; Coleman, J.R.I.; Hotopf, M.; Lewis, C.M.; Pariante, C.M. Elevated C-Reactive Protein in Patients with Depression, Independent of Genetic, Health, and Psychosocial Factors: Results From the UK Biobank. Am. J. Psychiatry 2021, 178, 522–529. [Google Scholar] [CrossRef]
- Whooley, M.A.; Wong, J.M. Depression and cardiovascular disorders. Annu. Rev. Clin. Psychol. 2013, 9, 327–354. [Google Scholar] [CrossRef]
- Shao, M.; Lin, X.; Jiang, D.; Tian, H.; Xu, Y.; Wang, L.; Ji, F.; Zhou, C.; Song, X.; Zhuo, C. Depression and cardiovascular disease: Shared molecular mechanisms and clinical implications. Psychiatry Res. 2020, 285, 112802. [Google Scholar] [CrossRef]
- Lee, S.M.; Baek, J.C. Serum Vitamin Levels, Cardiovascular Disease Risk Factors, and Their Association with Depression in Korean Women: A Cross-Sectional Study of a Nationally Representative Sample. Medicina 2023, 59, 2183. [Google Scholar] [CrossRef]
- Krittanawong, C.; Maitra, N.S.; Qadeer, Y.K.; Wang, Z.; Fogg, S.; Storch, E.A.; Celano, C.M.; Huffman, J.C.; Jha, M.; Charney, D.S.; et al. Association of Depression and Cardiovascular Disease. Am. J. Med. 2023, 136, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jackson, S.L.; Gillespie, C.; Merritt, R.; Yang, Q. Depressive Symptoms and Mortality Among US Adults. JAMA Netw. Open. 2023, 6, e2337011. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Liu, T.; Liang, J.; Hu, D.; Yang, B. Depression and Risk of Sudden Cardiac Death and Arrhythmias: A Meta-Analysis. Psychosom. Med. 2017, 79, 153–161. [Google Scholar] [CrossRef]
- Tolentino, J.C.; Schmidt, S.L. Association between depression severity and cardiac autonomic modulation. J. Psychosom. Res. 2016, 85, 9–11. [Google Scholar] [CrossRef]
- Taylor, C.B. Depression, heart rate related variables and cardiovascular disease. Int. J. Psychophysiol. 2010, 78, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Brunckhorst, C.B.; Holzmeister, J.; Scharf, C.; Binggeli, C.; Duru, F. Stress, Depression und kardiale Arrhythmien. Stress, depression and cardiac arrhythmias. Ther. Umsch. 2003, 60, 673–681. [Google Scholar] [CrossRef]
