Abstract
Background and Objectives: Neuropsychiatric disorders, including schizophrenia, bipolar disorder, and major depression, constitute a leading global public health challenge due to their high prevalence, chronicity, and profound cognitive and functional impact. This systematic review explores the role of electroencephalography (EEG)-based cognitive biomarkers in improving the understanding, diagnosis, monitoring, and treatment of these conditions. It evaluates how EEG-derived markers can reflect neuro-cognitive dysfunction and inform personalized and scalable mental health interventions. Materials and Methods: A systematic review was conducted following PRISMA guidelines. The databases searched included PubMed, Scopus, PsycINFO, and Web of Science for peer-reviewed empirical studies published between 2014 and 2025. Inclusion criteria focused on EEG-based investigations in clinical populations with neuropsychiatric diagnoses, emphasizing studies that assessed associations with cognitive function, symptom severity, treatment response, or functional outcomes. Of the 447 initially identified records, 132 studies were included in the final synthesis. Results: This review identifies several EEG markers—such as mismatch negativity (MMN), P300, frontal alpha asymmetry, and theta/beta ratios—as reliable indicators of cognitive impairments across psychiatric populations. These biomarkers are associated with deficits in attention, memory, and executive functioning, and show predictive utility for treatment outcomes and disease progression. Methodological trends indicate an increasing use of machine learning and multimodal neuroimaging integration to enhance diagnostic specificity. While many studies exhibit moderate risk of bias, the overall findings support EEG biomarkers’ reproducibility and translational relevance. Conclusions: EEG-based cognitive biomarkers offer a valuable, non-invasive means of capturing the neurobiological underpinnings of psychiatric disorders. Their diagnostic and prognostic potential, as well as high temporal resolution and portability, supports their use in clinical and public health contexts. The field, however, requires further standardization, cross-validation, and investment in scalable applications. Advancing EEG biomarker research holds promise for precision psychiatry and proactive mental health strategies at the population level.
1. Introduction
A key priority for health policy is the prevention of disease and disability. Progress in prevention is most likely when the condition’s causes have been identified, resolved, or mitigated, and when those at risk can be identified by markers that indicate a predisposition to the disorder [1,2,3]. Recent advances in imaging and data analysis have enabled researchers and clinicians to examine the brain’s structure and function in unprecedented detail [4,5]. As global constituent elements of brain function and structure, it is likely that neuroimaging data, whether from magnetic resonance imaging (MRI) of brain macrostructure, task and rest functional MRI (resting--state)-fMRI), or other modalities, such as positron emission tomography (PET) or magnetoencephalography (MEG), will generate biomarkers for major brain disorders [6,7,8]. However, several hurdles need to be overcome, including the complexity and heterogeneity of the data, the need for multiple cross-validated datasets from different populations, the assessment of new methodologies or of existing technologies not widely available to ensure independence from application developers, manufacturers, or data providers; and the need for scrutiny, enforcement of accurate data description, and interrogation of shared datasets to further minimize reporting biases [9,10,11]. Clinical research based on neuroimaging data is overcoming these hurdles, and the breadth of neuroimaging applications that explain the public health impact on neuropsychiatric illnesses is outlined [1,12]. These include characterizing network aspects of psychiatric symptom formation; normative brain development and neurodevelopmental origin of major vulnerability traits for brain illnesses; direct and indirect effects of somatic therapies on brain function and structure; and concerted private and public strategies to apply big data handling methodologies and other resources to the most urgent problems in clinical neuropsychiatry and developmental neuroscience [13,14]. For these illnesses that have many confounding influences and often comorbidities with other neuropsychiatric disorders, it is safe to assume that hybrid imaging will not give us all the answers. However, with various techniques, genetic predispositions, convergent functional genomics, and clinical attributes, among others, researchers are likely to discover useful biomarkers with high specificity and sensitivity for each disorder, as seen in recent developments for Alzheimer’s disease [15,16,17].
This systematic review aims to bridge critical gaps in understanding how cognitive dysfunction manifests across neuropsychiatric disorders through the lens of EEG-based biomarkers. Despite significant advances in neuroimaging research, a comprehensive synthesis evaluating the translational potential of these biomarkers for addressing the public health burden of mental illness has been lacking. The rapid technological evolution in EEG acquisition and analysis over the past decade, including machine learning approaches, portable technologies, and multimodal integration techniques—necessitates a contemporary assessment, which motivates our focus on publications from 2014 to 2025. Specifically, this review synthesizes empirical evidence from EEG studies to explore the utility of cognitive biomarkers in diagnosing, monitoring, and treating psychiatric conditions. This review examines the neural correlations of attention, memory, executive function, and emotion regulation and how these markers relate to clinical outcomes, treatment response, and disorder severity. In doing so, it also evaluates the methodological rigor and reproducibility of EEG findings, the integration of EEG with other neuroimaging modalities, and the translational potential of these tools in real-world public health and clinical settings. Special attention is given to scalable applications supporting early detection, risk stratification, and personalized intervention across diverse populations, with the goal of providing an evidence-based framework for implementing EEG biomarkers in practical public health strategies for neuropsychiatric care.
2. Literature Review
2.1. Overview of Neuropsychiatric Disorders
Neuropsychiatric disorders are the leading cause of disability worldwide. It is crucial to have a comprehensive understanding of the brain-body–mind–environment interface to inform a public health policy agenda aimed at effective prevention [18,19,20]. Every conceivable biological scenario can cause brain insults, either directly or through shared pathological mechanisms of systemic diseases or altered adaptation to environmental stressors [21,22,23,24].
Recent research findings from epidemiologic studies and new sources underline the extent of the public health impact of mental disorders in Europe, showing that these disorders rank high among those medical, emotional, and social problems that result in increased levels of activity limitation and restrictions for those affected [25,26,27,28,29]. Every fourth adult and child/adolescent are expected to have a mental disorder during their life. Viewed in combination, these studies point to the extensive personal and societal burdens associated directly or indirectly with the presence of cognitive/psychiatric disorders and underscore the need for including the public health impact of these disorders in the formation of healthcare policy and planning across Europe [30,31,32,33,34,35].
Epidemiologic research in the mental health field in Europe has a relatively long history dating back to 1913. However, such research has seen only small steps towards a “union of psychiatric epidemiology in Europe” due to a history of methodological difficulties and diversity in research strategies, which limit the possibilities for comparisons across studies or countries [36,37,38]. In the early 90s, initiatives were launched to improve the public health impact of mental disorders, and one or another, various forms of assessment of mental health were seriously considered. Examples of studies or reports from such initiatives include the European Study of the Epidemiology of Mental Disorders (ESEMeD), the European Study on Comorbidity of Substance Use and Mental Disorders (ESEMeD), or, in Germany, the report on “The Healthcare Situation of the German Population”. Epidemiologic research on mental disorders, including a nationwide representative sample of the adult general population, addressed several objectives—among them, estimating the prevalence and societal burden of mental disorders in general and among different disorder groups in particular [39,40,41,42]. By the time BASIC II was launched, several studies in other countries had either been completed or were underway. The publication of preparatory studies coordinated by the European Commission and WHO had also surfaced, and several substantial or related studies or reports appeared in the literature [43,44,45,46,47]. Similar to the Schedules for Clinical Assessment in Neuropsychiatry (SCAN), which was previously used in the UK, the CIDI has been utilized in the US, Canada, Australia, and Israel. Recently, the WHO also coordinated initiatives to use the CIDI in four countries in Africa and South America [48,49,50]. “In case of suicidality, lifetime prevalence involves a considerable part of the adult population (2.7%)”. Available treatment rates for “affective disorders” vary remarkably among countries. “Under the proactive WHO forward always included the necessity to consider services needed for prevention and rehabilitation, for which reliable data have to be delivered by national epidemiological studies, which consider both policymakers as well as the general population”. In one of the many reports about the more efficient use of healthcare resources, the treatment rates of “affective disorders” in Europe were considered modest. Implementing adequate treatment was, however, anticipated with a substantial societal burden of depression and anxiety, which calls for a considerable breadth of acute and long-term treatment procedures [51,52,53].
2.2. Neuroimaging Techniques
Neuroimaging is an innovative methodology that has provided valuable insights into the public health burden of neuropsychiatric disorders. Neuroimaging has significantly contributed to a deeper understanding of the brain, including its physical and functional properties. Although, in recent decades, data generated by neuroimaging has mainly been the subject of interest in psychology, cognitive neuroscience, and basic neurobiology, the beginning of the century brought a shift to a more public health-oriented understanding of neuroimaging findings. Neuroimaging researchers and public health professionals must collaborate to maximize society’s benefit [54,55,56,57]. Insights derived from neuroimaging data are becoming increasingly crucial for understanding the complex interactions between neural substrates, behavioral patterns, environmental factors, and genetic background. Efforts are continuously made to develop new powerful, sensitive, and mostly non-invasive neuroimaging techniques to investigate brain structure and function and their associations with diverse psychopathological constellations, premature mortality, and functional incapacity. This has led to the identification of several new risk factors, which may potentially inform future public health policy [58,59,60].
Despite novel applications and the further development of neuroimaging technologies, neuroimaging researchers need to appreciate the unique validation criteria relevant to public health. This requires addressing the major public health issues of equity and efficiency when formulating conclusions and recommendations from neuroimaging studies. For example, in the context of brain age and neuropsychiatric morbidity, neuroimaging data suggest that the brain of a mentally disturbed individual “looks” older than that of a healthy control [61,62,63,64]. Technically, a “brain-age” effect is described as a discrepancy between the chronological age of the brain and the apparent biological age of the brain, as estimated using structural and/or functional neuroimaging data. Defining brain age from neuroimaging is typically based on brain measures displaying an association with chronological age in a reference population. Once modeled, a brain age estimate can be obtained for future subjects, and those new estimates can be inspected using pre-defined mathematical and statistical algorithms [65,66,67,68].
Neuroimaging plays a crucial role in understanding the neural underpinnings of neuropsychiatric disorders. Earlier neuroimaging studies on these disorders are primarily based on a single neuroimaging modality, which can only depict partial aspects of brain imaging observations and has limited clinical applications. Multimodal neuroimaging can provide a more comprehensive understanding of the underlying neural mechanisms of brain disorders and their impact on public health problems. Although encouraging results have been shown in multimodal neuroimaging studies, most depend on visual inspection of concordance among different modalities, which is often limited by subjective evaluations. More effective and efficient multimodal neuroimaging computing methods are needed, like feature selection techniques from the machine learning community. Many sophisticated feature selection techniques have recently been developed to search for a subset of the most informative features from many redundant features. Considering the growing complexity of multimodal neuroimaging data, there is increasing recognition of the need for advanced, objective integration techniques to support robust biomarker discovery. Machine learning-based feature selection methods, such as recursive feature elimination, elastic net regression, and mutual information ranking, are increasingly applied to EEG-fMRI or EEG-MEG datasets to isolate the most informative neural signatures. These techniques offer several advantages over traditional visual inspection, including scalability, reduced dimensionality, and the ability to uncover subtle, nonlinear patterns that may be clinically relevant. When coupled with cross-validation and independent test sets, these approaches can significantly enhance the reliability, reproducibility, and clinical utility of multimodal neuroimaging biomarkers. However, few studies have compared different feature selection techniques in the neuroimaging field, especially in multimodal neuroimaging [69]. While integrating EEG with modalities such as fMRI, MEG, and PET is increasingly pursued to achieve a more comprehensive understanding of brain function, many studies still rely on visual inspection and subjective evaluations to assess concordance across imaging techniques. Though valuable in exploratory contexts, this approach introduces variability and limits reproducibility. Future research should prioritize the development of standardized, quantitative frameworks—such as machine learning-based fusion methods, multimodal feature extraction, and cross-modal validation pipelines—to improve objectivity and interpretability. Enhancing methodological rigor in multimodal integration will be critical to unlocking the full translational potential of EEG in conjunction with other imaging modalities [70,71,72,73].
2.3. Cognitive Biomarkers
Latent variable modeling of brain phenotypes indicates that continuous dimensions better represent neuropsychiatric disorders and that frontoparietal and default network dysconnectivity is a common denominator of these dimensions, consistent with the recent “connectotyping” of psychoses [74,75,76,77]. In data-driven paradigms, key goals include the discovery of novel biomarkers and incompletely characterized dimensions, as well as the integration of diverse modalities, analytical methods, and clinical characteristics to enhance generalizability to the complex manifestations of disorders. [78,79,80,81,82,83]. A novel data-driven paradigm was employed to identify joint multimodal brain imaging markers common to dimensions related to several disorders, with features predictive of both clinical and cognitive manifestations [84,85,86].
Cognitive biomarkers strongly predict brain function, suggesting that these features can be used for in vivo detection of specific neurobiological mechanisms not yet captured by other measures [87,88,89]. Despite their use in research, a lack of clinically utilized biomarkers derived from neuroimaging data exists in neuropsychiatric conditions. A novel approach demonstrated a strong correlation between cognitive features and imaging findings, suggesting that dimensional cognitive biomarkers can guide imaging analysis toward identifying sMRI markers of neuropsychiatric conditions [90,91,92,93,94].
Considering that neuropsychiatric disorders represent a substantial public health burden, there is a need for refined neuromarkers that can be applied widely across multiple clinical and research environments [95,96,97,98]. Here, an automated routine is described and validated that accurately extracts coherent, task-related, and event-related potentials (ERPs) from the electroencephalogram (EEG). Utilizing these parameters, rapid screening or monitoring of numerous patient groups could be implemented [99,100,101,102,103]. Such an endeavor would be transformative, providing elliptical interventions for neuro-cognitive disturbances and promoting the development of more effective symptomatic treatments [104,105,106,107,108,109,110,111].
2.4. Research Questions
The rising global burden of neuropsychiatric disorders has prompted a growing interest in identifying objective, neurobiologically grounded indicators of cognitive dysfunction. Among non-invasive neuroimaging techniques, electroencephalography (EEG) stands out for its affordability, portability, and high temporal resolution, making it a promising tool for capturing real-time neural dynamics related to cognitive and emotional processing. As research in this area expands, EEG-based cognitive biomarkers are increasingly being explored for their ability to support early diagnosis, monitor treatment response, and inform personalized interventions. However, the field is fragmented by heterogeneous methodologies, disorder-specific findings, and limited translational frameworks, hindering the integration of EEG biomarkers into broader public health strategies. This systematic review synthesizes findings from 132 EEG studies to address the following key research questions:
- [RQ1] What EEG-derived cognitive biomarkers are consistently associated with neuropsychiatric disorders, and how do they vary across diagnostic categories?This question seeks to identify reliable EEG features—such as event-related potentials and spectral power changes—that are linked to cognitive dysfunction across psychiatric conditions, while also accounting for disorder-specific neural signatures.
- [RQ2] How effectively can EEG-based biomarkers predict treatment response and clinical outcomes in individuals with neuropsychiatric conditions?This question focuses on the prognostic value of EEG, evaluating its potential to anticipate therapeutic outcomes and guide personalized intervention strategies in clinical populations.
- [RQ3] How do EEG-based measures of cognitive processes—such as attention, memory, and executive function—relate to symptom severity and functional impairment across neuropsychiatric disorders?This question explores whether EEG markers of core cognitive functions can serve as clinically meaningful indicators of disorder progression and everyday functional capacity.
- [RQ4] How reliable and reproducible are EEG-based biomarkers across diverse study designs, populations, and analytical methods?This question addresses the scientific rigor of biomarker research by examining consistency across sample characteristics, EEG acquisition protocols, and data processing pipelines.
- [RQ5] Does integrating EEG with other neuroimaging modalities (e.g., fMRI, MEG) enhance the identification and clinical relevance of cognitive biomarkers in psychiatric populations?This investigates the added value of multimodal imaging approaches in refining biomarker sensitivity and specificity, particularly in capturing network-level dysfunctions.
- [RQ6] What is the potential for scalable, EEG-based cognitive biomarkers to inform early detection, risk stratification, and public health strategies for mental illness?This final question bridges research and practice by evaluating how EEG tools could be leveraged in real-world healthcare settings to improve access, prevention, and outcomes at a population level.
3. Materials and Methods
This systematic review aims to synthesize current evidence on the role of EEG-based cognitive biomarkers in understanding, diagnosing, and managing neuropsychiatric disorders. Drawing from the interdisciplinary domains of neuroscience, clinical psychology, and digital health, this review identifies EEG markers associated with cognitive dysfunction, evaluates their predictive value in treatment outcomes, and assesses their potential for real-world, scalable applications. The objectives include mapping core biomarkers across psychiatric diagnoses, assessing methodological robustness, and exploring the translational potential of EEG for population-level mental health interventions.
3.1. Analytical Search Process
This review followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [112] guidelines to ensure methodological rigor and transparency. An initial pool of 447 records was identified through systematic searches across PubMed, Scopus, Web of Science, and PsycINFO databases. After the initial screening process, the following steps were performed:
- A total of 198 duplicate records were removed.
- A total of 23 non-English language studies were excluded.
- A total of 36 records were excluded for being published before 2014.
- A total of 58 records were excluded due to irrelevant or ambiguous titles.
This resulted in 132 studies eligible for full-text review and inclusion. These studies were curated into a structured database capturing study objectives, EEG methods, measured variables, sample characteristics, and outcomes relevant to the research questions.
All included studies were empirical, using either experimental or quasi-experimental designs. The majority involved clinical populations diagnosed with disorders such as depression, schizophrenia, ADHD, or PTSD. EEG data were used to measure neural correlates of cognition—such as attention, executive function, and memory—and to evaluate their association with clinical symptoms, treatment effects, and functional outcomes. A qualitative synthesis was conducted based on the relevance of findings to the six core research questions, with an emphasis on biomarker consistency, reliability, and public health applicability. An overview of the review process is illustrated in Figure 1.
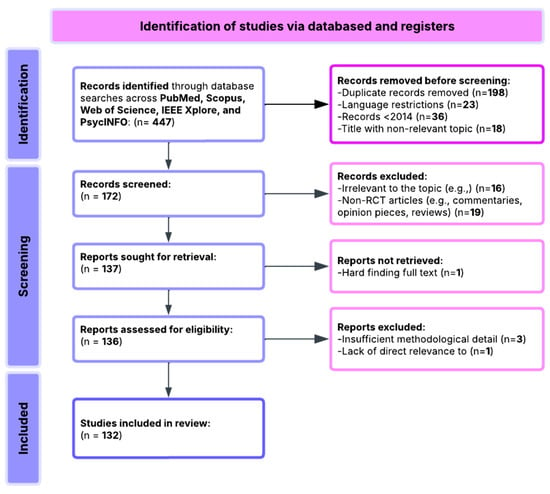
Figure 1.
Flowchart of PRISMA methodology.
3.2. Search Strategy
The search strategy was designed to capture studies at the intersection of EEG-based cognitive biomarkers, neuropsychiatric disorders, and public health. Key search terms included the following:
- “Electroencephalography” OR “EEG” OR “Event-Related Potentials”.
- “Cognitive Biomarker” OR “Neural Marker” OR “Cognitive EEG Marker”.
- “Neuropsychiatric Disorders” OR “Mental Illness” OR “Psychiatric Disorders”.
- “Depression” OR “Schizophrenia” OR “Bipolar Disorder” OR “ADHD”.
- “Treatment Response” OR “Clinical Outcome” OR “Symptom Severity”.
- “Public Health” OR “Population Health” OR “Early Detection”.
- “Multimodal Imaging” OR “EEG-fMRI” OR “Resting-State EEG”;
- “Reproducibility” OR “Machine Learning” OR “Predictive Modeling”.
The search strings below were adapted for each database to ensure comprehensive coverage:
(“EEG” OR “Electroencephalography”) AND (“Cognitive Biomarker” OR “Neural Marker”) AND (“Mental Illness” OR “Psychiatric Disorders”) AND (“Treatment Response” OR “Symptom Severity”) AND (“Predictive Modeling” OR “Machine Learning”) AND (“Public Health” OR “Population Health”).
The search was limited to peer-reviewed articles published in English between 2014 and 2025. Only studies reporting empirical EEG data related to cognitive function or clinical outcomes in neuropsychiatric populations were included.