- Aro, A.L.; Reinier, K.; Rusinaru, C.; Uy-Evanado, A.; Darouian, N.; Phan, D.; Mack, W.J.; Jui, J.; Soliman, E.Z.; Tereshchenko, L.G.; et al. Electrical risk score beyond the left ventricular ejection fraction: Prediction of sudden cardiac death in the Oregon Sudden Unexpected Death Study and the Atherosclerosis Risk in Communities Study. Eur. Heart J. 2017, 38, 3017–3025. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Mastropietri, F.; Di Iorio, C.; Mariani, M.V.; Fabietti, M.; Stricchiola, G.M.; Parrotta, I.; Sardella, G.; Mancone, M.; et al. Possible predictive role of electrical risk score on transcatheter aortic valve replacement outcomes in older patients: Preliminary data. Clin. Interv. Aging 2018, 13, 1657–1667. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Fabietti, M.; Di Iorio, C.; Mastropietri, F.; Sabatino, T.; Crapanzano, D.; Bertani, G.; Zaccagnini, G.; Lospinuso, I.; et al. Age, gender and drug therapy influences on Tpeak-tend interval and on electrical risk score. J. Electrocardiol. 2020, 59, 88–92. [Google Scholar] [CrossRef]
- Pham, H.N.; Holmstrom, L.; Chugh, H.; Uy-Evanado, A.; Nakamura, K.; Zhang, Z.; Salvucci, A.; Jui, J.; Reinier, K.; Chugh, S.S. Dynamic electrocardiogram changes are a novel risk marker for sudden cardiac death. Eur. Heart J. 2024, 45, 809–819. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Cerea, P. A paradigm change in sudden cardiac death risk prediction: ‘static’ goes out, ‘dynamic’ comes in. Eur. Heart J. 2024, 45, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Yilmaz, S. Major depressive disorder is an independent predictor of the electrocardiographic frontal QRS-T angle. Bratisl. Lek. Listy. 2023, 124, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, D.M.; Bunker, S.J.; Clarke, D.M.; Glozier, N.; Hare, D.L.; Hickie, I.B.; Tatoulis, J.; Thompson, D.R.; Tofler, G.H.; Wilson, A.; et al. Screening, referral and treatment for depression in patients with coronary heart disease. Med. J. Aust. 2013, 198, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.L.; Su, T.P.; Chen, T.J.; Chou, Y.H.; Bai, Y.M. Comorbidity of cardiovascular diseases with mood and anxiety disorder: A population based 4-year study. Psychiatry Clin. Neurosci. 2009, 63, 401–409. [Google Scholar] [CrossRef]
- Carney, R.M.; Freedland, K.E. Depression and coronary heart disease. Nat. Rev. Cardiol. 2017, 4, 145–155. [Google Scholar] [CrossRef]
- Rautaharju, P.M.; Surawicz, B.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Gorgels, A.; Hancock, E.W.; Josephson, M.; Kligfield, P.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 982–991. [Google Scholar] [CrossRef]
- You, Z.; He, T.; Ding, Y.; Yang, L.; Jiang, X.; Huang, L. Predictive value of electrocardiographic left ventricular hypertrophy in the general population: A meta-analysis. J. Electrocardiol. 2020, 62, 14–19. [Google Scholar] [CrossRef]
- Mirvis, D.M.; Goldberger, A.L. Electrocardiography. In Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine, 8th ed.; Libby, P., Bonow, R.O., Mann, D.L., Zipes, D.P., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2008; pp. 149–193. [Google Scholar]
- Colluoglu, T.; Tanriverdi, Z.; Unal, B.; Ozcan, E.E.; Dursun, H.; Kaya, D. The role of baseline and post-procedural frontal plane QRS-T angles for cardiac risk assessment in patients with acute STEMI. Ann. Noninvasive Electrocardiol. 2018, 23, e12558. [Google Scholar] [CrossRef]
- Khan, A.R.; Golwala, H.; Tripathi, A.; Bin Abdulhak, A.A.; Bavishi, C.; Riaz, H.; Mallipedi, V.; Pandey, A.; Bhatt, D.L. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: A systematic review and meta-analysis. Eur. Heart J. 2017, 38, 3082–3089. [Google Scholar] [CrossRef]