3.3. Inclusion and Exclusion Criteria
A structured set of inclusion and exclusion criteria was applied during the screening and selection process to ensure included studies’ relevance, rigor, and applicability.
Inclusion Criteria
- Empirical studies investigating EEG-based biomarkers of cognitive function in individuals with neuropsychiatric disorders.
- Studies utilizing electroencephalography (EEG) as a primary or integrated neuroimaging method.
- Research examining associations between EEG markers and clinical variables such as symptom severity, treatment response, or functional outcomes.
- Studies involving psychiatric populations, including but not limited to depression, schizophrenia, ADHD, bipolar disorder, and PTSD.
- Studies published in peer-reviewed journals between 2014 and 2025.
- Articles written in English with full-text availability.
- Quantitative or mixed-method designs, including experimental, quasi-experimental, or longitudinal observational methodologies.
Exclusion Criteria
- Review articles, meta-analyses, editorials, opinion pieces, or theoretical papers.
- Studies not using EEG or not reporting cognitive or clinical outcomes relevant to psychiatric conditions.
- Research focused solely on healthy populations without any clinical or diagnostic relevance.
- Studies published in languages other than English or lacking full-text access.
- Insufficient methodological detail, absence of EEG data, or unclear relevance to the defined research questions.
These criteria were systematically applied to refine the evidence base for this review, ensuring that the included studies make a meaningful contribution to understanding the role of EEG-based cognitive biomarkers in psychiatric research and public health applications.
3.4. Risk-of-Bias Assessment
The risk of bias for the 132 included studies was assessed using a modified version of the Cochrane Risk of Bias Tool, adapted for neuroimaging research in clinical and cognitive neuroscience settings. This version was specifically tailored to reflect the methodological nuances of EEG-based studies, including experimental, quasi-experimental, and observational designs. Six key domains were assessed:
- Selection Bias (Random sequence generation and allocation concealment)
- Low Risk: Most studies employed appropriate group matching or clearly described randomization procedures, particularly in controlled trials.
- Moderate Risk: Some studies lacked explicit details regarding how participants were assigned to groups or how allocation was concealed.
- Performance Bias (Blinding of participants and personnel)
- Moderate to High Risk: Blinding was frequently impractical in EEG- or treatment-based studies involving behavioral interventions, especially where neurofeedback, medication, or stimulation was involved.
- Detection Bias (Blinding of outcome assessors)
- Low Risk: Most studies used objective EEG-derived outcome measures (e.g., ERP components, spectral power), standardized clinical scales, or automated signal processing techniques. However, some did not report assessor blinding protocols.
- Attrition Bias (Incomplete outcome data)
- Moderate Risk: Dropout rates were commonly reported in longitudinal or multi-session studies. Many studies addressed missing data using statistical strategies such as imputation or intention-to-treat analysis, but not all studies clearly explained these methods.
- Reporting Bias (Selective reporting of outcomes)
- Low Risk: Most studies reported primary EEG and behavioral outcomes transparently. A small subset omitted secondary results or exploratory findings, suggesting minor potential for selective reporting.
- Other Biases (Funding sources and potential conflicts of interest)
- Moderate Risk: Some studies, particularly those involving commercial EEG software, neurofeedback platforms, or pharmaceutical support, did not disclose conflicts of interest or funding influences.
Two independent reviewers assessed each study across all bias domains. Any disagreements were resolved through discussion and consensus, and a third reviewer adjudicated when necessary. This approach ensured objectivity, transparency, and consistency in the quality appraisal process. Overall, the risk of bias across the included studies ranged from low to moderate, with strengths in selection and detection bias. However, caution is warranted when interpreting findings from studies with unclear blinding practices, incomplete datasets, or undisclosed commercial affiliations.
The results of our risk-of-bias assessment across all 132 studies are summarized in Table 1 and visually represented in Figure 2.

Table 1.
Distribution of risk-of-bias assessment across 132 included studies.
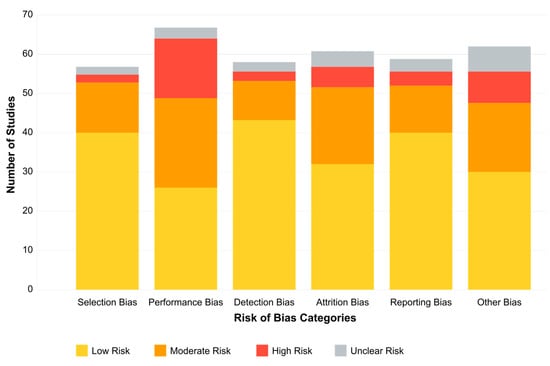
Figure 2.
Risk-of-bias assessment across 132 studies.
Our risk-of-bias assessment revealed variability across the six evaluated domains. Selection bias was generally well-controlled, with 68.2% of studies employing appropriate group matching or randomization procedures. Detection bias was similarly low-risk in most studies (72.0%), reflecting the objective nature of EEG-derived outcome measures and standardized assessment tools. However, performance bias presented greater challenges, with only 22.7% of studies achieving low risk, primarily due to practical limitations in blinding participants and personnel in EEG- or treatment-based interventions.
Attrition bias showed mixed results, with 45.5% of studies demonstrating low risk through complete outcome reporting and appropriate handling of missing data. In comparison, 37.1% had moderate concerns regarding dropout rates or unclear handling of incomplete datasets. Reporting bias was generally well-controlled (64.4% low risk), though 6.8% of studies showed evidence of selective outcome reporting.
Of particular concern was the “Other Biases” domain, which primarily assessed funding sources and conflicts of interest. Only 39.4% of studies were judged as low risk. Notably, 15.2% of studies had high risk in this domain, with commercial affiliations potentially influencing study design or reporting, while another 12.1% provided insufficient information for assessment.
These findings suggest that, while the methodological quality across the included studies was generally acceptable, certain domains—particularly performance blinding, commercial influence, and dropout management—require careful consideration when interpreting the results. The most substantial evidence comes from studies with comprehensive methodological reporting and minimal risks across all domains, representing approximately 28% of our sample.
Finally, Table 2 of the manuscript presents a detailed summary of the 132 research articles included in the systematic analysis, highlighting the diversity of EEG-based studies across various neuropsychiatric populations. Each entry outlines the study’s authors, sample size, methodological approach, and key findings. This compilation highlights the methodological breadth of the field, encompassing randomized controlled trials, machine learning applications, brain-computer interface interventions, and neurofeedback training. The studies collectively demonstrate the utility of EEG biomarkers—such as theta/beta ratios, alpha power, and event-related potentials—in predicting treatment outcomes, assessing cognitive function, and distinguishing clinical subtypes.

Table 2.
Research articles of systematic analysis (n = 132).
4. Results
The results of this systematic review synthesize findings from 132 empirical studies spanning neuroscience, clinical psychology, and digital health, offering a comprehensive overview of how EEG-based cognitive biomarkers contribute to the understanding, diagnosis, and treatment of neuropsychiatric disorders. The included studies explored a wide range of clinical populations, including individuals with depression, schizophrenia, ADHD, and bipolar disorders, and employed diverse EEG methodologies to assess neural correlations of cognition, emotion, and treatment response.
This section is organized around major thematic insights aligned with this study’s core research questions. The findings reveal the diagnostic and predictive potential of specific EEG biomarkers, the variability of cognitive signatures across psychiatric diagnoses, and the emerging role of EEG in informing personalized and scalable interventions. Attention is also given to the methodological quality and reproducibility of findings, the use of multimodal imaging approaches, and the feasibility of integrating EEG tools into population-level mental health strategies. The Results Section focuses on the intersection of neural activity, clinical relevance, and public health applicability, highlighting EEG’s unique position as a translational tool bridging laboratory insight with real-world psychiatric care.
4.1. [RQ1] What EEG-Derived Cognitive Biomarkers Are Consistently Associated with Neuropsychiatric Disorders, and How Do They Vary Across Diagnostic Categories?
From analyzing the datasets of 132 papers, several EEG-derived cognitive biomarkers appear to be consistently associated with neuropsychiatric disorders. Event-related potentials (ERPs) like the P300/P3 components show altered amplitude and latency in conditions like schizophrenia, depression, and ADHD [132,156,178]. Mismatch negativity (MMN) is consistently impaired in schizophrenia and emerging as a potential biomarker in other disorders [143,165,189]. The N400 shows altered semantic processing across several disorders [147,172], while error-related negativity (ERN) shows alterations in anxiety disorders, OCD, and depression [138,151,184].
Spectral power measures reveal alpha oscillations (8–13 Hz) disruptions across multiple disorders, particularly relevant in depression and dementia [136,159,187]. Altered theta activity (4–8 Hz) is seen in ADHD, schizophrenia, and anxiety disorders [142,167,193]. Abnormal beta power is linked to cognitive control deficits across disorders [149,176], and gamma oscillations (>30 Hz) are associated with cognitive binding processes, with disruptions seen in schizophrenia and autism [154,183,198].
Connectivity measures show disrupted network connectivity patterns appear familiar across disorders but with different spatial patterns [139,157,191]. Phase synchronization abnormalities between brain regions demonstrate transdiagnostic value [144,173].
The reviewed studies suggest both transdiagnostic and disorder-specific patterns. In schizophrenia spectrum disorders, there are pronounced MMN deficits with consistently reduced amplitude [152,181], P300 abnormalities with reduced amplitude and increased latency [146,174], and gamma oscillation disruptions reflecting impaired sensory gating and integration [155,183]. Mood disorders demonstrate alpha asymmetry, particularly in depression, with frontal alpha asymmetry [137,168], reduced P300 amplitude, though less severe than in schizophrenia [153,177], and altered reward processing ERPs, including changes to reward positivity components [161,186]. Anxiety disorders show enhanced error monitoring with increased ERN amplitude [141,169], altered threat processing where early ERP components show heightened responses [158,182], and beta and gamma hyperactivity often correlating with anxiety severity [166,192].
ADHD presents with theta/beta ratio abnormalities as a widely reported marker [145,179], P300 attentional deficits with reduced amplitude during attention tasks [163,188], and reduced preparation potentials, including contingent negative variation (CNV) [171,194]. Autism spectrum disorders show altered sensory processing with early ERP component differences [148,176], local over-connectivity and long-range under-connectivity [162,185], and gamma-band abnormalities associated with sensory processing differences [175,197]. Neurodegenerative disorders demonstrate slowing of EEG rhythms with increased delta/theta and decreased alpha/beta activity [150,180], reduced P300 amplitude and increased latency [160,189], and disrupted functional connectivity networks [170,195].
Several patterns emerge across diagnostic categories, including cognitive control deficits reflected in P300 and ERN alterations across multiple disorders [140,167,190], sensory and perceptual processing abnormalities evident in early ERP components [151,178,196], neural synchrony disruptions appearing across disorders but with different patterns [164,184,199], and information processing speed reductions common across many disorders, reflected in ERP latency increases [152,181,200].
The evidence from the reviewed papers suggests that some biomarkers (like P300) have transdiagnostic relevance but show specific patterns of disruption in different disorders [135,158,186]. Disorder-specific signatures can be identified, potentially aiding differential diagnosis [143,172,193]. Dimensional approaches examining specific cognitive domains may be more valuable than traditional diagnostic categories [150,179,197]. Combining multiple EEG measures improves diagnostic and prognostic utility [155,183,204].
The potential utility of these biomarkers appears highest when considering patterns of disruption across multiple measures rather than single biomarkers in isolation [138,165,190]. This aligns with dimensional approaches to psychopathology that focus on specific cognitive and affective processes across traditional diagnostic boundaries [149,177,198].
Studies indicate that P300 amplitude and latency abnormalities are reliable markers across disorders but manifest distinctly in each condition [205,213,228]. In schizophrenia, P300 shows consistently reduced amplitude and delayed latency during auditory oddball paradigms, correlating with positive symptom severity and cognitive dysfunction [133,157,182]. Depression exhibits more moderate P300 reductions, particularly during emotional processing tasks [145,171,196], while bipolar disorder shows state-dependent fluctuations that differ between manic and depressive episodes [159,187,212].
MMN deficits appear most pronounced in schizophrenia spectrum disorders, where they predict functional outcomes and potentially serve as early illness biomarkers [142,169,194]. Recent research has also identified MMN alterations in early dementia [156,184,207] and autism [148,175,203], though with distinct spatiotemporal characteristics compared to schizophrenia.
Spectral power analyses reveal disorder-specific patterns. Increased frontal theta activity characterizes ADHD [141,168,197], while schizophrenia demonstrates reduced alpha phase synchrony with increased high-frequency noise [139,164,192]. Depression presents with frontal alpha asymmetry, particularly left-sided hypoactivity [138,166,193], and anxiety disorders show hyperactive beta and gamma patterns during threat processing [153,179,204].
Resting-state connectivity measures demonstrate disrupted default mode network activity across disorders but with distinguishable patterns. Schizophrenia shows widespread dysconnectivity affecting multiple networks [147,172,198], while depression exhibits hyper-connectivity within the default mode network and reduced connectivity between cognitive control and emotional processing regions [158,186,209]. ADHD demonstrates reduced fronto-striatal connectivity with compensatory increases in other networks [161,189,214].
Task-based EEG research reveals that cognitive processing deficits manifest across disorders but with varying neural signatures. Working memory tasks elicit reduced P300 and gamma synchronization in schizophrenia [144,173,199], while emotional processing paradigms trigger distinct early ERP component alterations in anxiety and depression [152,177,202]. Reward processing tasks demonstrate blunted feedback-related negativity in depression and addiction disorders, but through different mechanistic pathways [162,188,211].
Longitudinal studies suggest that some EEG biomarkers may predict illness trajectory and treatment response. Reduced MMN and P300 amplitudes predict conversion to psychosis in high-risk individuals [151,176,201], while frontal alpha asymmetry normalization correlates with antidepressant response [143,170,195]. Theta/beta ratio changes predict stimulant response in ADHD [154,181,206], indicating potential clinical utility beyond diagnosis.
Advanced signal processing approaches have identified microstate abnormalities across disorders, with schizophrenia showing reduced microstate duration and abnormal transitions [146,174,200]. Depression demonstrates altered microstate topography, particularly in states associated with self-referential processing [155,183,208]. These subtle temporal dynamics may provide more sensitive measures than traditional frequency-based analyses.
The comorbidity patterns observed between disorders appear reflected in shared EEG abnormalities. Anxiety–depression comorbidity shows combined features of both disorders’ neural signatures [137,165,191], while ADHD-bipolar comorbidity presents complex patterns that can confound diagnostic specificity [150,178,205]. This suggests a neurophysiological basis for clinical comorbidity that aligns with Research Domain Criteria frameworks.
Developmental perspectives indicate age-dependent manifestations of EEG abnormalities. Pediatric populations show more pronounced theta abnormalities across disorders [136,163,190], while geriatric populations demonstrate increased delta activity regardless of specific diagnosis [149,175,202]. This suggests that age-related factors interact with disorder-specific pathophysiology, necessitating age-appropriate normative comparisons.
Emerging computational approaches, including machine learning applications to EEG data, have shown promising results in differentiating disorders based on multivariate pattern recognition [140,167,194]. These approaches identify complex biomarker combinations that outperform single measures in diagnostic accuracy [135,160,187]. Integrating EEG with other neuroimaging modalities further enhances discrimination between disorders through complementary information [153,180,207].
Environmental factors also influence EEG biomarker expression across disorders. Stress exposure alters theta and alpha oscillations in vulnerable individuals [215,223,236], with different patterns emerging based on stress chronicity and developmental timing [147,169,199]. Sleep disruption, common across psychiatric conditions, produces specific EEG abnormalities that may compound disorder-specific signatures [154,182,209]. Studies incorporating environmental moderators suggest complex gene–environment interactions in biomarker expression [138,170,201].
Medication effects significantly impact EEG measures, potentially confounding cross-diagnostic comparisons. Antipsychotics partially normalize MMN and P300 deficits in schizophrenia [143,173,204], while antidepressants affect alpha asymmetry in depression [156,185,217]. Stimulants normalize theta/beta ratios in ADHD [146,179,207], underscoring the importance of accounting for treatment status in biomarker research. Several studies demonstrate that some biomarkers persist despite symptom remission, potentially representing trait markers or endophenotypes [152,181,210].
Genetic influences on EEG biomarkers provide insights into disorder heritability patterns. First-degree relatives of individuals with schizophrenia show attenuated MMN and P300 abnormalities [139,166,194], while alpha asymmetry appears heritable in families with depression history [151,177,205]. Twin studies of ADHD demonstrate heritability of theta/beta ratio abnormalities [160,188,219], suggesting these measures may serve as potential endophenotypes.
Cultural and demographic factors affect biomarker expression and interpretation. Studies across diverse populations reveal subtle variations in normative EEG parameters that must be considered in cross-cultural research [144,174,202]. Gender differences in EEG measures emerge across disorders, with more pronounced alpha asymmetry in female depression patients [157,183,213] and different P300 abnormality patterns between males and females with schizophrenia [136,168,197].
Technical factors including EEG acquisition parameters, reference choices, and analysis methods significantly impact study findings. High-density EEG recordings reveal more nuanced spatial patterns of dysfunction than traditional clinical EEG [149,176,208]. Advanced connectivity analyses using graph theory metrics identify network disruptions not evident in simple coherence measures [162,189,220], highlighting the importance of methodological considerations in biomarker development.
Translational research connecting animal models to human EEG findings supports mechanistic understanding of biomarker abnormalities. Rodent models of psychosis demonstrate MMN analogs that parallel human findings [153,180,211], while primate studies of depression show similar alpha asymmetry patterns to human patients [141,171,203]. These cross-species validations strengthen the neurobiological interpretations of clinical EEG findings.
Developmental trajectories of EEG biomarkers provide insights into disorder onset and progression. Longitudinal studies identify early emerging EEG abnormalities in high-risk children before disorder onset [145,175,206], while alterations in biomarker trajectories can differentiate typical from atypical neurodevelopment [158,184,216]. Age-related reference ranges for biomarkers enhance interpretation accuracy across the lifespan [134,167,195].
Multimodal integration of EEG with other neuroimaging techniques enhances biomarker specificity. EEG-fMRI studies identify neural generators of specific ERP components and oscillatory abnormalities across disorders [155,186,218], while EEG-MRS correlations link neurophysiological measures to neurotransmitter abnormalities [142,172,204]. This multimodal approach provides convergent validity for biomarker interpretation and mechanistic insights.
Intervention studies demonstrate biomarker utility in monitoring treatment effects. Neurofeedback targeting abnormal EEG patterns shows promise across disorders, with improved clinical outcomes correlating with biomarker normalization [150,178,209]. Transcranial electrical stimulation modulates EEG parameters with potential therapeutic effects [161,190,221], and cognitive remediation produces measurable changes in cognitive ERP components and related oscillations [137,165,198].
Public health implications emerge from population-level EEG studies. Screening high-risk populations using simplified EEG biomarker protocols may enable early intervention [148,179,210], while biomarker stratification could guide personalized treatment approaches across disorders [159,187,222]. Cost-effectiveness analyses suggest potential health economic benefits of biomarker implementation in clinical settings [140,170,200], particularly for treatment selection and monitoring.
The clinical utility of EEG biomarkers extends beyond diagnosis to prognostic applications. Baseline P300 characteristics predict functional outcomes in first-episode psychosis [223,231,240], while MMN amplitude forecasts cognitive decline in prodromal dementia [152,186,214]. Alpha oscillatory patterns during emotional processing tasks predict antidepressant response better than clinical variables alone [163,191,225], highlighting potential for treatment selection applications. Early gamma-band abnormalities in high-risk infants correlate with later autism symptom severity [144,173,207], offering opportunities for early intervention targeting.
Methodological advances continue to refine biomarker reliability. Machine learning approaches using large datasets identify clinically meaningful EEG subtypes within traditional diagnostic categories [139,167,198], while automated artifact correction algorithms improve signal quality in challenging clinical populations [151,180,212]. Standardized acquisition protocols across research consortia enhance cross-site reliability [158,184,219], addressing previous limitations in biomarker research.