- Hamilton, M.A. Rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Akdemir, A.; Orsel, D.S.; Dag, İ.; Turkcapar, M.H.; Iscan, N.; Ozbay, H. Clinical use and the reliability and validty of the Turkish version of the Hamilton Depression Rating Scale (HDRS). 3 P Psikiyatr. Psikol. Psikofarmakol. Derg. 1996, 4, 251–259. [Google Scholar]
- Have, M.T.; de Graaf, R.; van Dorsselaer, S.; Tuithof, M.; Kleinjan, M.; Penninx, B.W.J.H. Recurrence and chronicity of major depressive disorder and their risk indicators in a population cohort. Acta Psychiatr. Scand. 2018, 137, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.; Schumacher, M.; Herrmann-Lingen, C. Depression as a risk factor for mortality in patients with coronary heart disease: A meta-analysis. Psychosom. Med. 2004, 66, 802–813. [Google Scholar] [CrossRef]
- Janszky, I.; Ahlbom, A.; Hallqvist, J.; Ahnve, S. Hospitalization for depression is associated with an increased risk for myocardial infarction not explained by lifestyle, lipids, coagulation, and inflammation: The SHEEP Study. Biol. Psychiatry 2007, 62, 25–32. [Google Scholar] [CrossRef]
- Shiga, T. Depression and cardiovascular diseases. J. Cardiol. 2023, 81, 485–490. [Google Scholar] [CrossRef]
- Nater, U.M.; Skoluda, N.; Strahler, J. Biomarkers of stress in behavioural medicine. Curr. Opin. Psychiatry 2013, 26, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Duivis, H.E.; de Jonge, P.; Penninx, B.W.; Na, B.Y.; Cohen, B.E.; Whooley, M.A. Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: Prospective findings from the heart and soul study. Am. J. Psychiatry 2011, 168, 913–920. [Google Scholar] [CrossRef]
- Koudouovoh-Tripp, P.; Hüfner, K.; Egeter, J.; Kandler, C.; Giesinger, J.M.; Sopper, S.; Humpel, C.; Sperner-Unterweger, B. Stress Enhances Proinflammatory Platelet Activity: The Impact of Acute and Chronic Mental Stress. J. Neuroimmune Pharmacol. 2021, 16, 500–512. [Google Scholar] [CrossRef]