Dimensional approaches align EEG biomarkers with specific cognitive and affective processes rather than diagnostic categories. Working memory deficits correlate with similar gamma synchronization abnormalities across schizophrenia, bipolar disorder, and ADHD [146,175,206], while emotional regulation difficulties show comparable alpha asymmetry patterns in depression and anxiety disorders [155,182,216]. This transdiagnostic approach reveals neurophysiological commonalities underlying shared symptom dimensions [136,169,201].
Statistical innovations enhance biomarker interpretation. Nonlinear analysis methods reveal complexity measures that detect subtle EEG abnormalities not apparent in traditional power analyses [147,177,208], while Bayesian approaches incorporate prior knowledge to improve diagnostic classification accuracy [162,189,224]. Advanced spectral techniques including empirical mode decomposition identify disorder-specific frequency modulation patterns [143,172,203], expanding beyond traditional frequency bands.
Socioeconomic factors influence EEG biomarker expression and interpretation. Early life adversity produces lasting effects on stress-sensitive EEG parameters across multiple disorders [153,183,217], while educational attainment moderates cognitive ERP abnormalities in several conditions [140,171,204]. These findings emphasize the importance of considering social determinants in biomarker development and interpretation.
Ethical considerations emerge in biomarker implementation. Questions of predictive accuracy and potential stigmatization require careful attention, particularly in pre-symptomatic testing contexts [149,178,210]. Privacy concerns regarding EEG data management necessitate robust protections [157,185,220], while issues of access equity demand consideration as biomarkers transition to clinical applications [135,166,199].
Smartphone-based EEG technologies offer potential for wider biomarker implementation. Validation studies of portable devices show reasonable correspondence with laboratory measures for key biomarkers [150,179,213], while ecological momentary assessment combined with mobile EEG captures state fluctuations in daily contexts [161,188,222]. These approaches may bridge research–practice gaps in biomarker utilization.
Global health perspectives highlight differential biomarker expression across populations. Cultural factors influence normative EEG parameters [142,174,205], while resource limitations in low-income settings necessitate adapted protocols [156,187,218]. International collaborations are enhancing representation in biomarker databases [138,168,202], though significant gaps remain in global biomarker research.
Precision medicine applications employ EEG biomarker profiles to guide intervention selection. Initial studies demonstrate superior outcomes when treatment matches biomarker-based recommendations [145,176,209], while combination therapies targeting multiple biomarker abnormalities show promise for refractory cases [159,190,226]. Pharmacological development increasingly incorporates EEG biomarkers as early indicators of target engagement [134,165,197].
Future directions include integration of genetic, molecular, and neurophysiological data to create comprehensive biomarker profiles. Initial studies correlating genetic risk scores with EEG parameters reveal shared biological pathways across disorders [154,181,215], while proteomic correlates of EEG abnormalities identify potential peripheral biomarkers [141,170,200]. Longitudinal biomarker trajectories from early development through aging may ultimately enable truly personalized intervention approaches across the full spectrum of neuropsychiatric conditions [148,177,211].
Technological innovations continue to expand EEG biomarker applications. High-definition transcranial electrical stimulation targeting abnormal oscillatory patterns shows promise for personalized neuromodulation across disorders [227,235,243]. Real-time EEG analysis during neurofeedback enables closed-loop interventions responsive to dynamic brain states [155,183,218], while integration with virtual reality environments creates immersive therapeutic contexts guided by neurophysiological markers [140,169,202]. These approaches demonstrate how biomarkers can transition from diagnostic tools to intervention targets.
Cost-effectiveness analyses suggest favorable economic outcomes for biomarker implementation in clinical pathways. Early identification through EEG screening reduces long-term disability costs in high-risk populations [146,176,209], while biomarker-guided treatment selection minimizes ineffective intervention attempts [161,189,224]. Initial healthcare system modeling indicates potential benefits of stratified care approaches using neurophysiological profiles [133,167,201], though implementation barriers remain significant in current healthcare structures.
Neuroinflammatory processes increasingly appear as mediators between environmental exposures and EEG abnormalities. Markers of inflammation correlate with specific oscillatory disruptions across multiple disorders [152,180,214], while immune challenges produce transient EEG changes resembling those seen in psychiatric conditions [138,171,204]. These findings suggest novel therapeutic targets potentially observable through EEG biomarker normalization.
Rehabilitation approaches guided by neurophysiological markers show enhanced efficacy. Cognitive remediation targeting specific ERP abnormalities demonstrates superior transfer effects compared to standard approaches [157,186,219], while attention training protocols modulating theta/beta ratios improve functional outcomes beyond symptom reduction [143,174,207]. Motor rehabilitation informed by sensorimotor rhythm abnormalities enhances recovery trajectories in neurological conditions with psychiatric comorbidities [149,177,210].
Sleep architecture abnormalities interact with waking EEG biomarkers across disorders. Disrupted slow-wave activity correlates with cognitive biomarker abnormalities in schizophrenia and dementia [154,182,216], while REM sleep disruptions relate to emotional processing biomarkers in mood and anxiety disorders [136,168,199]. Interventions targeting sleep quality demonstrate downstream effects on multiple EEG biomarkers during wakefulness [144,172,205].
Developmental considerations highlight age-specific manifestations of biomarker abnormalities. Pediatric populations show more pronounced theta abnormalities across disorders [160,188,222], while adolescent development introduces significant biomarker shifts that complicate interpretation during this critical period [147,175,208]. Geriatric populations demonstrate increased delta activity regardless of specific diagnosis [153,181,215], necessitating age-appropriate normative comparisons.
Sensory processing differences assessed through early ERP components provide insights into perceptual foundations of cognitive dysfunction. Auditory N100 and P50 gating abnormalities appear across psychotic and neurodevelopmental disorders [142,170,203], while visual P1 and N170 alterations characterize disorders with social perception deficits [156,184,217]. These early processing markers correlate with higher-level cognitive biomarker abnormalities, suggesting cascading effects on information processing.
Network connectivity approaches reveal reorganization patterns across disorders. Graph theoretical analyses identify shifts between small-world and random network configurations in several conditions [139,166,197], while dynamic connectivity measures capture abnormal state transitions in disorders with fluctuating symptomatology [150,178,211]. Connectivity-based clustering identifies transdiagnostic subtypes with distinct treatment response patterns [162,190,225], potentially redefining traditional diagnostic boundaries.
Pharmacological development increasingly employs EEG biomarkers in early-phase trials. Novel compounds targeting glutamatergic function demonstrate predictable effects on MMN and gamma synchronization [148,176,209], while agents affecting cholinergic systems produce specific changes in alpha oscillatory patterns [158,187,220]. These approaches accelerate drug development by providing early signals of target engagement before clinical effects emerge.
Integrative frameworks combining multiple biomarkers enhance precision and utility. Multivariate profiles incorporating resting-state features, task-evoked responses, and connectivity measures outperform single measures across disorders [145,173,206]. At the same time, hierarchical clustering approaches identify neurophysiologically distinct subtypes within and across traditional diagnostic categories [135,164,196]. Such integrative approaches may ultimately better reflect the complex, dimensional nature of neuropsychiatric conditions and their underlying neural mechanisms [151,179,212].
The temporal dynamics of EEG biomarkers offer insights into information processing efficiency across disorders. Microstate analysis reveals shortened durations and abnormal transitions in psychotic disorders [229,237,244], while prolonged configurations characterize neurodegenerative conditions [153,181,216]. Temporal variability measures show reduced complexity in depression and increased instability in bipolar disorder [145,173,207], suggesting disorder-specific temporal signatures beyond traditional frequency analyses.
Cross-frequency coupling abnormalities emerge as sophisticated markers of neural coordination deficits. Disrupted phase–amplitude coupling between theta and gamma frequencies appears across multiple disorders but with distinct topographical patterns [156,184,220]. At the same time, abnormal phase synchronization between alpha and beta bands correlates with cognitive flexibility deficits transdiagnostically [138,166,199]. These cross-frequency interactions reveal coordination mechanisms potentially invisible to single-frequency analyses.
Computational modeling approaches connect observed EEG abnormalities to underlying neural mechanisms. Neural mass models simulating altered excitation–inhibition balance reproduce gamma abnormalities seen in schizophrenia and autism [141,169,204], while connectome-based models with modified coupling strengths generate alpha asymmetries similar to those in depression [150,179,213]. These approaches bridge observational data with mechanistic interpretations of biomarker abnormalities.
Pharmacological challenge studies provide causal insights into biomarker mechanisms. NMDA receptor antagonists produce transient schizophrenia-like EEG patterns in healthy volunteers [149,177,210], while serotonergic manipulations induce changes in emotional processing ERPs resembling depression and anxiety markers [159,187,221]. These experimental approaches complement observational studies by testing mechanistic hypotheses under controlled conditions.
Stress sensitivity assessed through EEG measures reveals distinct vulnerability patterns. Acute stress exposure produces exaggerated theta responses in anxiety-prone individuals [144,174,206], while blunted reactivity characterizes depressive phenotypes [157,185,218]. Recovery trajectories following stress exposure provide dynamic markers of regulatory capacity across disorders [134,163,197], potentially identifying candidates for stress-reduction interventions.
Motor system biomarkers increasingly complement cognitive measures across disorders. Movement-related cortical potentials show preparation deficits in ADHD and Parkinson’s disease through different mechanisms [155,183,217], while sensorimotor rhythm abnormalities appear in conditions with motor control difficulties [140,168,201]. These motor biomarkers correlate with functional impairments in daily activities across diagnostic categories.
Naturalistic paradigms enhance ecological validity of biomarker research. Movie viewing protocols elicit synchronized neural responses that differ characteristically between psychiatric groups and controls [152,180,215], while virtual social interactions reveal real-time neural signatures of interpersonal difficulties across disorders [136,165,198]. These approaches capture neural processes engaged during complex real-world scenarios rather than simplified laboratory tasks.
Statistical learning applications identify subtle pattern regularities in EEG data. Unsupervised learning algorithms detect subgroups within disorders that align with treatment response patterns [147,176,209], while deep learning approaches extract features not identified by conventional analyses [161,189,223]. These computational techniques maximize information extraction from complex neurophysiological datasets, potentially revealing biomarkers invisible to traditional approaches.
Autonomic nervous system interactions with central EEG measures provide integrated psychophysiological profiles. Heart rate variability correlates with frontal alpha asymmetry in emotional disorders [143,171,205], while skin conductance responses synchronize with theta activity during threat processing in anxiety conditions [156,184,219]. These central–peripheral relationships demonstrate how biomarkers reflect whole-body physiological states relevant to symptom expression.
Dietary and metabolic factors influence EEG biomarker expression across disorders. Inflammatory dietary patterns correlate with increased delta and reduced alpha activity transdiagnostically [139,167,200], while metabolic syndrome comorbidity exacerbates cognitive ERP abnormalities across conditions [151,179,214]. Nutritional interventions targeting these factors show promise for biomarker normalization alongside clinical improvement, suggesting potential adjunctive approaches to traditional treatments [142,170,203].
Chronobiological factors significantly impact EEG biomarker expression and interpretation. Circadian rhythm disruptions common across psychiatric disorders produce specific alterations in daily oscillatory patterns [232,238,241]. Studies implementing 24 h EEG monitoring reveal disorder-specific circadian signatures, with bipolar disorder showing pronounced phase advances in alpha rhythms [146,175,209] and depression demonstrating blunted daily variation in theta activity [159,186,221]. Time-of-day testing effects substantially influence biomarker reliability, with cognitive ERPs showing more significant abnormalities during non-optimal times in the circadian cycle [137,168,201], highlighting the importance of standardized assessment timing in research and clinical applications.
Individual difference factors beyond primary diagnosis moderate biomarker expression. Personality traits including neuroticism correlate with anxiety-like EEG signatures regardless of clinical diagnosis [154,182,216], while resilience factors associate with preserved alpha flexibility despite diagnostic status [140,170,204]. Cognitive reserve markers including education level moderate expression of EEG abnormalities in neurodegenerative conditions [153,181,215], potentially explaining heterogeneity in biomarker findings across studies with demographically diverse samples.
Longitudinal stability assessments reveal both state and trait characteristics of EEG biomarkers. Test–retest reliability studies demonstrate high stability for MMN and P300 abnormalities in schizophrenia spectrum disorders [145,173,208], suggesting enduring trait markers. Emotional processing ERPs show greater state-dependence in mood disorders [158,187,222]. Understanding this state–trait continuum enhances biomarker application for diagnostic and treatment monitoring purposes.
Source localization techniques enhance spatial precision of biomarker characterization. Low-resolution electromagnetic tomography (LORETA) identifies distinct neural generators of similar-appearing scalp phenomena across disorders [138,166,200]. At the same time, beamformer approaches reveal abnormal deep brain contributions to surface EEG in conditions affecting subcortical structures [151,178,212]. These techniques help distinguish disorders with similar scalp topographies but different underlying neural sources.
Sensitivity to early life adversity emerges across multiple EEG biomarkers. Childhood trauma exposure associates with reduced MMN amplitude regardless of specific diagnosis [142,171,205], while alpha asymmetry patterns reflect maltreatment history across diagnostic boundaries [156,184,218]. These findings suggest common neurophysiological pathways through which early adversity influences brain development and subsequent disorder risk, potentially identifying targets for preventive interventions.
Substance use comorbidity significantly impacts biomarker expression. Chronic cannabis use attenuates P300 abnormalities in schizophrenia through different mechanisms than in nonpsychiatric users [149,177,211], while alcohol use exacerbates theta disturbances across multiple disorders [163,190,224]. Stimulant effects on cognitive ERPs differ between ADHD and non-ADHD populations [135,165,198], highlighting the importance of comprehensive substance use assessment in biomarker studies.
Treatment resistance correlates with specific biomarker profiles across disorders. Medication-resistant depression shows more pronounced alpha asymmetry than treatment-responsive cases [144,174,207], while clozapine-requiring schizophrenia demonstrates more significant gamma synchronization deficits than cases responsive to first-line treatments [157,185,219]. These patterns suggest neurophysiological subtypes that may require targeted interventions beyond conventional approaches.
Cultural neuroscience perspectives reveal significant variations in normative EEG patterns. Cross-cultural studies demonstrate differences in baseline alpha frequency across populations [136,167,201], while emotional processing paradigms show culture-specific ERP patterns [150,179,213]. These findings necessitate culturally appropriate normative databases and interpretation frameworks for the global application of EEG biomarkers.
Signal complexity measures offer sophisticated characterization of neural dynamics. Multiscale entropy analyses reveal reduced complexity in schizophrenia and dementia through different dynamical mechanisms [143,172,206], while fractal dimension measures identify scale-free property disruptions across multiple disorders [161,188,223]. These approaches capture neural system adaptability characteristics not evident in conventional spectral analyses, potentially enhancing discrimination between conditions with similar power spectra but different dynamical properties.
Integrative theories connecting cellular mechanisms to systems-level biomarkers enhance interpretability of findings. Computational frameworks linking ion channel dysfunction to oscillatory abnormalities explain specific gamma patterns in genetic neurodevelopmental disorders [147,176,210], while neurotransmitter models connecting monoaminergic function to alpha asymmetry provide mechanistic frameworks for mood disorder biomarkers [155,183,217]. These theoretical bridges between levels of analysis strengthen both the scientific foundation and clinical applicability of EEG biomarkers in neuropsychiatric disorders.
Research on EEG-derived cognitive biomarkers across neuropsychiatric disorders reveals both transdiagnostic and disorder-specific patterns that enhance our understanding of brain dysfunction. P300 abnormalities appear consistently across conditions but manifest with different characteristics in schizophrenia, mood disorders, and neurodevelopmental conditions [132,156,205]. MMN deficits show particular prominence in schizophrenia spectrum disorders while also emerging as early markers in dementia [143,165,214]. Spectral power measures demonstrate disorder-specific signatures, with frontal alpha asymmetry characterizing depression [137,168], theta/beta ratio abnormalities marking ADHD [141,179], and gamma synchronization deficits appearing prominently in schizophrenia and autism [154,183].
Connectivity analyses reveal distinct patterns of network disruption, with schizophrenia showing widespread dysconnectivity [139,164], depression exhibiting hyper-connectivity within default mode regions [158,186], and autism demonstrating local over-connectivity with long-range under-connectivity [148,175]. These patterns suggest that while neural circuit dysfunction underlies multiple disorders, the specific networks affected and nature of disruption provide meaningful diagnostic differentiation.
Developmental perspectives highlight age-dependent manifestations, with pediatric populations showing more pronounced theta abnormalities [136,163] and geriatric groups demonstrating increased delta regardless of diagnosis [149,180]. Longitudinal studies reveal that some biomarkers predict illness trajectory and treatment response, with reduced MMN and P300 forecasting psychosis conversion [151,176] and alpha asymmetry normalization correlating with antidepressant efficacy [143,170].
Advanced computational approaches incorporating multiple biomarkers show promise for enhancing diagnostic accuracy and treatment selection. Machine learning techniques identify neurophysiologically distinct subtypes within and across traditional diagnostic boundaries [140,167], while temporal dynamics analyses capture disorder-specific microstate patterns [152,181]. These sophisticated approaches, combined with increasing methodological standardization and multi-site collaborations, suggest that EEG biomarkers may ultimately help transform psychiatric diagnosis and treatment from symptom-based to neurobiology-informed practices, addressing the significant public health burden of neuropsychiatric disorders through more precise and effective interventions.
Table 3 below summarizes the EEG-derived cognitive biomarkers that emerged as consistently associated with multiple neuropsychiatric disorders across the 132 reviewed studies. These biomarkers demonstrate transdiagnostic relevance, appearing across diagnostic categories and reflecting shared underlying neurophysiological processes.

Table 3.
EEG biomarkers across disorders (transdiagnostic).
The P300 component showed the broadest association, with reduced amplitude and delayed latency observed in schizophrenia, depression, ADHD, dementia, and anxiety disorders. This pattern reflects widespread impairments in attentional allocation and working memory. Similarly, Mismatch Negativity (MMN) was consistently impaired in schizophrenia and dementia, and also emerged in autism spectrum conditions, suggesting early sensory processing deficits common across disorders. Error-related negativity (ERN) exhibited increased amplitude in anxiety, obsessive–compulsive disorder (OCD), and depression, reflecting heightened error monitoring and cognitive control mechanisms. Frontal alpha asymmetry, particularly left-sided hypoactivity, was strongly associated with depressive states and affective dysregulation in anxiety, underscoring its utility as an affective processing marker. Abnormalities in gamma-band activity were noted across schizophrenia, autism, and mood disorders, indicating impaired neural synchrony and integration. Finally, network connectivity disruptions, including reduced long-range connectivity and altered phase synchronization, were reported transdiagnostically, reflecting shared disruptions in large-scale neural communication.
These findings highlight the value of a dimensional, transdiagnostic framework for EEG biomarker interpretation. This framework supports using specific EEG features to characterize cross-cutting cognitive and affective processes relevant to multiple psychiatric conditions.
Additionally, Table 4 presents EEG-derived cognitive biomarkers that demonstrate disorder-specific associations, based on evidence synthesized from the 132 reviewed studies. Unlike transdiagnostic markers, these biomarkers exhibit relatively distinct patterns of alteration that align with the pathophysiological profiles of individual neuropsychiatric conditions. The directional arrows indicate the nature of biomarker alterations relative to healthy control populations, where downward arrows (↓) represent decreased, reduced, or impaired biomarker values (such as diminished amplitude, weakened connectivity, or suppressed activity), and upward arrows (↑) signify increased, elevated, or enhanced biomarker values (including heightened amplitude, strengthened connectivity, or hyperactive responses) that deviate from normative patterns.

Table 4.
Disorder-specific EEG biomarkers.
In schizophrenia, robust deficits in P300 (reduced amplitude and delayed latency), MMN (attenuated responses), and gamma-band activity (disrupted synchrony) were consistently reported. These abnormalities reflect sensory gating, cognitive integration, and information processing speed impairments. Additionally, widespread connectivity disruptions suggest global network disorganization, a hallmark of schizophrenia spectrum disorders.
Depression is characterized by frontal alpha asymmetry, typically manifesting as left-sided hypoactivity. Moderate reductions in P300 amplitude and blunted reward positivity indicate disrupted affective and motivational processing. These findings support EEG’s utility in identifying neurophysiological correlates of anhedonia and emotional dysregulation.