- Birnbaum, Y.; Rankinen, J.; Jneid, H.; Atar, D.; Nikus, K. The Role of ECG in the Diagnosis and Risk Stratification of Acute Coronary Syndromes: An Old but Indispensable Tool. Curr. Cardiol. Rep. 2022, 24, 109–118. [Google Scholar] [CrossRef]
- Elmas, A.N.; Fedai, H.; Toprak, K.; Taşcanov, M.B.; Altıparmak, İ.H.; Biçer, A.; Demirbağ, R.; Tanrıverdi, Z. The Association of Electrical Risk Score with Prognosis in Patients with Non-ST Elevation Myocardial Infarction Undergoing Coronary Angiography. Anatol. J. Cardiol. 2024, 29, 11–18. [Google Scholar] [CrossRef]
- Oehler, A.; Feldman, T.; Henrikson, C.A.; Tereshchenko, L.G. QRS-T angle: A review. Ann. Noninvasive Electrocardiol. 2014, 19, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Won, E.; Kim, Y.K. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.M.; Freedland, K.E.; Miller, G.E.; Jaffe, A.S. Depression as a risk factor for cardiac mortality and morbidity: A review of potential mechanisms. J. Psychosom. Res. 2002, 53, 897–902. [Google Scholar] [CrossRef] [PubMed]
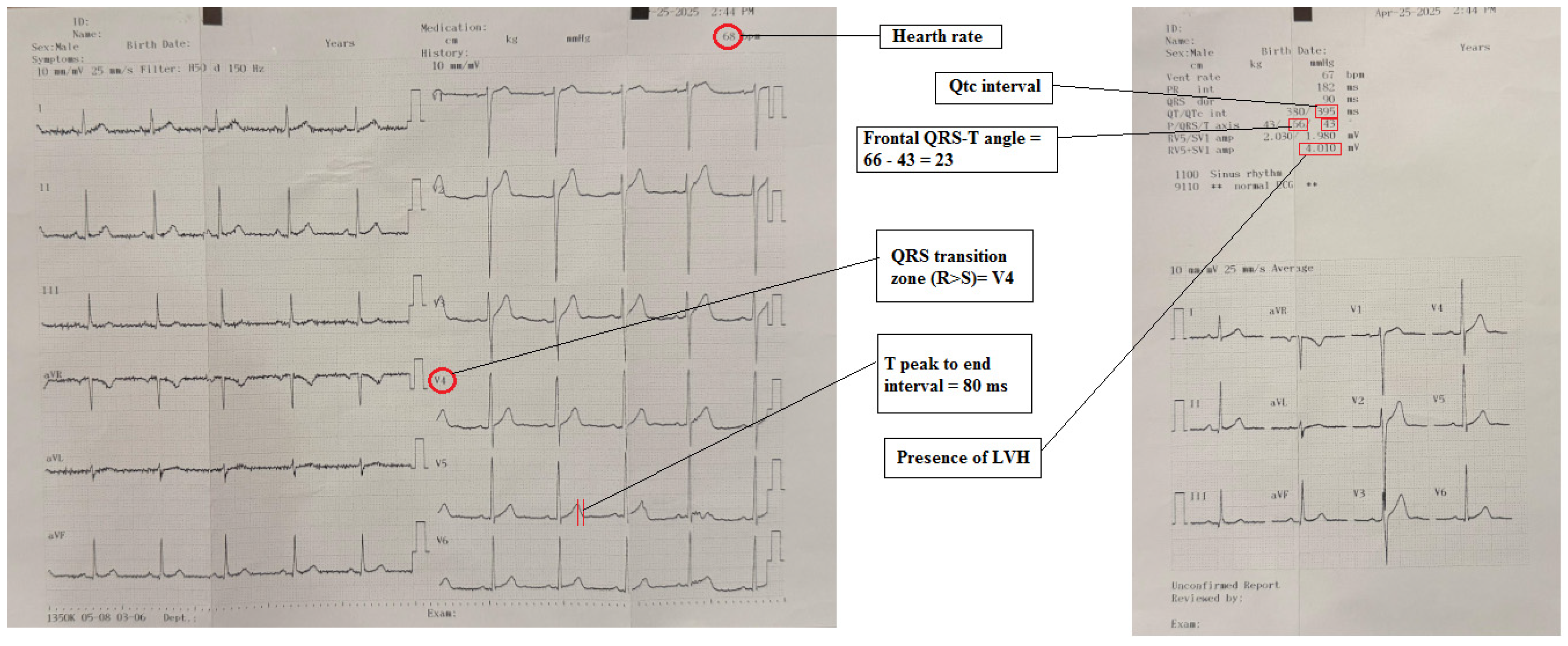

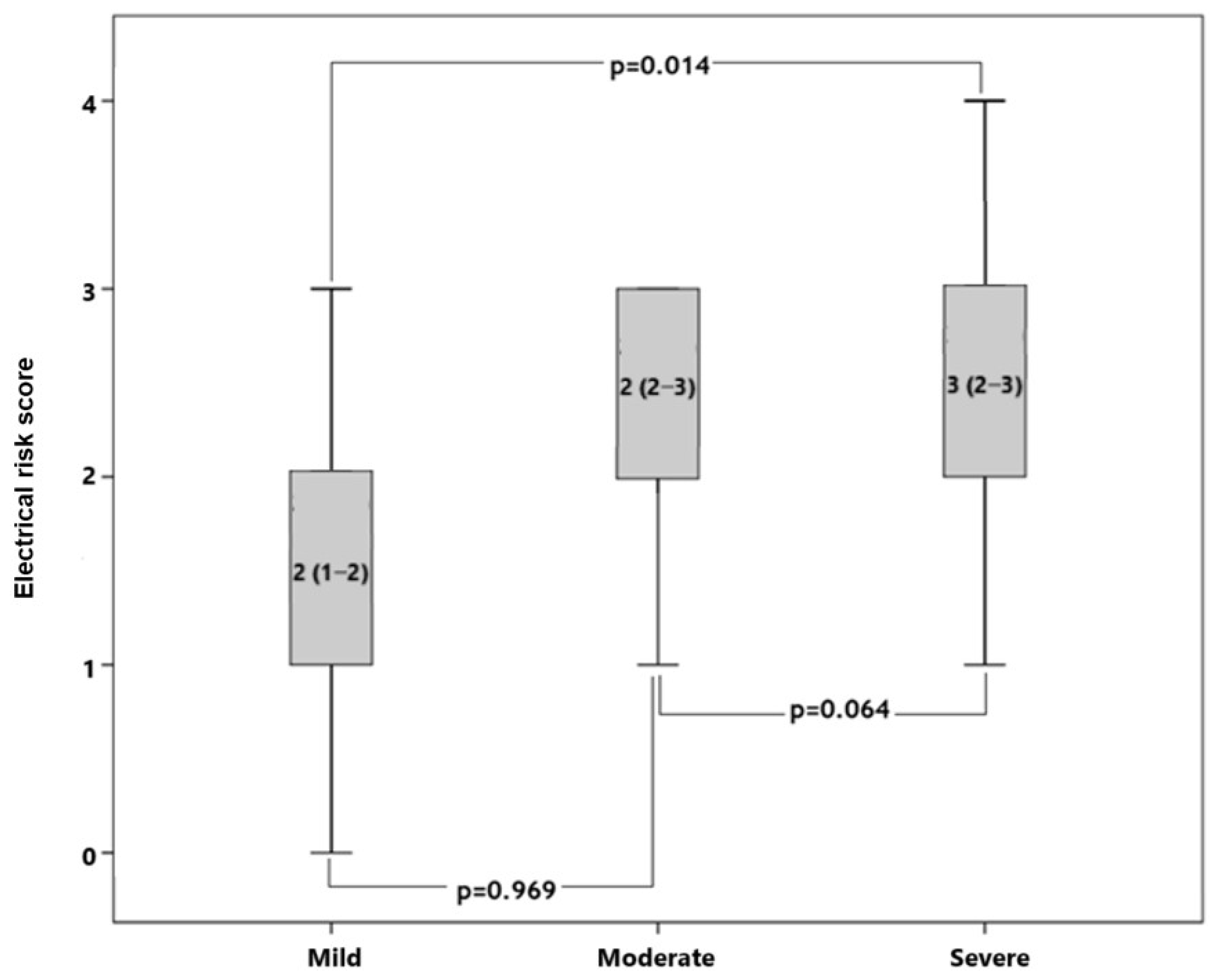
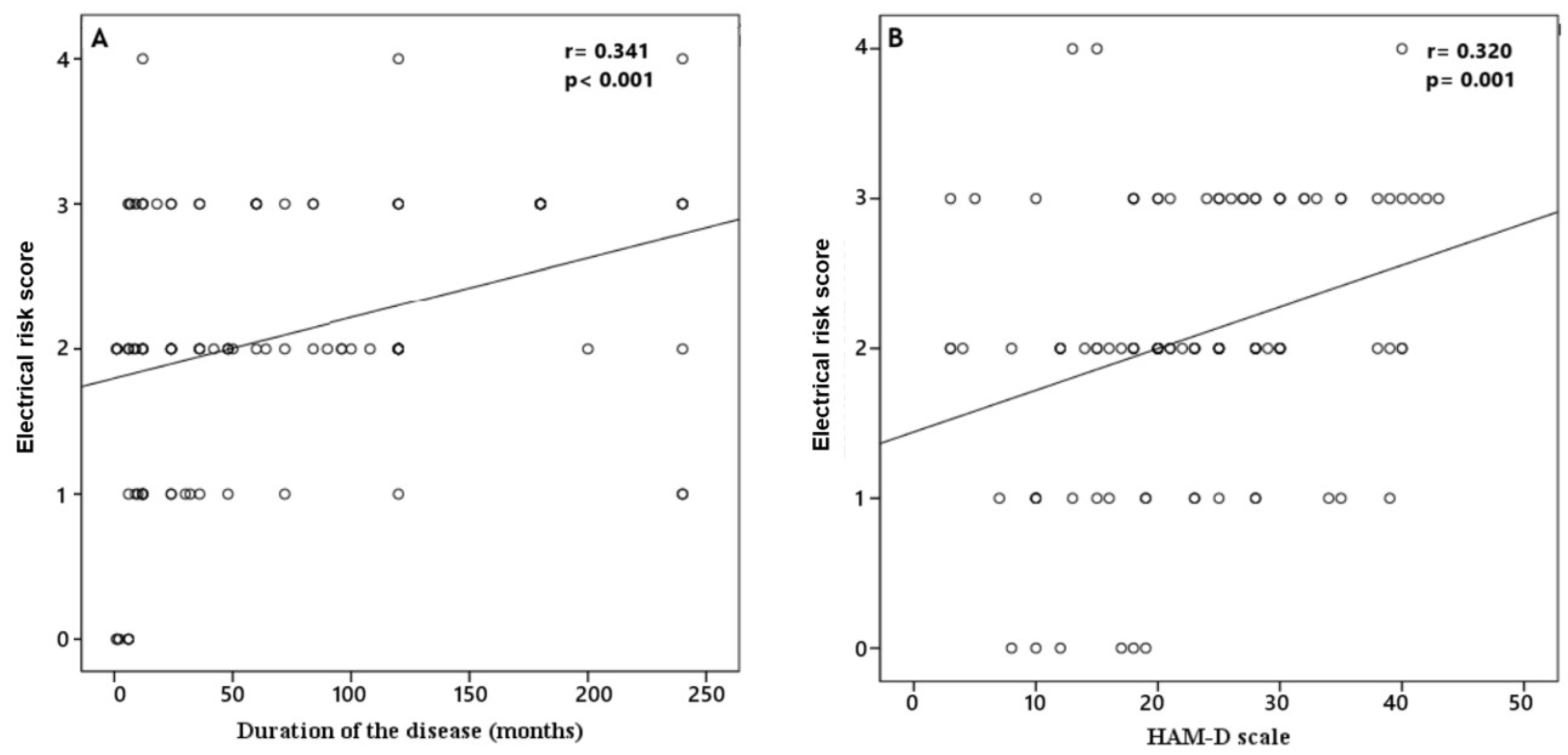
| Variables | Patients (n = 102) | Control (n = 62) | p |
|---|---|---|---|
| Age, years | 39.0 (28.8–51.3) | 35.5 (27.0–48.3) | 0.465 |
| Gender, female (%) | 62 (60.8) | 36 (58.1) | 0.731 |
| Smoking (%) | 34 (33.3) | 28 (45.2) | 0.130 |
| SBP, mmHg | 119.9 ± 7.7 | 120.9 ± 8.3 | 0.425 |
| DBP, mmHg | 72.0 ± 6.6 | 71.1 ± 6.3 | 0.375 |
| Creatinine, mg/dL | 0.79 ± 0.18 | 0.81 ± 0.19 | 0.449 |
| Total-cholesterol, mg/dl | 219.1 ± 48.7 | 220.8 ± 53.7 | 0.829 |
| Triglyceride, mg/dL | 134.5 (83.3–199.5) | 126.5 (77.8–168.0) | 0.391 |
| LDL-cholesterol, mg/dL | 137.2 ± 38.7 | 135.0 ± 39.4 | 0.735 |
| HDL-cholesterol, mg/dL | 53.6 ± 13.3 | 56.2 ± 14.5 | 0.251 |
| Hemoglobin, g/dL | 13.9 ± 1.7 | 13.4 ± 1.6 | 0.067 |
| Platelets, ×103/µL | 269.2 ± 63.3 | 267.2 ± 67.9 | 0.853 |
| Leukocytes, ×103/µL | 7.6 ± 1.6 | 7.2 ± 1.9 | 0.103 |
| TSH, mIU/L | 1.9 (1.2–2.5) | 1.8 (1.3–2.5) | 0.948 |