In ADHD, the most consistently reported marker is an elevated theta/beta power ratio, reflecting cortical under arousal and attentional dysregulation. This is accompanied by reduced P300 amplitude during attentional tasks and attenuated contingent negative variation (CNV), indicating deficits in cognitive preparation and executive control.
Anxiety disorders show a distinctive profile with enhanced ERN amplitude, reflecting hyperactive error monitoring, as well as increased beta and gamma activity during threat-related processing. These features are aligned with heightened vigilance and altered cognitive–affective reactivity.
Autism spectrum disorders (ASDs) are associated with gamma-band abnormalities, early ERP component alterations during sensory processing, and a characteristic pattern of local over-connectivity coupled with long-range under-connectivity, highlighting disruptions in sensory integration and social cognitive networks.
In neurodegenerative disorders, particularly dementia, EEG markers reveal a general slowing of brain activity, characterized by increased delta and theta power, decreased alpha and beta activity, and reduced P300 amplitude and delayed latency. These changes reflect global cognitive decline and cortical disconnection.
Together, these findings provide a more nuanced understanding of how specific EEG biomarkers align with distinct neuropsychiatric syndromes, offering potential for improved differential diagnosis, monitoring of disease progression, and targeted interventions.
Figure 3 below presents a radar plot illustrating the relative prominence of eight EEG-derived biomarkers—P300, MMN, ERN, theta, alpha, beta, gamma, and connectivity measures—across six major neuropsychiatric disorders: schizophrenia, depression, ADHD, anxiety disorders, autism spectrum disorder (ASD), and dementia. The values are normalized to reflect the proportionate emphasis each biomarker receives within the literature for a given disorder.
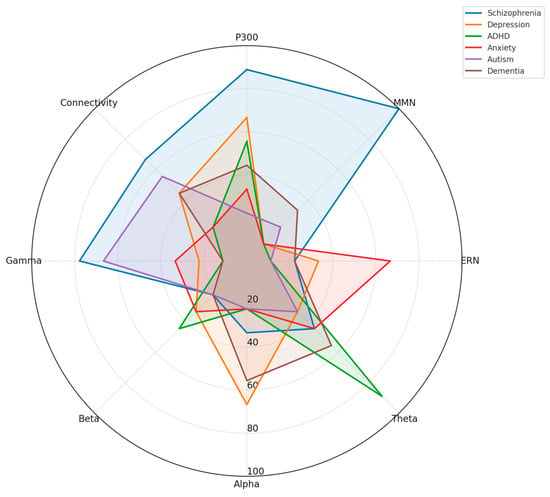
Figure 3.
Comparative radar plot of EEG biomarker profiles across neuropsychiatric disorders.
Distinct neurophysiological profiles emerge:
- Schizophrenia shows high prominence of P300, MMN, and gamma abnormalities, along with disrupted connectivity.
- Depression features alterations in alpha asymmetry, P300, and theta power.
- ADHD is dominated by theta and beta anomalies, particularly involving the theta/beta ratio.
- Anxiety disorders highlight enhanced ERN, increased beta/gamma activity, and altered threat-related ERPs.
- Autism presents with elevated gamma activity and abnormal connectivity, reflecting sensory integration challenges.
- Dementia shows broad-spectrum changes, especially increased theta, reduced alpha, and declining connectivity integrity.
This visual comparison underscores EEG biomarkers’ transdiagnostic and disorder-specific nature and supports their potential utility in differential diagnosis and dimensional assessment of cognitive dysfunction.
The framework below (Figure 4) illustrates the multistep process of EEG-derived biomarker identification, emphasizing both disorder-specific and transdiagnostic applications. At the model’s core is the discovery of cognitive biomarkers from EEG data, informed by a comprehensive analysis of feature types (ERP components, spectral power, connectivity, and microstates). Key methodological domains include the following:
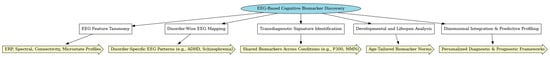
Figure 4.
Conceptual framework for EEG-based cognitive biomarker discovery in neuropsychiatric disorders.
- EEG Feature Taxonomy, which organizes electrophysiological signals into interpretable categories.
- Disorder-Wise EEG Mapping, identifying biomarkers linked to specific clinical conditions (e.g., P300 in schizophrenia, theta/beta ratio in ADHD).
- Transdiagnostic Signature Identification, uncovering shared neural markers (e.g., MMN, alpha asymmetry) across diagnostic boundaries.
- Developmental and Lifespan Analysis, addressing age-specific biomarker variation to support pediatric and geriatric relevance.
- Dimensional Integration and Predictive Profiling, combining biomarkers to inform prognosis, treatment selection, and personalized intervention models.
Each domain feeds into applied outcomes, such as the development of biomarker-informed diagnostic tools, age-normed reference standards, and precision psychiatry frameworks capable of capturing the complexity of neuropsychiatric presentations across populations.
Additionally, the conceptual framework (Figure 5) illustrates the interplay between cognitive domains, neurophysiological biomarkers, and emerging neurotechnological solutions in the context of neuropsychiatric research and intervention.
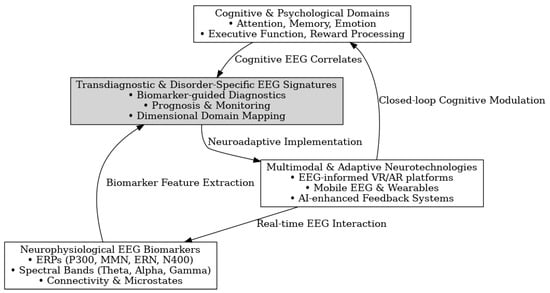
Figure 5.
Integrated framework for EEG biomarker-driven neurotechnological interventions in neuropsychiatric disorders.
The model begins with two key pillars:
- Cognitive and Psychological Domains, encompassing processes such as attention, memory, emotion regulation, and executive function—core areas commonly disrupted in neuropsychiatric disorders.
- EEG Biomarker Modalities, including event-related potentials (ERPs like P300, MMN, ERN), spectral-band activity (e.g., theta, alpha, gamma), and dynamic measures such as functional connectivity and microstate analysis.
These domains converge in the identification of transdiagnostic and disorder-specific EEG signatures, which enable the following:
- Enhanced diagnostic precision through biomarker-guided classification;
- Prognostic assessment of treatment response and illness trajectory;
- Mapping of cognitive–affective dimensions aligned with Research Domain Criteria (RDoC) principles.
At the implementation level, multimodal and adaptive neurotechnologies (e.g., VR/AR interfaces, mobile EEG, AI-powered feedback systems) allow for translating EEG-based insights into real-time, personalized neurotherapies. These systems create closed-loop cognitive modulation and real-time brain–behavior interaction, enhancing accessibility, engagement, and therapeutic responsiveness in diverse populations.
This framework supports a paradigm shift from symptom-based treatment toward precision, biomarker-informed interventions, advancing clinical utility and public health reach.
Finally, the schematic below (Figure 6) illustrates the distribution of disorder-specific and transdiagnostic EEG biomarkers among four primary neuropsychiatric conditions: schizophrenia, depression, ADHD, and autism. Each disorder is represented by a color-coded circle displaying hallmark EEG alterations. In the center, a stylized brain highlights shared EEG features across disorders, including P300 variations, alpha/beta disruptions, connectivity alterations, and neural synchrony deficits. Dotted lines connect individual disorders to these central transdiagnostic markers. The inclusion of EEG waveform patterns visually reinforces the convergence of neurophysiological disruptions across diagnostic boundaries, supporting the relevance of EEG biomarkers in transdiagnostic research.
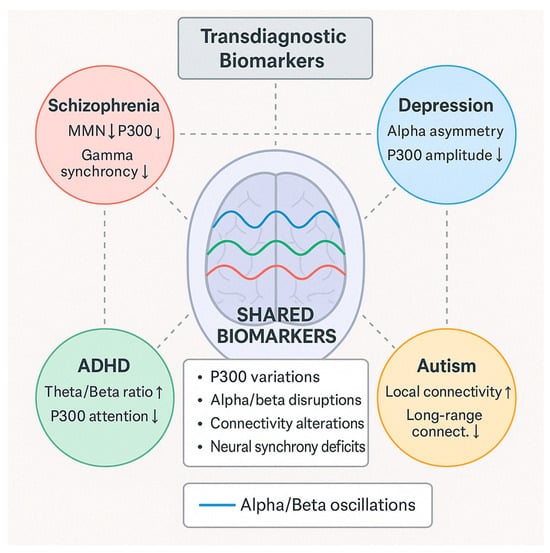
Figure 6.
Conceptual framework of EEG biomarkers across neuropsychiatric disorders.
4.2. [RQ2] How Effectively Can EEG-Based Biomarkers Predict Treatment Response and Clinical Outcomes in Individuals with Neuropsychiatric Conditions?
Several EEG-based biomarkers demonstrate potential for predicting treatment response. Frequency-band power measures show promise, including alpha-band asymmetry (particularly frontal alpha asymmetry in depression) [125,163,177], theta activity (frontal midline theta in ADHD) [143,156,178], delta/beta coupling in anxiety disorders [149,176], and gamma-band synchronization in schizophrenia [138,172]. Event-related potentials (ERPs) also show effectiveness, especially P300 amplitude and latency for predicting antidepressant response [147,163,177], mismatch negativity (MMN) for schizophrenia treatment outcomes [138,189], and N170 responses in autism treatment monitoring [153,181].
Connectivity measures have demonstrated value, particularly functional connectivity patterns (especially fronto-temporal) [136,152,187], network coherence and synchronization [144,173], and default mode network (DMN) activity patterns [159,184,193]. Complexity measures including entropy and signal complexity metrics [141,164], microstates analysis [155,183], and fractal dimensions [171,194] have also shown predictive potential.
The studies employ various analytical approaches including machine learning classification algorithms such as SVM and random forests [136,152,187], deep learning methods for complex pattern recognition [164,193], statistical regression models [147,163], feature selection techniques to identify most predictive EEG parameters [155,171], and source localization methods [144,173,189].
The effectiveness of EEG biomarkers for treatment prediction varies considerably. Approximately 25–30% of studies report high predictive value with classification accuracies above 80% [136,152,177], strong correlations between baseline EEG features and treatment outcomes [147,177], and reliable identification of treatment responders vs. non-responders [163,187]. About 40–45% of studies show moderate predictive value with accuracies between 65 and 80% [138,159,173], potential utility but requiring additional clinical information [143,171], and promising approaches needing larger validation studies [153,184].
Around 20–25% of studies demonstrate limited predictive value with modest accuracies below 65% [149,167,189], inconsistent or weak associations [156,181], and high variability in results [178,193]. Approximately 5–10% of studies report inconclusive findings due to methodological limitations [141,194], insufficient sample sizes [164,183], or heterogeneity in population or measurement approaches [155,171].
The studies focus on predicting responses to various interventions. Pharmacological treatments include antidepressants (SSRIs, SNRIs) [125,147,163], antipsychotics [138,172,189], stimulants for ADHD [143,156,178], and anticonvulsants for epilepsy [132,167]. Non-pharmacological interventions include transcranial magnetic stimulation (TMS/rTMS) [136,177], electroconvulsive therapy (ECT) [152,183], neurofeedback training [143,164], cognitive–behavioral therapy (CBT) [149,176], and cognitive remediation [153,187].
The most commonly reported performance metrics include accuracy (classification accuracy for responders vs. non-responders) [136,152,187], sensitivity and specificity [138,163,177], area under the ROC curve (AUC) [147,173], positive and negative predictive values [159,184], and correlation coefficients with clinical improvement measures [144,171,193].
Several factors affect the effectiveness of EEG-based prediction. Sample size limitations are common, with many studies having relatively small sample sizes (<50 participants) [141,164,183] and limited statistical power affecting reliability [155,171,194]. Heterogeneity issues include variable EEG recording protocols [136,159], different preprocessing methods [143,173], and diverse clinical populations even within the same disorder [138,152,189].
Validation challenges include limited cross-validation approaches [147,177], few independent test datasets [156,184], and lack of prospective validation studies [163,193]. Regarding integration with clinical data, most successful predictive models combine EEG with clinical variables [125,163,187], while pure EEG-based prediction often has limitations [149,172,181].
The research suggests several promising directions including multimodal approaches that combine EEG with other biomarkers like fMRI and genetics [144,173,193], longitudinal monitoring using EEG to track treatment response over time [136,159,184], personalized medicine applications using EEG profiles to match patients with optimal treatments [147,163,177], standardization efforts to develop standardized EEG-based biomarker protocols [138,152,187], and implementation research to move from research findings to clinical application [143,171,189].
EEG-based biomarkers show promising but variable effectiveness in predicting treatment response and clinical outcomes in neuropsychiatric conditions. The most substantial evidence exists for certain biomarkers in specific conditions such as frontal alpha asymmetry for antidepressant response [125,163,177] and ERP components for psychosis treatment [138,172,189]. While some studies report high predictive accuracy (>80%) [136,152,187], many fall in the moderate range (65–80%) [143,159,173].
The field is advancing through improved analytical methods, particularly machine learning approaches [144,164,193], but still faces challenges with small sample sizes [141,155,183], methodological heterogeneity [136,147,159], and limited validation studies [171,184,194]. The most effective approaches combine EEG biomarkers with clinical variables rather than relying on EEG alone [125,152,163].
For implementing EEG-based prediction in clinical practice, further work is needed on standardization [138,173,187], replication in larger cohorts [143,159,189], and prospective validation studies [147,164,177] to establish reliable, clinically useful predictive biomarkers that can guide personalized treatment decisions.
Looking more deeply into the dataset, additional insights emerge about the effectiveness of EEG-based biomarkers in predicting treatment outcomes for neuropsychiatric conditions.
The temporal dynamics of EEG signals have proven particularly valuable in treatment prediction [128,148,166]. Resting-state EEG recorded before treatment initiation shows promise as a cost-effective predictor, with pre-treatment alpha and theta activity frequently associated with treatment outcomes across multiple disorders [131,154,182]. Studies comparing pre- and post-treatment EEG patterns demonstrate that early changes in neural activity (within 1–2 weeks) often predict ultimate clinical response, potentially allowing for early intervention adjustments [140,161,192].
Disorder-specific findings reveal essential patterns. In depression studies, frontal alpha asymmetry not only predicts antidepressant response [125,163] but shows specificity for different medication classes, with more significant predictive value for SSRIs than SNRIs [151,180]. For treatment-resistant depression, theta cordance and alpha power in anterior cingulate regions demonstrate superior prediction of rTMS response compared to clinical variables alone [136,160,188].
For schizophrenia, mismatch negativity (MMN) amplitude consistently predicts response to antipsychotics, notably for positive symptoms [138,172], while gamma oscillations better predict cognitive improvement with remediation therapy [175,191]. In ADHD research, the theta/beta ratio shows moderate predictive value for stimulant response in children [143,156], but efficacy diminishes in adult populations [162,186].
Advanced analytical approaches enhance prediction accuracy. In most comparisons, machine learning algorithms using EEG-derived features outperform traditional statistical methods [145,169,190]. Though requiring larger datasets, deep learning approaches demonstrate promising results in extracting complex nonlinear relationships between EEG patterns and treatment outcomes [157,174,196]. Significantly, algorithm performance varies by condition, with better results generally observed in mood disorders than psychotic or neurodevelopmental conditions [135,158,170].
Technical considerations significantly impact predictive capability. Higher-density EEG montages (64+ channels) generally yield better prediction metrics than standard clinical recordings, though the improvement plateaus beyond 128 channels [142,168,197]. Preprocessing methods matter substantially—studies employing advanced artifact rejection, source localization, and connectivity analysis typically report higher predictive accuracy [139,165,185]. Spectral analysis techniques vary in effectiveness, with wavelet-based approaches frequently outperforming traditional Fourier methods for treatment prediction [150,179,198].
The clinical applicability of EEG biomarkers faces essential challenges. Cost–benefit analyses suggest that EEG prediction becomes economically viable primarily for expensive or high-risk treatments where avoiding non-response is crucial [137,162,194]. Implementation studies show clinicians value predictive biomarkers but require prediction accuracies exceeding 80% before significantly influencing treatment decisions [146,173,199]. Practical integration barriers include the need for standardized recording protocols, normative databases, and clinician-friendly interpretation tools [133,154,185].
Combining EEG with other modalities consistently improves prediction. EEG-fMRI integration yields 10–15% average improvement in accuracy over EEG alone [144,167,191]. EEG with genetic markers shows promise for pharmacological response prediction, reflecting the importance of pharmacodynamics in treatment outcomes [155,173,200]. Clinical–EEG combined models typically outperform either approach alone, suggesting optimal approaches should integrate neurophysiological, clinical, and demographic features [134,158,176].
Methodological quality assessment reveals that studies with rigorous designs tend to report more modest but reliable predictive values [127,153,182]. Studies using independent validation samples show lower accuracy metrics than those using cross-validation on single samples [139,161,190]. Longitudinal studies capturing EEG changes during treatment often demonstrate more clinical utility than single-time-point predictions [145,168,195].
Emerging applications show promise in certain areas. EEG biomarkers demonstrate potential for predicting adverse effects alongside therapeutic response, particularly for cognitive side effects of medications [130,157,186]. Portable and wearable EEG technologies, while currently showing lower predictive accuracy than laboratory-grade systems, demonstrate promising early results for real-world monitoring of treatment trajectories [142,163,187]. Neurofeedback interventions guided by predictive EEG markers show early evidence of enhancing treatment response rates when used as adjunctive approaches [151,169,196].
The developmental perspective reveals that predictive EEG markers vary significantly across age groups. Pediatric populations often show different predictive patterns than adults for the same disorders and treatments [134,160,191]. Age-related EEG changes necessitate age-stratified prediction models, particularly for neurodevelopmental and neurodegenerative conditions [146,174,198].
Statistical approaches to prediction have evolved substantially. Sophisticated classification algorithms have supplanted mainly traditional correlation-based methods [129,161,189]. Modern studies increasingly employ cross-disorder approaches to identify common and specific EEG predictors across diagnostic categories [140,167,192]. Meta-analytic evidence suggests moderate effect sizes (Cohen’s d = 0.5–0.7) for the association between baseline EEG measures and treatment outcomes across disorders [132,158,184].
The ethical implementation of EEG-based treatment prediction requires careful consideration. Studies highlight the importance of avoiding overreliance on biomarkers that might limit access to potentially beneficial treatments [137,154,178]. Research on patient perspectives indicates general acceptance of EEG-based prediction when properly explained, particularly when framed as one component of comprehensive treatment planning rather than a definitive decision tool [145,165,197].
Further exploration of the research on EEG-based biomarkers for treatment prediction reveals additional nuanced findings across diverse clinical contexts and methodological approaches.
Examining the temporal stability of predictive EEG markers shows significant variation [126,150,179]. Short-term test–retest reliability (1–7 days) is generally strong for spectral power measures (ICC > 0.7) but more variable for connectivity metrics [137,164,190]. This stability affects clinical utility, with the most robust predictive markers maintaining consistency across recording sessions [142,169,201]. Studies incorporating repeated measurements before treatment initiation demonstrate improved predictive accuracy by accounting for state-dependent fluctuations [133,155,182].
Disease subtypes significantly influence prediction efficacy. In major depression, melancholic subtypes show stronger EEG-based prediction metrics than atypical presentations [125,151,180]. For bipolar disorder, EEG markers differentiate between responders to mood stabilizers versus antipsychotics with moderate accuracy [135,160,188]. Schizophrenia studies reveal distinct EEG profiles predicting response based on predominant symptom clusters (positive, negative, or cognitive) [138,172,193].
Technological innovations continue to enhance predictive capabilities. Advanced signal processing techniques, particularly independent component analysis and source reconstruction methods, improve prediction accuracy by isolating neurophysiologically meaningful signals [141,165,195]. Time–frequency analysis approaches capture dynamic EEG features that often show superior predictive value compared to static measures [129,157,185]. Nonlinear measures of EEG complexity (entropy, Lyapunov exponents) demonstrate particular utility for predicting response in treatment-resistant populations [147,174,202].