| Medication (%) | 55 (53.9) | ||
| n (min–max mg/day) | |||
| Venlafaxine | 34 (75–375) | ||
| Duloxetine | 2 (60–120) | ||
| Sertraline | 14 (50–200) | ||
| Essitaloprame | 1 (10) | ||
| Fluoxetine | 4 (40) | ||
| Paroxetine | 3 (20–40) | ||
| Mirtazapine | 18 (15–45) | ||
| Trazodone | 4 (50–100) | ||
| Bupropione | 7 (150–300) | ||
| Klomipramine | 1 (75) | ||
| Olanzapine | 12 (2.5–10) | ||
| Quetipine | 16 (25–300) | ||
| Aripiprazole | 2 (2.5–5) | ||
| Sulpırıde | 4 (50–100) |
| Patients (n = 102) | Control (n = 62) | p | |
|---|---|---|---|
| Heart rate, /bpm | 83.1 ± 16.3 | 70.8 ± 9.5 | <0.001 |
| Sokolow–Lyon voltage criteria, mV | 17.8 (12.9–21.9) | 18.0 (15.0–22.3) | 0.205 |
| QTc interval, ms | 414.8 ± 29.0 | 408.3 ± 18.4 | 0.081 |
| Tp-e interval, ms | 81.3 ± 21.7 | 70.1 ± 14.0 | <0.001 |
| Frontal QRS-T angle, o | 28.0 (11.8–53.0) | 14.5 (9.0–30.3) | 0.013 |
| ERS | 2 (2–3) | 0 (0–1) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atilan Fedai, U.; Fedai, H.; Tanriverdi, Z. Cardiac Clues in Major Depressive Disorder: Evaluating Electrical Risk Score as a Predictive Electrocardiography Biomarker. Medicina 2025, 61, 1026. https://doi.org/10.3390/medicina61061026
Atilan Fedai U, Fedai H, Tanriverdi Z. Cardiac Clues in Major Depressive Disorder: Evaluating Electrical Risk Score as a Predictive Electrocardiography Biomarker. Medicina. 2025; 61(6):1026. https://doi.org/10.3390/medicina61061026
Chicago/Turabian StyleAtilan Fedai, Ulker, Halil Fedai, and Zulkif Tanriverdi. 2025. "Cardiac Clues in Major Depressive Disorder: Evaluating Electrical Risk Score as a Predictive Electrocardiography Biomarker" Medicina 61, no. 6: 1026. https://doi.org/10.3390/medicina61061026
APA StyleAtilan Fedai, U., Fedai, H., & Tanriverdi, Z. (2025). Cardiac Clues in Major Depressive Disorder: Evaluating Electrical Risk Score as a Predictive Electrocardiography Biomarker. Medicina, 61(6), 1026. https://doi.org/10.3390/medicina61061026