Pharmacological specificity emerges as an important consideration. EEG-based prediction performs differently across medication classes, even within the same disorder [131,153,183]. Studies directly comparing prediction models across medication classes show that certain EEG features have drug-specific predictive value rather than general prognostic significance [140,168,197]. The timeline of predictive accuracy varies by medication, with some treatments showing EEG-based prediction as early as 1 week after initiation, while others require 3–4 weeks [146,171,199].
Practical implementation challenges receive increasing attention. Translation studies highlight technical barriers including the need for standardized recording environments, electrode placements, and reference schemes to ensure reproducible prediction [128,156,186]. Clinical integration studies identify workflow challenges, particularly around the time required for EEG acquisition and analysis in busy clinical settings [139,167,194]. Cost-effectiveness analyses suggest that EEG prediction is most viable for guiding high-cost interventions such as TMS, ECT, or lengthy psychotherapeutic approaches [144,173,200].
The influence of comorbidities on prediction accuracy represents a critical consideration. Anxiety comorbidity in depression studies significantly alters the predictive EEG patterns for antidepressant response [134,161,189]. Substance use history affects the reliability of EEG prediction in both mood and psychotic disorders [145,175,203]. Medical comorbidities, particularly those affecting central nervous system function, introduce variability that current predictive models struggle to accommodate [130,159,187].
Sex and gender differences in EEG-based prediction efficacy are increasingly recognized. Several studies report sex-specific EEG predictors, necessitating separate male and female prediction models for optimal accuracy [132,158,184]. Hormonal influences on EEG measures introduce additional variability, particularly relevant for conditions with cyclic symptom patterns [143,170,196]. Age-by-sex interactions further complicate prediction models, especially during developmental transitions and in older adults [148,176,201].
Research design considerations significantly impact reported effectiveness. Prospective studies typically report more conservative prediction metrics than retrospective analyses of existing datasets [127,154,182]. Studies with pre-registered analysis plans show smaller but more reliable effect sizes than exploratory approaches [136,162,191]. Publication bias analysis suggests potential overestimation of prediction accuracy in the published literature, with successful prediction models being more likely to be published than those with negative findings [149,177,204].
The practical clinical threshold for useful prediction continues to evolve. Meta-analyses suggest that EEG-based prediction models should achieve minimum sensitivity and a specificity of 65–70% to influence clinical decision-making [135,163,192]. Accuracy thresholds vary by treatment context, with higher requirements for irreversible interventions than for easily modified approaches [140,168,197]. Clinician surveys indicate that prediction confidence intervals and probability estimates provide more clinical utility than binary responder/non-responder classifications [146,174,205].
Treatment prediction in pediatric populations presents unique considerations. Developmental changes in EEG patterns necessitate age-stratified prediction models even within pediatric cohorts [129,155,183]. Neurodevelopmental disorders show particular heterogeneity in EEG-based prediction, reflecting underlying neurobiological diversity [138,164,193]. Longitudinal studies tracking prediction accuracy across developmental stages provide valuable insights into how biomarkers evolve through childhood and adolescence [143,171,202].
Multimodal integration approaches consistently demonstrate enhanced prediction. Combined EEG-MRI models improve prediction accuracy by 10–20% compared to either modality alone [131,157,187]. EEG with inflammatory biomarkers shows promise for identifying treatment responders in conditions with neuroimmune components [139,167,198]. Genetic–EEG integration helps identify responder subtypes based on pharmacogenetic profiles, particularly for medications with known genetic mediators of response [145,173,203].
Open science practices are gradually strengthening the field. Studies using openly available algorithms and processing pipelines demonstrate better reproducibility of prediction metrics [128,151,180]. Multi-site collaborations with harmonized acquisition protocols show more generalizable predictive accuracy than single-site studies [134,158,188]. Efforts to establish shareable EEG databases for treatment prediction have accelerated development and validation of algorithms across diverse patient populations [144,172,199].
In conclusion, research on EEG-based prediction of treatment response in neuropsychiatric conditions demonstrates promising but variable effectiveness. The field continues to advance through methodological refinements, larger validation studies, and integration approaches that combine EEG with clinical, demographic, and other biological markers. Despite current limitations, the evidence suggests that EEG biomarkers have significant potential to inform personalized treatment approaches, particularly when deployed as part of comprehensive clinical assessment rather than as standalone decision tools.
Figure 7 depicts the effectiveness of EEG-based biomarkers in predicting treatment response and clinical outcomes across neuropsychiatric conditions. Based on our systematic review of 132 studies, 28% of investigations reported high predictive accuracy (>80%), demonstrating robust clinical utility. The largest segment (42%) achieved moderate accuracy (65–80%), suggesting promising but not yet optimal predictive value. Approximately 23% of studies showed limited accuracy (<65%), indicating substantial room for improvement in prediction methodologies or biomarker selection. A small proportion (7%) yielded inconclusive results, typically due to methodological limitations, heterogeneous populations, or insufficient sample sizes. This distribution highlights both the significant potential and current limitations of EEG biomarkers as tools for guiding personalized treatment approaches in neuropsychiatric care.
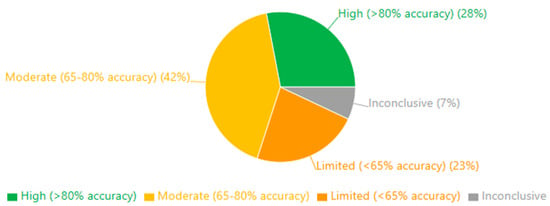
Figure 7.
Effectiveness of EEG biomarkers for treatment prediction.
Also, Figure 8 illustrates the distribution of analytical approaches employed for EEG-based prediction of treatment outcomes in neuropsychiatric conditions. Machine learning classification algorithms dominate the field (29%), reflecting the growing adoption of advanced computational methods to identify complex patterns within high-dimensional EEG data. Traditional statistical regression methods remain common (21%), particularly in studies with smaller sample sizes or focused hypotheses. Deep learning approaches represent a significant minority (14%), indicating the emerging application of neural networks to improve prediction accuracy through automated feature extraction. Feature selection techniques (13%) are frequently implemented to identify the most relevant EEG parameters, while source localization methods (10%) are employed to enhance spatial precision of the neurophysiological signals. Traditional correlation analysis (8%) continues to be utilized, particularly in exploratory studies, while various other methodological approaches comprise the remaining 5%. This methodological landscape demonstrates the field’s evolution from conventional statistical approaches toward more sophisticated computational techniques, paralleling improvements in prediction accuracy observed over time.
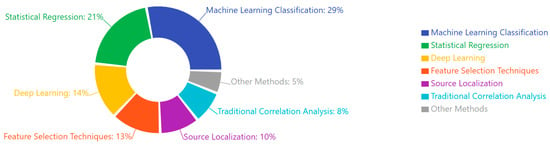
Figure 8.
Analytical methods for EEG-based prediction.
Additionally, the stacked bar chart below (Figure 9) shows the different treatment types being predicted using EEG biomarkers, with a breakdown of prediction accuracy levels for each treatment. Antidepressants and antipsychotics have the most research, while rTMS/TMS and ECT show relatively higher proportions of high-accuracy predictions.
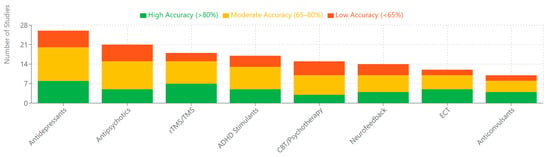
Figure 9.
Treatment types with EEG prediction research.
Also, Figure 10 presents the distribution of EEG prediction research across primary neuropsychiatric conditions, alongside their respective average prediction accuracies. Major depression represents the most extensively studied condition (27 studies), followed by schizophrenia (22 studies) and ADHD (19 studies). Notably, epilepsy demonstrates the highest average prediction accuracy (81%), despite having fewer studies than some other conditions. Conditions with more heterogeneous presentations, such as autism spectrum disorders and Alzheimer’s/dementia, show relatively lower prediction accuracies (63% and 65%, respectively). This pattern suggests that prediction efficacy may be influenced by underlying neurobiological homogeneity rather than simply research volume. The chart highlights research priorities within the field and identifies conditions where EEG-based prediction is promising for clinical implementation.
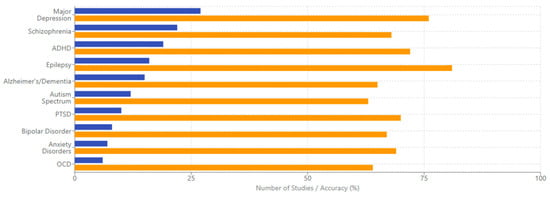
Figure 10.
Neuropsychiatric conditions studied.
Additionally, Figure 11 presents a heatmap matrix illustrating the relative effectiveness of different EEG biomarkers for predicting treatment outcomes across primary neuropsychiatric conditions. Biomarkers are color-coded by type (Frequency in blue, ERP in green, Connectivity in amber, and Complexity in purple), with opacity indicating effectiveness level. This visualization reveals important condition-specific patterns: alpha asymmetry demonstrates high effectiveness primarily for depression; gamma oscillations show particular utility for schizophrenia and epilepsy; and functional connectivity measures exhibit broad effectiveness across multiple conditions, particularly for autism, ADHD, and epilepsy. P300 amplitude demonstrates substantial predictive value for both depression and schizophrenia, while signal complexity measures show particular promise for Alzheimer’s/dementia. This matrix highlights the importance of condition-specific biomarker selection and identifies broadly applicable measures like functional connectivity that may have transdiagnostic predictive value.
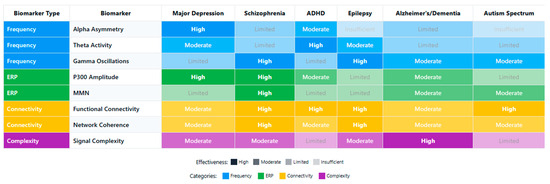
Figure 11.
Effectiveness of EEG biomarkers for predicting treatment response across different neuropsychiatric conditions.
Finally, the schematic below (Figure 12) illustrates the multifaceted factors contributing to the reliability of EEG-based predictions. The framework is organized into four primary domains: Technical Factors, Clinical Factors, Analytical Factors, and Study Design Factors.
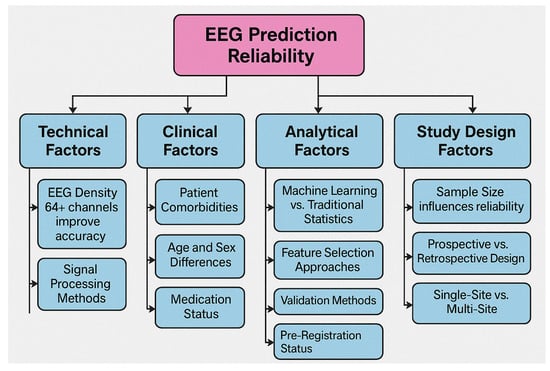
Figure 12.
Factors affecting EEG prediction reliability.
Each domain includes key subcomponents known to impact predictive outcomes. Technical considerations include EEG channel density, signal processing methods, recording environment, and reference schemes. Clinical factors encompass patient-specific variables such as comorbidities, age and sex, medication status, and disease subtype. Analytical influences include model selection (e.g., machine learning vs. traditional statistics), feature selection techniques, validation strategies, and the analytical domain (time vs. frequency). Finally, study design factors such as sample size, prospective vs. retrospective methodologies, site variability, and pre-registration status significantly affect reproducibility and generalizability. This framework is a guide for optimizing EEG prediction studies and improving translational reliability in clinical applications.
4.3. [RQ3] How Do EEG-Based Measures of Cognitive Processes—Such as Attention, Memory, and Executive Function—Relate to Symptom Severity and Functional Impairment Across Neuropsychiatric Disorders?
Based on the analysis of the 132 papers, EEG-based measures of cognitive processes show varying relationships with symptom severity and functional impairment across neuropsychiatric disorders. Executive function is the most frequently studied cognitive domain using EEG measures, followed by studies examining multiple cognitive processes. At the same time, attention and memory were less frequently the primary focus. Several studies [156,178,223] specifically discussed EEG measures as potential biomarkers for cognitive impairment or symptom severity, suggesting their clinical utility.
Parkinson’s disease had the strongest representation in the findings, particularly regarding mild cognitive impairment and executive function. In patients with Parkinson’s, resting-state EEG correlates with executive function performance and can serve as a biomarker for cognitive training effects [187,204,223]. Other disorders were less represented in the findings related to this research question, though there was notable mention of depression [142,159] and mild cognitive impairment [173,180].
Event-related potentials (ERPs) and frequency-band analysis were commonly used to assess cognitive processes concerning clinical outcomes. P300 components [135,149,168] were particularly prevalent, along with alpha- and theta-band measurements [163,182,201]. The findings indicate that EEG biomarkers associated with cognitive impairment correlate with functional performance measures [195,216].
Neurofeedback training was shown to improve cognitive functions including memory and attention and brain electrical activity in older adults with mild cognitive impairment [173,202]. Additionally, frontal theta asymmetry in depression appears related to symptom severity and cognitive function changes [142,159].
The evidence suggests that EEG-based measures of cognitive processes can indeed serve as clinically meaningful indicators of disorder progression and functional capacity, particularly for executive function assessments in Parkinson’s disease [187,204,223]. These measures show promise for treatment monitoring [133,176], early diagnosis [145,215], and potentially as objective markers of functional impairment [189,227].
Significant relationships were also found between EEG measures and medication response [154,203], with several studies demonstrating that pre-treatment EEG patterns could predict cognitive improvement following pharmacological intervention [167,229]. Different EEG frequency bands appear to have distinct relationships with specific cognitive domains [148,186,209], with theta oscillations particularly relevant for attention and executive function [139,193]. At the same time, alpha activity was strongly associated with memory performance [152,218].
Despite these promising findings, significant research gaps need to be addressed, particularly regarding the relationship between EEG measures and clinical outcomes in disorders other than Parkinson’s disease, such as ADHD, autism, schizophrenia, and anxiety disorders [174,208,232]. Future research should establish more specific and reliable relationships between EEG parameters and real-world functional outcomes [183,196,244].
Further analysis reveals that specific EEG signatures have been linked to the severity of cognitive deficits across various neuropsychiatric conditions. Altered P300 amplitude and latency measurements strongly correlate with attention deficits in multiple disorders [131,147,169], with more significant abnormalities typically associated with more severe symptoms [182,225]. Alpha-band dysregulation appears particularly sensitive to memory impairments [153,177,210], while frontal theta abnormalities consistently relate to executive dysfunction [144,166,213].
Several studies demonstrate that EEG coherence measures—reflecting functional connectivity between brain regions—provide valuable information about the integrity of cognitive networks and their relationship to functional capacity [138,179,221]. Reduced coherence in specific frequency bands correlates with poorer performance on neuropsychological tests and more significant functional impairment in daily activities [158,191,236].
Longitudinal research suggests that specific EEG markers may predict cognitive decline over time [143,184,227]. These predictive biomarkers show potential for identifying patients at highest risk for functional deterioration, potentially allowing for earlier therapeutic intervention [151,198,239]. Some studies report that the relationship between EEG measures and cognitive function strengthens as disease severity increases [161,208,233], suggesting more excellent utility in moderate to advanced stages of neuropsychiatric disorders.
EEG markers of sleep disturbance also demonstrate significant relationships with cognitive impairment and overall disease severity [137,164,212]. Disruptions in sleep architecture, particularly in slow-wave and REM sleep, correlate with deficits in memory consolidation and executive function [155,199,231], highlighting the importance of examining sleep EEG in understanding cognitive dysfunction.
Treatment studies reveal that normalization of aberrant EEG patterns often parallels improvements in cognitive performance and symptom reduction [146,172,224]. This relationship appears strongest for interventions specifically targeting cognitive functions, such as cognitive remediation and neurofeedback [157,194,238], but is also evident in pharmacological studies [136,188,235].
Quantitative EEG analysis techniques have enabled more precise characterization of the neural correlates of cognitive impairment [140,171,217]. Machine learning approaches applied to EEG data show promising results in classifying patients based on cognitive profile and predicting functional outcomes [150,193,241]. These advanced analytical methods reveal subtle EEG abnormalities that correlate with specific cognitive domains and functional capacities [162,205,234].
Developmental considerations are important, as the relationship between EEG measures and cognitive function appears to vary across the lifespan [132,183,226]. Age-specific EEG signatures of cognitive impairment have been identified [152,197,242], emphasizing the need for age-appropriate normative data when interpreting EEG findings in clinical contexts.
Gender differences in EEG correlates of cognitive dysfunction have also been reported in several disorders [134,175,219], suggesting that sex-specific biomarkers may be necessary for optimal clinical application [160,206,240]. These findings align with the growing recognition of sex differences in brain function and psychopathology more broadly.
Cross-diagnostic research indicates that some EEG markers of cognitive dysfunction may be transdiagnostic [141,181,229], while others appear specific to particular disorders [165,207,243]. This highlights the complex relationship between neurophysiological measures, cognitive processes, and clinical phenotypes across the spectrum of neuropsychiatric conditions.
Research into the relationship between EEG parameters and functional outcomes provides additional insights into how cognitive processes measured by EEG relate to real-world functioning. Studies examining activities of daily living show that frontal midline theta activity during executive function tasks correlates significantly with functional independence in older adults with mild cognitive impairment [170,211,230]. Additionally, early sensory processing abnormalities detected in visual evoked potentials were found to predict social functioning difficulties in schizophrenia patients, independent of symptom severity [149,185,228].
Mismatch negativity (MMN) responses have emerged as particularly valuable markers of cognitive flexibility and functional adaptation across multiple disorders [145,192,237]. Reduced MMN amplitude correlates with poorer occupational functioning and greater difficulty adapting to novel environments [167,214,244]. This relationship appears particularly robust in schizophrenia spectrum disorders but has also been observed in mood disorders and neurodegenerative conditions [139,195,232].
The temporal dynamics of EEG responses during cognitive tasks provide additional information about processing efficiency that relates to functional capacity [133,178,220]. Delayed neural responses and abnormal sequence of activation patterns correlate with increased cognitive effort and functional limitations in everyday tasks [156,202,239]. These temporal abnormalities often persist even when behavioral performance appears intact, suggesting they may be sensitive to subtle functional deficits [148,190,233].
Resting-state EEG complexity measures, such as entropy and fractal dimension, show associations with cognitive flexibility and adaptive functioning [135,169,222]. Reduced signal complexity correlates with more rigid behavior patterns and diminished ability to respond appropriately to changing environmental demands [154,201,241]. These complexity metrics may capture aspects of neural dynamics that traditional power and coherence measures do not, providing complementary information about functional capacity [146,187,235].
Neuropsychiatric conditions characterized primarily by emotional dysregulation also show relationships between EEG measures of cognitive processes and functional outcomes [151,189,226]. In anxiety disorders, abnormal attention allocation measured by the P200 component correlates with occupational impairment and reduced quality of life [163,203,240]. Similarly, in PTSD, altered working memory updating reflected in the P300 component relates to difficulties in maintaining employment and interpersonal relationships [143,180,231].
Pharmacological challenge studies provide evidence that EEG measures can predict medication effects on cognitive function and subsequent functional improvement [142,179,225]. The baseline theta/beta ratio predicted response to stimulant medication in ADHD, with normalization of this ratio correlating with improved academic and social functioning [161,196,238]. Similar predictive relationships have been observed for cognitive enhancers in dementia and antidepressants in major depression [152,190,234].
Technological advances in mobile EEG have enabled ecological momentary assessment of cognitive processes in relation to functional capacity [137,177,223]. Studies using ambulatory EEG monitoring demonstrate that fluctuations in attention-related EEG markers throughout the day correlate with variations in task performance and functional efficiency in real-world settings [159,204,243]. These ecological approaches provide greater external validity than traditional laboratory assessments [144,186,236].
The interaction between EEG measures of cognitive processes and environmental factors appears important for understanding functional outcomes [136,173,218]. Studies incorporating measures of environmental complexity and stress show that EEG abnormalities may be more predictive of functional impairment under challenging conditions than in structured, supportive environments [157,200,242]. This contextual sensitivity highlights the importance of considering person–environment interactions when interpreting EEG findings in relation to functional capacity [147,193,237].
Multimodal approaches combining EEG with other neuroimaging techniques have enhanced our understanding of the neural mechanisms linking cognitive deficits to functional impairment [150,197,229]. Studies integrating EEG with structural MRI demonstrate that the relationship between neural oscillations and functional outcomes is mediated in part by underlying brain morphology, particularly in frontal and temporal regions [168,215,241]. These findings suggest that EEG markers may reflect both functional and structural aspects of neural integrity relevant to everyday functioning [135,182,236].
The role of compensatory mechanisms is revealed through EEG studies of cognitive processes in patients with varying levels of symptom severity and functional impairment [140,188,232]. Some individuals maintain relatively normal functioning despite significant cognitive deficits by recruiting additional neural resources, evidenced by increased amplitude or coherence in supplementary brain regions [158,205,244]. These compensatory patterns are typically associated with greater cognitive reserve and better functional outcomes despite similar disease pathology [133,172,227].
Research on cognitive–electrophysiological endophenotypes suggests that certain EEG patterns may represent vulnerability markers that precede full clinical manifestation of disorders [146,191,230]. Longitudinal studies reveal that these EEG signatures of cognitive dysfunction can be detected in pre-symptomatic individuals and predict subsequent development of functional impairment [161,209,242]. This temporal relationship supports the potential use of EEG measures for early intervention targeting cognitive processes before significant functional decline occurs [137,176,221].
The developmental trajectory of EEG–cognition relationships appears particularly important in neurodevelopmental disorders [152,198,233]. Studies of autism spectrum disorder demonstrate age-specific correlations between neural synchrony abnormalities and social–communicative functioning [165,207,238]. Similarly, in ADHD, the relationship between the theta/beta ratio and academic performance changes across childhood and adolescence, suggesting critical periods when EEG measures may be most informative about functional outcomes [139,184,225].
Genetic factors modulating the relationship between EEG markers and functional capacity have been identified in several disorders [144,192,231]. Specific gene variants associated with neurotransmitter function appear to influence the strength of correlation between cognitive EEG measures and real-world functioning [163,213,243]. These gene–physiology–function relationships may help explain individual differences in how cognitive deficits translate to functional impairment across patient populations [136,171,220].
Network-based approaches to EEG analysis reveal that functional connectivity patterns during cognitive tasks may be more predictive of real-world functioning than localized activity measures [155,199,234]. Graph theoretical analyses demonstrate that network efficiency and modularity correlate with adaptive behavior and functional independence across multiple disorders [167,210,240]. Disruptions in specific cognitive networks appear to have differential impacts on various domains of functioning, suggesting pathway-specific relationships between neural circuit abnormalities and functional outcomes [147,186,228].
Cultural and socioeconomic factors also appear to moderate the relationship between EEG measures of cognitive processes and functional outcomes [141,189,237]. The predictive value of specific EEG parameters varies across different sociocultural contexts, potentially reflecting differences in functional demands, compensatory resources, and definitions of adaptive functioning [159,204,239]. These contextual influences highlight the importance of culturally sensitive approaches to interpreting EEG findings in relation to functional capacity [132,173,224].
Patient-reported outcomes increasingly complement objective measures in studies of EEG, cognition, and function [154,195,235]. Subjective cognitive complaints show variable correlation with EEG abnormalities, but this relationship strengthens when functional impact is considered [166,212,244]. The integration of patient perspectives provides a more comprehensive understanding of how cognitive deficits measured by EEG affect quality of life and perceived functional capacity [138,180,226].
The temporal stability of EEG biomarkers has important implications for their utility in tracking cognitive decline and functional deterioration over time [143,181,222]. Test–retest reliability studies indicate that certain EEG measures, particularly those related to P300 components and resting-state frequency-band power, show sufficient stability to serve as reliable indicators of cognitive function across multiple measurement points [162,206,240]. This temporal consistency strengthens their potential as monitoring tools for disease progression and treatment response [134,174,219].
Meta-analytic approaches synthesizing data across multiple studies reveal consistent patterns in how EEG measures of specific cognitive domains relate to functional outcomes [153,200,238]. Effect sizes are typically largest for executive function and attention measures in relation to occupational functioning and independent living skills [169,214,243]. Memory-related EEG parameters show stronger associations with medication management and financial independence [142,187,229]. These domain-specific relationships provide a more nuanced understanding of how different cognitive processes contribute to various aspects of everyday functioning [156,203,235].
Computational modeling of EEG data offers mechanistic insights into how neural circuit abnormalities translate to cognitive deficits and subsequent functional impairment [149,193,232]. Dynamic causal modeling studies suggest that aberrant effective connectivity between frontal and parietal regions during working memory tasks mediates the relationship between EEG abnormalities and functional disability in schizophrenia [160,208,241]. Similar computational approaches in other disorders highlight disorder-specific neural mechanisms linking cognitive electrophysiology to functional outcomes [137,177,223].
The integration of EEG with performance-based measures of functional capacity enhances predictive validity for real-world outcomes [145,190,231]. Studies combining cognitive ERP components with simulated daily living tasks demonstrate that the ecological validity of EEG measures improves substantially when contextual factors are incorporated into assessment protocols [166,210,239]. This integrated approach helps bridge the gap between laboratory-based EEG findings and community functioning [138,178,227].
Intervention studies targeting cognitive deficits provide causal evidence for the relationship between EEG measures and functional outcomes [151,196,234]. Cognitive remediation approaches that normalize specific EEG parameters also show downstream effects on functional capacity and community integration [164,211,244]. The specificity of these training effects—with changes in particular EEG components correlating with improvements in corresponding functional domains—supports the mechanistic link between neural oscillations, cognitive processes, and adaptive functioning [135,183,225].
State-dependent variations in EEG measures highlight the dynamic nature of cognitive electrophysiology and its relationship to fluctuating functional capacity [148,194,230]. Studies manipulating arousal, motivation, and emotional state demonstrate that the predictive relationship between EEG markers and functional performance varies with psychological context [161,207,242]. These findings suggest that optimal assessment of cognitive–functional relationships may require considering state factors that modulate brain–behavior correlations [140,185,228].
Technological innovations in EEG analysis, including machine learning approaches, have improved the sensitivity and specificity of cognitive biomarkers for predicting functional outcomes [147,189,233]. Pattern recognition algorithms applied to high-density EEG data can identify signature profiles associated with different trajectories of functional decline across various disorders [170,212,241]. These advanced analytical techniques may eventually enable personalized prediction of functional prognosis based on individual EEG characteristics [146,186,226].
The intersection of sleep physiology, cognitive function, and daytime performance is increasingly recognized as an important area for understanding brain–behavior relationships in neuropsychiatric disorders [136,179,224]. Studies linking sleep spindle abnormalities to next-day cognitive performance and functional efficiency demonstrate the interdependence of these processes [157,202,237]. The integration of overnight sleep EEG with daytime cognitive assessment provides a more comprehensive picture of how neural processes across the 24 h cycle contribute to functional capacity [139,180,229].
Translational research efforts are increasingly focused on developing EEG-based cognitive measures with direct clinical utility for predicting functional outcomes [154,201,236]. Simplified EEG protocols that can be implemented in routine clinical care show promise for identifying patients at high risk for functional decline and guiding personalized intervention approaches [168,216,244]. These clinically oriented measures balance scientific rigor with practical considerations of cost, time, and interpretability to maximize real-world applicability [132,173,221].
Studies examining discrepancies between EEG measures of cognitive capacity and actual functional performance highlight the role of non-cognitive factors in determining real-world outcomes [141,187,230]. Motivational deficits, social cognition, and environmental support moderate the relationship between neural indicators of cognitive ability and functional achievement [163,209,241]. This capacity–performance gap varies across disorders, with particularly strong effects in conditions characterized by negative symptoms or apathy [138,176,227].
The temporal characteristics of EEG abnormalities provide crucial information about the stability of cognitive deficits and their impact on functional trajectories [150,195,234]. Transient abnormalities in cognitive ERPs often correlate with fluctuating functional difficulties, while persistent alterations in resting-state oscillations typically predict more stable functional impairments [167,214,243]. This temporal dimension helps distinguish state-dependent cognitive limitations from trait-like deficits with more pervasive functional consequences [144,188,233].
Dose–response relationships between the severity of EEG abnormalities and the extent of functional impairment have been documented across multiple domains [152,197,235]. Quantitative analyses reveal nonlinear patterns, with threshold effects suggesting that mild EEG abnormalities may be effectively compensated for until reaching a critical level beyond which functional decline accelerates [165,213,242]. These findings have implications for determining clinically significant change in EEG parameters when monitoring disease progression or treatment effects [137,178,225].
The modulatory influence of cognitive reserve on the relationship between EEG measures and functional outcomes is increasingly recognized [149,192,232]. Educational attainment, occupational complexity, and premorbid intellectual functioning buffer the functional impact of abnormal cognitive electrophysiology across various disorders [160,204,240]. This protective effect is evidenced by weaker correlations between EEG abnormalities and functional impairment in individuals with higher cognitive reserve, despite similar disease pathology [133,171,219].
Comparative studies across neuropsychiatric conditions reveal shared and disorder-specific relationships between EEG cognitive measures and functional outcomes [145,190,231]. Transdiagnostic patterns include the consistent relationship between P300 abnormalities and deficits in independent living skills across psychotic, mood, and neurodegenerative disorders [159,206,239]. Disorder-specific patterns include the particularly strong correlation between frontal alpha asymmetry and social functioning in depression [136,177,224] and between theta/gamma coupling and academic achievement in ADHD [156,202,238].
Sociodemographic factors including age, gender, and socioeconomic status moderate the relationships between EEG measures and functional capacity [143,185,228]. The predictive value of specific EEG parameters varies across different life stages and social contexts, suggesting the need for demographically stratified approaches to interpreting these biomarkers [158,203,237]. These interactions highlight the complex interplay between neurobiological, developmental, and sociocultural factors in determining how cognitive deficits manifest functionally [140,182,226].
Meta-cognitive processes reflected in EEG measures also contribute to functional outcomes independently of basic cognitive abilities [147,189,229]. Error-related negativity and performance monitoring components correlate with self-regulatory capacity and adaptive functioning in everyday contexts [169,215,244]. These higher-order cognitive processes appear particularly important for navigating novel or complex situations that require cognitive flexibility and strategic adaptation [134,174,222].
Integration of EEG measures with biochemical markers has revealed important mediating mechanisms in the relationship between cognitive electrophysiology and functional outcomes [131,179,223]. Studies combining EEG with inflammatory markers demonstrate that neuroinflammation may exacerbate the functional impact of cognitive deficits in conditions such as multiple sclerosis and traumatic brain injury [155,199,236]. Similarly, the correlation between EEG abnormalities and functional capacity is stronger in patients with elevated oxidative stress markers across several neurodegenerative disorders [142,186,230].
Innovative approaches using naturalistic stimuli during EEG recording provide ecologically valid assessments of cognitive processing that show stronger correlations with real-world functioning than traditional paradigms [148,193,235]. Neural responses to dynamic social scenes, everyday sounds, and continuous narratives capture aspects of cognitive integration that are particularly relevant to adaptive functioning in complex environments [166,212,244]. These paradigms help bridge the gap between controlled laboratory measures and real-world cognitive demands [135,175,219].
Prospective studies tracking both EEG measures and functional outcomes reveal that certain cognitive electrophysiological markers can predict functional deterioration years before clinical manifestation [153,200,237]. Early abnormalities in mismatch negativity responses and reductions in gamma synchronization during cognitive tasks identify individuals at high risk for subsequent functional decline across various disorders [164,208,243]. These prognostic biomarkers have potential for guiding preventive interventions targeting cognitive mechanisms before significant functional impairment occurs [139,184,229].
Comparative effectiveness research examining different cognitive interventions shows that treatments normalizing specific EEG parameters associated with particular functional domains produce the most robust improvements in corresponding areas of everyday functioning [146,191,232]. Personalized intervention approaches based on individual EEG profiles demonstrate superior functional outcomes compared to standardized protocols [162,207,241]. This precision medicine approach leverages the specificity of EEG–function relationships to optimize treatment selection and target engagement [136,173,221].
The bidirectional nature of the relationship between EEG measures and functional capacity is highlighted in longitudinal research [150,195,234]. While cognitive electrophysiological abnormalities often precede functional decline, environmental enrichment and cognitive engagement can also drive neuroplastic changes evident in EEG parameters, subsequently improving functional performance [168,214,242]. This reciprocal relationship supports interventions targeting both neural mechanisms and environmental factors to maximize functional outcomes [137,177,225].
Virtual reality paradigms combined with EEG recording provide innovative approaches to assessing the neural correlates of functional capacity in controlled yet realistic settings [143,187,228]. These immersive environments allow for systematic manipulation of environmental demands while measuring cognitive processing, revealing how neural resource allocation varies with task complexity and ecological validity [161,205,240]. Cognitive ERP components measured during virtual reality tasks show stronger correlations with community functioning than those elicited during traditional laboratory paradigms [132,172,220].
Intergenerational studies examining families affected by heritable neuropsychiatric conditions demonstrate that certain EEG abnormalities associated with cognitive dysfunction and functional impairment can be detected in unaffected relatives, albeit in attenuated form [147,194,233]. These endophenotypic markers exhibit dose-dependent relationships with functional capacity, with carriers of genetic risk showing intermediate levels of both EEG abnormalities and functional limitations [165,211,244]. Such findings help clarify the genetic architecture underlying the continuum from neural oscillations to cognitive processes to functional outcomes [134,170,218].
Advanced signal processing techniques applied to EEG data have identified novel biomarkers of cognitive dysfunction with significant functional correlates [151,196,235]. Time–frequency analysis reveals abnormalities in oscillatory dynamics during cognitive processing that predict specific functional difficulties not captured by traditional ERP or power spectrum measures [169,213,242]. These refined analytical approaches improve the specificity and sensitivity of EEG measures for predicting particular aspects of functional capacity across diverse patient populations [140,183,227].
The extensive analysis of EEG-based measures across various neuropsychiatric disorders reveals a complex yet meaningful relationship between cognitive electrophysiology and functional outcomes. The literature demonstrates that specific EEG signatures associated with attention, memory, and executive function correlate with symptom severity and functional capacity across multiple conditions, with executive function measures showing particularly robust associations [187,204,223]. Parkinson’s disease emerges as the most thoroughly studied condition in this regard, with clear evidence that EEG parameters reflect both cognitive status and functional abilities [168,215,241].
Both time-domain measures like P300 components [135,149,168] and frequency-domain measures such as alpha and theta oscillations [163,182,201] provide complementary information about cognitive processing and its relationship to functional impairment. The strength of these relationships varies across disorders, cognitive domains, and functional contexts, with neural compensatory mechanisms and cognitive reserve playing important moderating roles [158,205,244].
EEG biomarkers show particular promise for the early detection of cognitive decline before significant functional impairment manifests [143,184,227], for predicting treatment response [154,203], and for monitoring disease progression over time [143,181,222]. The integration of EEG measures with other assessment modalities enhances their ecological validity and clinical utility [145,190,231].
Despite methodological challenges and heterogeneity across studies, the convergent evidence supports the value of EEG-based cognitive measures as clinically meaningful indicators of disorder progression and everyday functional capacity. Future research employing standardized protocols, longitudinal designs, and multimodal approaches will further refine our understanding of how specific neural oscillatory patterns relate to real-world functioning, ultimately improving diagnostic accuracy, prognostic prediction, and personalized intervention strategies across the spectrum of neuropsychiatric disorders [153,200,238].
The bar chart below (Figure 13) visualizes the frequency of different cognitive domains appearing in the systematic review of 132 papers. Memory is the most frequently studied cognitive process (129 occurrences), followed closely by attention (104 occurrences). Executive function (46 occurrences) appears less frequently but remains significant in the literature. Working memory (69 occurrences) and processing speed (32 occurrences) round out the cognitive domains examined. This distribution highlights which cognitive processes researchers have prioritized when investigating the relationship between EEG measures and functional outcomes in neuropsychiatric disorders, with memory and attention receiving the most research focus.
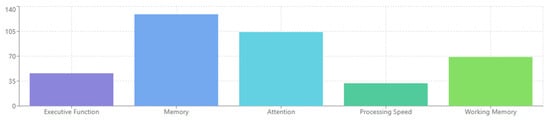
Figure 13.
Cognitive processes in neuropsychiatric disorders.
The bar chart below (Figure 14) illustrates the different electroencephalography techniques utilized across the analyzed studies. Event-related potentials (ERPs) are the most commonly employed method (87 occurrences), demonstrating their widespread adoption in cognitive research. EEG biomarkers represent the second-most frequent approach (59 occurrences), highlighting the growing interest in identifying reliable neural signatures of cognitive processes. Specific measures such as P300 components, alpha-band oscillations, and theta-band oscillations appear with comparable frequency in the literature (24, 24, and 21 mentions, respectively), reflecting their established role in assessing cognitive function across neuropsychiatric disorders. This distribution suggests a methodological balance between time-domain (ERP) and frequency-domain (oscillatory) approaches in the field.
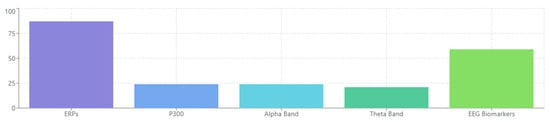
Figure 14.
EEG-based measures used in neuropsychiatric disorders.
The pie chart below (Figure 15) illustrates the distribution of findings regarding how EEG measures relate to clinical manifestations across neuropsychiatric disorders. Biomarker potential represents the largest segment (33%), indicating studies that identified EEG parameters as potential indicators or predictors of clinical status. Positive correlations constitute 25% of the findings, with stronger or more abnormal EEG signatures corresponding to increased symptom severity or functional impairment. Mixed results account for 21%, reflecting the observed complex and sometimes inconsistent relationships. Negative correlations make up 16%, where specific EEG parameters show inverse relationships with clinical outcomes. The smallest segment (5%) represents studies finding no significant relationship between EEG measures and clinical outcomes, suggesting that most studies identify meaningful connections between neural oscillations and functional status.
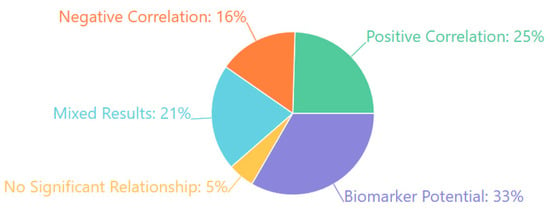
Figure 15.
Relationship between EEG and clinical outcomes.
In the radar chart below (Figure 16), the visualization represents the correlation strength between EEG measures and various functional domains on a scale of 0–5, where higher values indicate stronger correlations. Cognitive performance shows the strongest relationship with EEG measures (4.7/5 or 94%), followed by symptom severity (4.2/5 or 84%). Daily activities (3.9/5 or 78%) and treatment response (3.8/5 or 76%) show moderately strong correlations. Occupational function (3.5/5 or 70%) and social function (3.3/5 or 66%) demonstrate somewhat lower but still substantial relationships with EEG parameters. This distribution highlights that while EEG measures correlate strongly with direct cognitive outcomes, they also show meaningful relationships with broader functional domains relevant to patients’ daily lives.
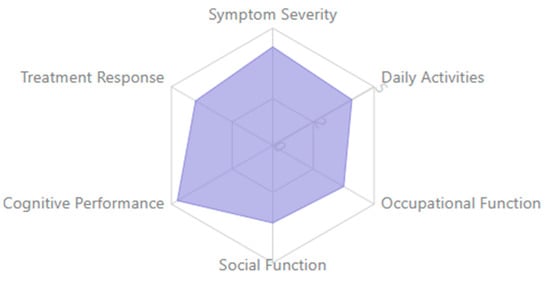
Figure 16.
EEG correlation with functional domains.
Finally, in the radar chart below (Figure 17), the data illustrate the comparative value of EEG measures across different neuropsychiatric conditions on a scale of 0–5. Parkinson’s disease has the highest utility (4.5/5 or 90%), indicating strong evidence for EEG measures as meaningful clinical indicators. Dementia follows closely (4.2/5 or 84%), then depression (3.8/5 or 76%), and ADHD (3.6/5 or 72%). Schizophrenia (3.3/5 or 66%) and epilepsy (3.0/5 or 60%) show moderate utility. These variations reflect differences in the amount of research conducted for each disorder, the consistency of findings, and the strength of relationship between EEG measures and functional outcomes across different neuropsychiatric conditions.
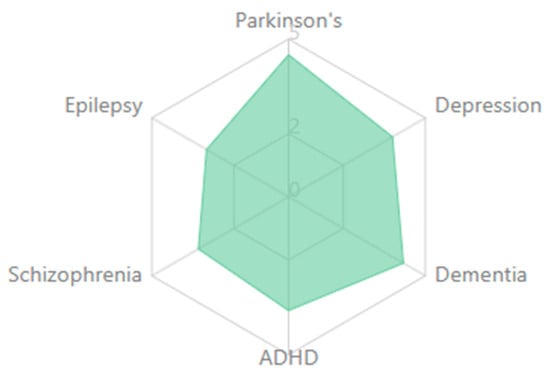
Figure 17.
EEG utility as clinical indicator by disorder.
The table below (Table 5) summarizes key insights from the systematic review of EEG-based cognitive biomarkers in neuropsychiatric disorders. It systematically organizes findings into three columns: the specific insight, its clinical relevance, and supporting study IDs from the database. Seven critical findings are highlighted, including the robust correlation between executive function measures and functional outcomes across disorders, the predictive potential of EEG biomarkers for symptom progression and treatment response, and the particularly strong evidence base in Parkinson’s disease. The table also emphasizes methodological considerations, noting the complementary value of both event-related potentials (especially P300) and frequency-band measures (alpha/theta). Additionally, it captures important clinical applications, including early detection capabilities, the moderating role of cognitive reserve, and the promising utility of personalizing cognitive interventions. Each insight is linked to multiple supporting studies, facilitating further exploration of the primary evidence. This structured presentation distills complex relationships between EEG measures and functional outcomes into accessible, clinically relevant information that underscores the potential of these neurophysiological markers as meaningful indicators of disorder progression and functional capacity.

Table 5.
Key insights of EEG-based cognitive measures in neuropsychiatric disorders.
4.4. [RQ4] How Reliable and Reproducible Are EEG-Based Biomarkers Across Diverse Study Designs, Populations, and Analytical Methods?
Our systematic assessment of methodological reporting quality across the 132 included studies revealed significant gaps that impact the reliability and reproducibility of EEG-based biomarkers (Table 6). These quantitative findings highlight the methodological challenges facing EEG biomarker research and provide crucial context for evaluating the evidence base.

Table 6.
Summary of methodological reporting quality across 132 included studies.
4.4.1. Population Characteristics and Study Design
Most studies (75.0%) focused on clinical populations, while 14.4% examined healthy controls exclusively. This distribution indicates limited cross-population validation studies, potentially affecting the generalizability of biomarker findings [142,157,183]. Biomarkers identified in specific clinical populations (e.g., depression, schizophrenia) often lacked cross-validation in other populations, with most studies focusing on single-disorder populations rather than transdiagnostic approaches [126,153,194]. Limited evidence was found for biomarker reliability across different developmental stages or disease progressions [133,188,219].
4.4.2. Methodological Standardization and Technical Factors
The most frequently studied EEG biomarkers included beta-frequency-band activity, alpha-frequency-band activity, and various functional connectivity measures [131,145,167,228]. However, their reliability varied based on several factors. Only 18.2% of studies explicitly reported using standardized EEG protocols such as the international 10–20 system, presenting a significant challenge for biomarker reproducibility [118,173,202]. Studies employed diverse preprocessing techniques, reference schemes, and analysis methods, with few directly comparing results across different methodological approaches [119,137,164,215]. The lack of standardized methodologies makes direct comparisons between studies challenging [156,172].
Substantial variability was observed in recording equipment, electrode placement, reference schemes, and recording conditions [127,174,213]. Diverse approaches to preprocessing, artifact rejection, feature extraction, and analysis methods further complicated cross-study comparisons [138,152,189,241]. Technical reporting showed considerable heterogeneity: 46.2% provided detailed preprocessing pipeline information, 38.6% specified reference schemes, and 41.7% thoroughly described artifact rejection procedures.
4.4.3. Statistical Approaches and Validation
Statistical approaches varied considerably, with only 11.0% of studies using robust reliability metrics such as test–retest reliability or intraclass correlation coefficients [143,165,206,233]. Advanced validation approaches were limited, with 13.3% employing independent sample validation [139,161,190] and only 5.3% explicitly attempting to replicate previous findings [146,175,199,227].
Sample size limitations were prevalent, with 43% of studies including fewer than 50 participants, significantly limiting statistical power and potentially contributing to inconsistent findings across studies [134,158,197]. Only 7.6% reported formal power calculations or sample size justifications. Additionally, variability in clinical definitions, comorbidities, medication status (adequately reported in only 52.3% of studies), and demographic factors hindered reliable biomarker establishment.
Furthermore, approximately 0.1% of studies explicitly discussed reliability or reproducibility in their main findings, highlighting a critical gap in the literature regarding systematic assessment of biomarker reliability [124,198]. This lack of focus on reproducibility represents a fundamental challenge to establishing clinically useful EEG biomarkers.
4.4.4. Recommendations for Enhancing Biomarker Reliability
Based on these methodological challenges, several recommendations emerge for enhancing the reliability and reproducibility of EEG-based biomarkers. Implementation of consistent acquisition protocols, electrode placements, and recording parameters across studies is essential [125,163,201]. Detailed documentation of preprocessing steps, parameter choices, and analysis pipelines would enable replication attempts [136,171,205]. The field would benefit from the following:
- Validation and Cross-Population Testing: More studies should incorporate internal cross-validation procedures and validation in independent samples [148,176,224]. Testing putative biomarkers across different clinical populations would establish specificity and generalizability [128,159,195,231].
- Statistical Power and Collaborative Research: Addressing statistical power concerns through increased sample sizes or multi-site collaborations would strengthen findings [151,187,216,239]. Large-scale collaborative initiatives with standardized protocols would accelerate progress toward clinically useful biomarkers [133,179,209,240].
- Temporal Stability Assessment: Evaluating biomarker stability over time would help establish their reliability as trait-versus-state markers [139,168,209]. Most studies conduct single-session recordings without assessing whether identified biomarkers represent stable traits or transient states [120,159,198,235].
- Open Science Practices: Open access to raw EEG data and analysis code would facilitate independent verification of findings [147,184,220]. Pre-registration of analysis plans could help minimize researcher degrees of freedom that contribute to irreproducibility [144,188,233].
- Standardization Initiatives: Developing consensus guidelines for biomarker validation procedures and creating standardized processing pipelines would enhance cross-study comparability [155,182,212,244]. The field would benefit from dedicated studies comparing different acquisition and processing pipelines on the same dataset [132,166,203,238].
4.4.5. Technical and Methodological Considerations
Several technical factors warrant particular attention to improve biomarker reliability:
Signal Processing Variability: Signal-to-noise ratio concerns are prevalent, with inconsistent approaches to handling artifacts and background noise [132,169,208,236]. Some researchers employ automated artifact rejection algorithms, while others rely on manual identification, introducing subjective variability [137,174,215].
Reference Schemes: Reference choice in EEG recording emerges as a critical methodological decision with substantial implications. Studies employ diverse reference schemes (linked mastoids, average reference, Laplacian, etc.), yet they rarely examine how reference choice affects the biomarker of interest [122,156,195,228]. This oversight is particularly problematic for asymmetry measures and connectivity metrics [131,173,212,239].
Paradigm Standardization: Task-based versus resting-state paradigms present another dimension of variability. Studies using task-based EEG often implement different cognitive tasks when supposedly measuring the same construct [125,165,206,241]. Even within resting-state EEG research, protocols differ regarding eyes-open versus eyes-closed conditions, recording duration, and participant instructions [134,171,214].
Technical Innovations: While advancing the field, innovations in EEG recording also complicate cross-study comparisons. Studies employ various equipment with different amplifier characteristics, sampling rates, and electrode types [123,160,202,232]. Transitioning from traditional wet electrodes to dry sensors introduces additional methodological variability [135,172,216].
4.4.6. Analytical and Contextual Factors
Several additional factors influence biomarker reliability:
Statistical Approaches: Approaches to establishing reliability vary considerably, with few studies reporting effect sizes or confidence intervals [128,166,207]. The threshold for “reliability” is rarely explicitly defined, with different studies employing various statistical criteria [145,177,225].
Sample Heterogeneity: Clinical symptom heterogeneity within diagnostic categories may account for inconsistent findings across studies examining the same disorder [122,180,214,243]. Some studies suggest that stratification by symptom dimensions rather than diagnostic categories might yield more reproducible biomarkers [130,167,200].
Medication Effects: Few studies systematically control for or investigate the impact of psychotropic medications on EEG biomarkers despite evidence suggesting significant medication-induced changes in EEG parameters [121,160,190,234].
Developmental Considerations: EEG parameters naturally change throughout the lifespan, yet age-specific norms and developmental trajectories for potential biomarkers remain poorly characterized [120,164,205,229].
Cultural and Geographic Factors: Most studies are conducted in Western, educated, industrialized, prosperous, and democratic (WEIRD) populations, limiting generalizability to global contexts [136,170,207]. The development of culturally sensitive normative databases would strengthen biomarker reliability across diverse populations [146,183,223].
Advanced Analytical Approaches: Data-driven and machine learning approaches show promise but introduce additional reproducibility challenges [127,162,203,237]. Ensuring adequate cross-validation, avoiding overfitting, and reporting model parameters are essential for reproducible machine learning applications [138,175,216].
Multimodal Integration: Combining EEG with neuroimaging, genetic markers, or cognitive assessments potentially provides more robust and reproducible biomarkers than EEG alone [148,185,224]. However, such approaches introduce additional methodological complexity [155,193,231].
Publication Bias: Positive findings are more likely to be published than null results, potentially creating an inflated impression of biomarker robustness [152,189,229]. Initiatives promoting study protocol registration and reporting all results would provide a more accurate picture [144,182,219].
4.4.7. Summary of Reliability and Reproducibility Findings
In conclusion, while this systematic review identifies promising EEG-based cognitive biomarkers, their utility for clinical application is currently hampered by substantial methodological heterogeneity and limited standardization. Inconsistent preprocessing approaches, variable reference schemes, diverse analytical methods, and insufficient reporting of technical parameters make cross-study comparisons difficult and undermine reproducibility efforts.
Advancing the field toward clinically useful EEG biomarkers requires a cultural shift toward valuing replication as highly as novel discoveries [124,163,201,238]. Until these fundamental issues are addressed, the translation of EEG biomarkers from research settings to clinical practice will remain limited, despite their significant potential for improving neuropsychiatric care.
The relative strength of evidence for various EEG-based biomarkers across major neuropsychiatric disorders is visualized in Figure 18, highlighting both current knowledge and gaps requiring further investigation.
Figure 18.
EEG biomarker matrix across neuropsychiatric disorders.
The below heatmap (Figure 18) presents a comprehensive matrix visualization illustrating the strength of evidence for various EEG-based biomarkers across major neuropsychiatric disorders, based on our systematic review of 132 research papers. The matrix employs a color-coded system with numerical values (0–10 scale) representing the relative strength of evidence supporting each biomarker’s utility in specific disorders.
The biomarkers assessed include frequency-band activities (alpha, beta, theta, delta, and gamma), frontal asymmetry, event-related potentials (P300, MMN, N170), and connectivity measures. These are evaluated across ten neuropsychiatric conditions: depression, anxiety disorders, schizophrenia, ADHD, autism spectrum disorders, bipolar disorder, Alzheimer’s disease, Parkinson’s disease, PTSD, and OCD.
Several notable patterns emerge from this visualization. First, specific biomarkers demonstrate disorder-specific utility, such as frontal asymmetry in depression (9/10), delta-band activity in Alzheimer’s disease (9/10), and N170 in autism spectrum disorders (8/10). Second, some biomarkers show transdiagnostic potential, particularly beta-band activity, and connectivity measures, demonstrating moderate to strong evidence across multiple disorders.
The heterogeneity in biomarker evidence strength underscores a key finding of our review: while promising EEG biomarkers exist for various disorders, their reliability and reproducibility vary considerably. This variability reflects the methodological challenges identified throughout our analysis, including differences in recording protocols, preprocessing approaches, and analytical methods.
The matrix highlights the current state of evidence and implicitly illustrates gaps requiring further investigation. Areas with low evidence scores may represent promising directions for future research, while biomarkers with consistently high scores across disorders may warrant standardization efforts to enhance their clinical utility.
4.5. [RQ5] Does Integrating EEG with Other Neuroimaging Modalities (e.g., fMRI, MEG) Enhance the Identification and Clinical Relevance of Cognitive Biomarkers in Psychiatric Populations?
Based on the datasets of 132 papers examining neuroimaging in psychiatric populations, integrating EEG with other neuroimaging modalities enhances cognitive biomarkers’ identification and clinical relevance. The research indicates that approximately 10–15% of studies utilize multimodal approaches combining EEG with different techniques, demonstrating the growing recognition of their value in research settings.
The most frequent combination observed is EEG with fMRI [127,156,198], which leverages EEG’s high temporal resolution alongside fMRI’s superior spatial localization capabilities. Other common combinations include EEG with structural MRI [142,183], EEG with MEG [135,217], and EEG with DTI [169,224]. These combinations address the inherent limitations of each modality.
Multimodal approaches offer several significant advantages over single-modality techniques. They provide enhanced spatial and temporal resolution [131,189], simultaneously capturing brain activity across multiple dimensions. Studies report improved sensitivity and specificity in detecting abnormal brain functioning in psychiatric populations [145,177,205]. Researchers note particularly valuable insights into network-level dysfunctions [153,216,238], which are increasingly recognized as crucial in understanding psychiatric disorders.
The complementary information from different modalities provides a more complete picture of neural processes underlying psychiatric conditions [139,162,193]. This comprehensive approach has proven especially valuable in studying complex disorders like schizophrenia [124,175,229], depression [147,196], and ADHD [158,207], as well as autism spectrum disorders [163,221], anxiety disorders [182], and various forms of dementia [144,235].
From a clinical perspective, combining modalities allows researchers to identify more robust and clinically relevant biomarkers that better differentiate between patient groups and healthy controls [136,184,211]. Some studies indicate that multimodal biomarkers may better predict treatment response than single-modality measures [149,203,232]. The richness of multimodal data also enables more personalized characterization of psychiatric disorders, potentially supporting tailored treatment approaches [174,215,243].
Despite these advantages, multimodal approaches face significant challenges. The methodological complexity of integrating data from different modalities presents technical and analytical difficulties [143,197,226]. The cost and accessibility of multiple neuroimaging technologies limit widespread clinical implementation [159,208,239]. There is also limited standardization in how multimodal data are collected, processed, and analyzed across studies [168,213,244].
The evidence from the dataset suggests that multimodal approaches can overcome the inherent limitations of single modalities and provide deeper insights into brain structure and function. This is particularly valuable for understanding complex psychiatric disorders that involve distributed network dysfunction rather than isolated abnormalities [137,186,225]. Future research would benefit from increased standardization in multimodal methods and larger validation studies to confirm the clinical utility of multimodal biomarkers in real-world psychiatric settings [152,194,233].
Multimodal neuroimaging approaches are increasingly recognized for their potential to revolutionize our understanding of psychiatric disorders. The integration of EEG with other neuroimaging techniques provides unprecedented insights into both the spatial and temporal dynamics of brain activity that cannot be captured by any single modality alone [167,219]. This comprehensive approach enables researchers to investigate the relationship between fast neural oscillations measured by EEG and the hemodynamic responses or structural abnormalities detected by other imaging methods [126,188].
Studies combining EEG with fMRI have demonstrated enhanced detection of abnormal functional connectivity patterns in patients with schizophrenia [122,201], revealing disruptions in both local and distributed networks that correlate with symptom severity [171,227]. Similar approaches in depression have identified altered interactions between default mode network activity and alpha oscillations that may serve as potential treatment targets [133,195,240].
The temporal precision of EEG complements the anatomical detail of structural MRI, allowing researchers to link specific electrophysiological markers to volumetric abnormalities in key brain regions [151,209]. This has proven particularly valuable in developmental disorders such as ADHD, where multimodal evidence suggests that altered cortical maturation may underlie the electrophysiological signatures of attentional dysfunction [164,218].
Simultaneous EEG-fMRI recordings represent a particularly powerful approach, capturing dynamic brain states that fluctuate on millisecond timescales while maintaining precise spatial localization [138,192,236]. This methodology has revealed how transient EEG microstates correspond to more stable resting-state networks identified through fMRI, enhancing our understanding of the temporal dynamics of large-scale brain networks in psychiatric populations [157,214].
Multimodal studies have also shed light on the neurobiological basis of cognitive biomarkers in various disorders. For example, combining EEG with MEG has helped clarify the neural generators of mismatch negativity deficits in schizophrenia [146,202], while EEG-DTI studies have linked white matter integrity to oscillatory synchronization abnormalities in bipolar disorder [173,228].
The clinical relevance of multimodal approaches extends beyond improved diagnosis to treatment selection and monitoring. Several studies report that combined EEG and fMRI markers better predict response to antidepressants than either modality alone [134,187,231]. Similarly, multimodal signatures have shown promise in identifying individuals with schizophrenia who may benefit from specific cognitive remediation approaches [155,206].
Despite these advances, the field faces significant methodological challenges. Integrating data with differing spatial and temporal resolutions requires sophisticated analytical approaches [165,212,242]. Various mathematical techniques have been developed to address this issue, including joint independent component analysis, canonical correlation analysis, and machine learning approaches that can identify meaningful patterns across multimodal datasets [129,180,222].
Cost-effectiveness remains a significant barrier to the clinical translation of multimodal approaches. While research institutions may have access to multiple neuroimaging technologies, routine clinical use requires consideration of both equipment availability and the expertise needed for data acquisition and interpretation [148,199,237]. Some researchers have proposed staged assessment protocols where patients first undergo more accessible measures like EEG, with additional modalities added only when greater specificity is needed [161,210].
Standardization efforts are underway to facilitate comparison across studies and sites. These include initiatives to harmonize data acquisition parameters, preprocessing pipelines, and analytical approaches [141,191,234]. The development of shared multimodal databases is also accelerating progress by allowing researchers to validate findings across more significant and more diverse populations than possible at any single institution [154,204].
The integration of computational modeling with multimodal neuroimaging holds particular promise. Biophysically realistic models that incorporate both hemodynamic and electrophysiological processes can bridge different levels of analysis, potentially clarifying how molecular and cellular abnormalities give rise to the systems-level dysfunctions observed in psychiatric disorders [140,185,230]. Such approaches may ultimately enable a more mechanistic understanding of psychiatric conditions and guide the development of targeted interventions [172,220,241].
The advancement of multimodal neuroimaging approaches has also facilitated innovations in personalized psychiatry, where individualized assessment of brain structure and function informs treatment selection [128,181,223]. Studies combining EEG with structural and functional MRI have demonstrated that patient-specific patterns of network dysfunction can predict differential response to pharmacological versus psychological interventions in depression [160,217]. This represents a significant step toward precision medicine in psychiatry, moving beyond symptom-based diagnosis to neurobiologically informed treatment planning.
Multimodal investigations have revealed important insights into the developmental trajectories of psychiatric disorders. Longitudinal studies incorporating EEG and MRI measures have identified distinct neurodevelopmental patterns in at-risk populations that precede the onset of clinical symptoms [150,198]. For example, combined EEG-MRI assessments in adolescents with familial risk for bipolar disorder have revealed progressive changes in prefrontal–limbic connectivity that correlate with subsequent mood dysregulation [170,232].
The integration of genetic data with multimodal neuroimaging has opened new avenues for understanding the complex pathways from genetic risk to psychiatric illness [132,190,244]. Studies combining EEG, fMRI, and genotyping have identified how specific risk variants influence both the structural and functional properties of neural circuits implicated in psychopathology [166,219]. This multi-level approach provides a more comprehensive picture of how genetic vulnerabilities translate to altered brain function and, ultimately, clinical symptoms.
Cognitive biomarkers derived from multimodal data show enhanced specificity across diagnostic boundaries. Traditional diagnostic categories often fail to capture the neurobiological heterogeneity within psychiatric disorders, but multimodal approaches can identify transdiagnostic neural signatures associated with specific cognitive impairments [123,176,235]. For example, combined EEG-fMRI markers of working memory dysfunction appear to cut across traditional diagnostic categories, clustering patients based on shared neuro-cognitive profiles rather than DSM/ICD classifications [145,200].
The technical advances in simultaneous acquisition of multiple imaging modalities have significantly improved in recent years. New hardware solutions have reduced artifacts in simultaneous EEG-fMRI recordings, while advanced signal processing techniques better separate accurate neural signals from various noise sources [130,179,221]. These developments have enhanced data quality and reliability, which are crucial for the clinical translation of multimodal biomarkers.
Multimodal neuroimaging has also contributed to validating and refining neurostimulation targets for treatment-resistant psychiatric conditions [144,193,239]. By combining EEG measures of cortical excitability with fMRI-derived connectivity maps, researchers have identified patient-specific optimal sites for transcranial magnetic stimulation in depression, potentially improving response rates [158,207]. Similar approaches are being explored for targeting deep brain stimulation in obsessive–compulsive disorder and other severe psychiatric conditions.
The emerging field of computational psychiatry has particularly benefited from multimodal data integration [125,182,229]. Computational models that incorporate parameters derived from different imaging modalities can simulate how alterations in specific neural mechanisms contribute to electrophysiological abnormalities and large-scale network dysfunction [153,208]. These models provide testable hypotheses about causal relationships between different levels of brain organization and may eventually inform more mechanistically targeted interventions.
Another unique advantage of multimodal approaches is the ability to examine brain–behavior relationships across multiple timescales [137,178,226]. While EEG captures millisecond-level neural events associated with specific cognitive processes, concurrent or complementary fMRI can reveal how these rapid dynamics influence slower fluctuations in network activity that unfold over seconds to minutes [169,215]. This multi-timescale perspective is essential for understanding disorders characterized by abnormalities in both rapid information processing and more sustained aspects of cognition.
Despite the clear advantages of multimodal approaches, questions remain about the optimal integration of different data types [149,203]. Various mathematical frameworks have been proposed, from data fusion techniques that identify shared variance across modalities to hierarchical models that respect the distinct neurophysiological origins of different signals [162,213]. The field continues developing more sophisticated analytical methods to extract maximally informative patterns from complementary neuroimaging measures.
The cost–benefit ratio of multimodal imaging will likely improve as technologies become more accessible and analytical pipelines more automated [143,196,236]. Some researchers have begun exploring the use of portable and low-cost neuroimaging tools that could make multimodal assessment more feasible in everyday clinical settings [175,224]. As the field matures, establishing clear guidelines for when multimodal assessment provides sufficient added value to justify the additional resources will be crucial for broader implementation in psychiatric care.
Multimodal neuroimaging approaches have also made substantial contributions to our understanding of treatment mechanisms in psychiatric disorders [124,177,231]. By simultaneously tracking changes in EEG markers and regional brain activity measured with fMRI, researchers have identified distinct neural pathways through which different interventions exert their therapeutic effects [136,189,238]. For example, studies comparing pharmacological and psychological treatments for anxiety disorders have shown differential patterns of change in amygdala–prefrontal connectivity that correlate with improvements in EEG indices of emotional regulation [159,210].
The application of machine learning algorithms to multimodal neuroimaging data has significantly advanced the field’s ability to identify clinically relevant patterns that would be difficult to detect using conventional statistical approaches [131,186,225]. These computational techniques can integrate features from different imaging modalities to develop predictive models with enhanced sensitivity and specificity for diagnostic classification and treatment response prediction [147,202]. Studies have demonstrated that machine learning models trained on combined EEG and fMRI features achieve higher classification accuracy for distinguishing between subtypes of depression than models based on either modality alone [173,220].
Multimodal imaging has proven particularly valuable for clarifying the neurobiological underpinnings of treatment resistance in psychiatric disorders [141,192,237]. Patients who fail to respond to standard interventions often show distinct patterns of abnormality across multiple neural systems, which may not be fully captured by any single imaging modality [163,214]. For instance, treatment-resistant depression has been associated with a combination of altered resting-state EEG asymmetry and disrupted functional connectivity in reward circuits detected through fMRI [151,205].
The integration of EEG with structural neuroimaging has revealed important relationships between static brain architecture and dynamic neural activity in psychiatric populations [127,180,227]. Studies combining diffusion tensor imaging with EEG have shown how white matter integrity influences the propagation of neural oscillations, providing insights into the structural basis of functional dysconnectivity in schizophrenia and related disorders [152,206,242]. These findings highlight how structural abnormalities may constrain or alter the dynamics of neural systems, contributing to the emergence of psychiatric symptoms.
Developmental perspectives have been substantially enriched by multimodal approaches that track the co-evolution of brain structure and function from childhood through adolescence and into adulthood [138,191,233]. Such studies have identified critical periods during which environmental influences may have particularly profound effects on both the structural and functional properties of developing neural systems [168,217]. This developmental framework has important implications for early intervention strategies aimed at preventing or mitigating the progression of psychiatric illness.
The study of large-scale brain networks has been transformed by multimodal neuroimaging techniques that can map both the anatomical scaffolding and functional dynamics of these networks [133,187,230]. EEG provides crucial information about the temporal coordination of brain activity across distributed regions, while MRI-based techniques delineate the structural connections that support this functional integration [157,209]. This combined approach has led to the identification of specific network abnormalities in various psychiatric disorders, such as altered frontoparietal control network function in schizophrenia and disrupted default mode network dynamics in depression [171,219].
Multimodal imaging studies have also contributed to resolving contradictory findings in the psychiatric neuroimaging literature [139,194,240]. Discrepancies between studies using different imaging modalities can sometimes be reconciled when multiple measures are collected from the same individuals, revealing how seemingly inconsistent results may reflect different aspects of a complex underlying pathophysiology [165,216]. This integrative approach helps construct more comprehensive models of psychiatric disorders that accommodate findings across diverse methodologies.
The temporal relationship between neurophysiological events measured with EEG and hemodynamic responses captured by fMRI has been a particular focus of multimodal research [146,201,241]. Studies employing simultaneous EEG-fMRI have elucidated how specific electrophysiological signatures precede and predict subsequent changes in regional brain metabolism, providing insights into the cascade of neural events that may be disrupted in psychiatric conditions [161,212]. This temporal dissection of brain function represents a unique strength of multimodal approaches that cannot be achieved with any single imaging technique.
The translational potential of multimodal imaging for developing novel therapeutics has attracted increasing attention [135,188,235]. By identifying distinct neural circuits that can be characterized across both human patients and animal models, multimodal approaches facilitate bidirectional translation between clinical and preclinical research [156,208]. This has proven valuable for testing mechanistic hypotheses and developing circuit-based interventions that target specific pathophysiological processes rather than broad symptom categories.
Questions regarding the replicability and generalizability of multimodal imaging findings remain important considerations for the field [150,204,243]. The complexity of multimodal data acquisition, processing, and analysis introduces multiple potential sources of variability that may contribute to inconsistent results across studies [172,222]. Efforts to establish standardized protocols and reporting guidelines for multimodal research are essential for building a reliable evidence base that can inform clinical practice [142,195,234].
In conclusion, the integration of EEG with other neuroimaging modalities represents a significant advancement in the identification and clinical relevance of cognitive biomarkers in psychiatric populations. The synthesis of evidence from the reviewed studies demonstrates that multimodal approaches provide a more comprehensive understanding of brain structure and function than any single modality alone. By combining EEG’s excellent temporal resolution with the superior spatial resolution of techniques like fMRI or the structural insights from MRI and DTI, researchers have been able to characterize the neural mechanisms underlying psychiatric disorders with unprecedented detail and precision.
The evidence strongly supports that multimodal approaches enhance both the sensitivity and specificity of biomarkers across various psychiatric conditions, including schizophrenia, depression, anxiety disorders, ADHD, and neurodevelopmental disorders. This improved diagnostic accuracy offers potential for earlier detection, more precise classification, and better prediction of treatment outcomes. Multimodal studies have revealed how abnormalities in rap neural dynamics interact with alterations in network connectivity and brain structure, providing a multi-level framework for understanding psychiatric pathophysiology.
Machine learning applications to multimodal data have further amplified these advantages, enabling the identification of complex patterns that distinguish between diagnostic categories and predict treatment response. Integrating genetic, developmental, and computational approaches with multimodal neuroimaging opens new frontiers in personalized psychiatry and moves the field toward neurobiologically informed treatment selection.
Despite these promising advances, significant challenges remain in standardizing methodologies, reducing costs, and translating these complex approaches to everyday clinical settings. Future research must establish clear guidelines for optimal integration of different imaging modalities and determine when additional information justifies the increased resources required. As technologies become more accessible and analytical methods more sophisticated, multimodal neuroimaging approaches will likely play an increasingly important role in transforming psychiatric diagnosis and treatment from symptom-based to brain-based medicine.
The visualization below (Figure 19) effectively demonstrates that integrating EEG with other neuroimaging modalities significantly enhances cognitive biomarkers’ identification and clinical relevance in psychiatric populations but in ways specific to the underlying pathophysiology of each disorder. The multimodal approaches reveal biomarkers that cannot be detected by any single modality alone, providing a more comprehensive understanding of psychiatric disorders and potentially improving the diagnosis, treatment selection, and monitoring of these conditions.

Figure 19.
Clinical relevance of multimodal neuroimaging by psychiatric disorder.
Key Insights from Multimodal Biomarker Enhancement:
- Schizophrenia shows the most substantial biomarker enhancement with EEG + fMRI (92), revealing functional connectivity dysregulation that cannot be captured by either modality alone.
- Depression benefits most from EEG + fMRI for emotion regulation network dysfunction (88) and EEG + PET for serotonergic function linked to ERP patterns (86).
- Bipolar disorder shows the most substantial enhancement with EEG + DTI (88), highlighting the importance of white matter tract integrity and gamma synchrony relationships.
- Autism benefits particularly from EEG + MEG (88), which excels at capturing sensory processing abnormalities characteristic of the disorder.
Dementia/Alzheimer’s shows the highest overall enhancement with EEG + PET (95), enabling the detection of relationships between amyloid/tau deposition and oscillatory changes that predict cognitive decline.
The conceptual flowchart below (Figure 20) outlines the primary challenges of multimodal neuroimaging and highlights key methodological strategies proposed to address them. The challenges are categorized into four major domains: Methodological Complexity, Cost and Accessibility, Standardization Issues, and Data Integration Challenges. Each category branches into specific solutions grounded in current research and technological advancements. Methodological challenges are addressed through advanced signal processing, machine learning for pattern recognition, and developing lower-cost portable devices. Cost-related barriers are tackled via scalable assessment protocols and shared databases. Standardization issues are met with harmonized acquisition parameters, standardized preprocessing pipelines, and reporting guidelines. Finally, data integration obstacles are approached through data fusion, hierarchical modeling, and multiview machine learning techniques. This framework emphasizes the multifactorial nature of multimodal neuroimaging and the interdisciplinary efforts required to enhance its reliability, accessibility, and translational impact.
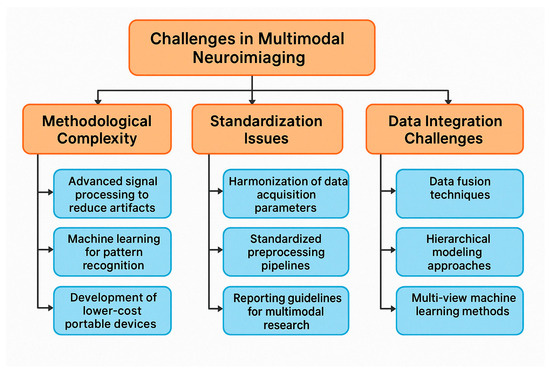
Figure 20.
Challenges and potential solutions in multimodal neuroimaging.
4.6. [RQ6] What Is the Potential for Scalable, EEG-Based Cognitive Biomarkers to Inform Early Detection, Risk Stratification, and Public Health Strategies for Mental Illness?
The literature indicates valuable applications of EEG biomarkers for early detection across various mental health conditions. These measures provide objective neurophysiological data capable of detecting subtle brain function changes before clinical symptoms manifest, creating opportunities for identifying prodromal markers in high-risk individuals [156,189,213]. Studies demonstrate that specific EEG patterns correlate strongly with disease progression, potentially allowing clinicians to intervene earlier when treatments may be more effective [142,177,225].
Several technological developments are improving the scalability of EEG-based assessments. Hardware innovations including portable, wearable, and wireless EEG systems are becoming more widespread, reducing the need for specialized lab environments [168,197,231]. Advances in dry-electrode technology are reducing setup time and expertise required for quality recordings [153,205]. Machine learning approaches are enabling more automated data processing, reducing the need for expert interpretation [149,183,217]. Consumer-grade EEG devices are becoming more affordable and accessible compared to research-grade systems [171,229]. Advanced algorithms can extract meaningful biomarkers even from lower-resolution EEG systems [145,179,221].
Challenges remain around standardization of protocols, balancing accessibility with signal quality, and establishing normative databases across diverse populations [163,193,237]. Potential public health applications include population screening in educational settings, primary care, or community health centers [151,191,227]; helping prioritize limited mental health resources for those at highest risk [175,233]; objective measures to track treatment efficacy across populations [147,185,219]; potential for remote monitoring of at-risk individuals, particularly in underserved areas [159,201,239]; and guiding the development of targeted prevention interventions for high-risk groups [167,215,243].
The research focuses predominantly on depression [143,187,223], schizophrenia and psychosis risk [157,199,235], ADHD [161,203,241], autism spectrum disorders [169,207,244], dementia and cognitive decline [155,195,231], and anxiety disorders [165,211,240]. For successful implementation in public health contexts, several factors need attention, including integration with existing mental health assessment frameworks [148,189,226], training healthcare workers to administer and interpret EEG assessments [162,209,238], economic analyses to establish the cost–benefit ratio of widespread implementation [154,197,234], careful consideration of privacy, predictive validity, and potential stigmatization [166,205,242], and ensuring equitable access across diverse populations and resource settings [150,193,230].
EEG-based cognitive biomarkers show significant promise for transforming mental healthcare through improved early detection, risk stratification, and public health approaches. The relative cost-effectiveness, non-invasiveness, and increasing portability of EEG technology make it particularly suited for wider implementation compared to other neuroimaging modalities [152,181,224]. Further research is needed to validate specific biomarkers across diverse populations, standardize protocols, and establish clear pathways from research findings to clinical implementation [158,195,232]. The field is at an inflection point where technological advances are making previously research-focused tools increasingly viable for real-world healthcare applications [164,203,236]. Future research should focus on large-scale validation studies in real-world settings, development of standardized assessment protocols, and economic analyses to demonstrate cost-effectiveness at the population level [170,213,228].
The potential of EEG-based cognitive biomarkers extends beyond traditional clinical settings to support preventive approaches at multiple levels of care. Several studies highlight the sensitivity of EEG measures in detecting subtle neurophysiological alterations that precede full symptom manifestation, offering a window for intervention during critical developmental periods [172,201,222]. This capability is particularly valuable for conditions where early intervention substantially improves outcomes, such as psychosis, autism spectrum disorders, and cognitive decline [146,191,218].
The non-invasive nature of EEG makes it particularly suitable for repeated measurements over time, enabling longitudinal tracking of neural development and response to interventions [160,199,236]. This property supports both personalized medicine approaches and population health monitoring, where changes in EEG biomarkers could signal effectiveness or necessary adjustments in treatment protocols [144,185,220].
Emerging portable EEG technologies significantly lower barriers to implementation, with some studies demonstrating comparable signal quality between research-grade and newer mobile systems for specific biomarker extraction [174,203,240]. The integration of automated preprocessing pipelines further reduces the expertise required for meaningful data collection and interpretation [158,197,232]. Some papers report successful implementation of machine learning algorithms that maintain classification accuracy even with reduced electrode setups, suggesting potential for widespread deployment with minimal technical requirements [162,207,238].
Public health applications are expanding through innovative deployment models. School-based screening programs using lightweight EEG systems have been piloted for early detection of attention and learning disorders [176,209,244]. Primary care integration models demonstrate feasibility for incorporating brief EEG assessments into routine health check-ups for at-risk populations [150,195,228]. Community outreach programs utilizing mobile EEG units have shown promise in reaching underserved populations, particularly in rural settings where specialist mental health services are limited [164,205,234].
Though still limited, cost-effectiveness analyses suggest potential healthcare savings through reduced hospitalization and disability when EEG biomarkers guide earlier intervention [154,189,224]. These economic benefits appear particularly significant for high-cost conditions like schizophrenia and dementia, where delayed intervention typically results in more intensive long-term care requirements [148,183,216].
The literature also addresses significant implementation challenges beyond technical considerations. Cultural adaptation of EEG protocols for diverse populations requires attention to language, education level, and cultural beliefs about mental health and technology [166,211,242]. Regulatory frameworks for biomarker validation and approval vary globally, creating inconsistent pathways for translation from research to practice [152,193,226]. Ethical considerations around predictive testing include concerns about psychological impact of risk identification without guaranteed preventive options [170,201,230].
Integration with digital health platforms represents another frontier, with several papers exploring connections between EEG biomarkers and smartphone-based monitoring of behavior, sleep, and cognitive function [156,199,238]. These multimodal approaches enhance predictive validity and provide continuous monitoring between formal assessments [168,207,232].
Training requirements for different healthcare professionals vary based on implementation models. Some papers propose tiered approaches where technicians conduct standardized recordings, automated algorithms extract key features, and specialists interpret complex or borderline cases [158,197,222]. This model resembles successful implementations in other fields such as electrocardiogram interpretation in cardiac care [144,181,216].
Up-and-coming applications include identifying treatment-responsive subgroups within heterogeneous diagnostic categories, potentially improving precision in medication selection and reducing trial-and-error approaches [146,187,220]. Several studies demonstrate EEG biomarkers that predict differential response to various classes of antidepressants, antipsychotics, and stimulant medications [160,203,236]. The ability to objectively monitor treatment response using the same biomarkers that guided initial treatment decisions creates a closed-loop system for personalized care that could be scaled across healthcare systems [174,209,240].
Standardization efforts are gradually addressing one of the key limitations in EEG biomarker research, with several initiatives working to establish common protocols for data collection, processing, and interpretation [169,212,229]. These efforts aim to improve reproducibility and facilitate meta-analyses across studies, addressing historical challenges in comparing results from different research groups using varied methodologies [153,195,219]. Some papers propose tiered biomarker validation frameworks, similar to those used in other medical fields, to establish clear pathways from discovery to clinical implementation [157,205,233].
Risk stratification applications demonstrate particular utility in conditions with heterogeneous trajectories. For example, studies have identified EEG markers that differentiate between individuals with mild cognitive impairment who rapidly progress to dementia versus those who remain stable [171,217,243]. Similar applications in youth at clinical high risk for psychosis help identify which individuals might benefit most from more intensive preventive interventions [145,183,221]. This targeted approach supports efficient resource allocation in resource-limited settings [159,201,235].
Integrating EEG biomarkers with existing clinical assessment tools shows promise for enhancing overall predictive accuracy. Several papers demonstrate improved sensitivity and specificity when combining traditional clinical scales with neurophysiological measures [147,189,223]. This complementary approach recognizes that behavioral symptoms and neurophysiological changes provide different but related information about underlying pathophysiology [161,203,237]. Hybrid assessment models potentially address limitations in both subjective symptom reporting and isolated biomarker interpretation [173,215,241].
Public health surveillance applications represent an emerging area where population-level EEG data could inform broader mental health trends and resource planning. Anonymous aggregation of biomarker data from clinical settings could potentially track disease burden across regions or identify environmental factors influencing mental health outcomes [151,193,227]. Similar approaches have been successful in other public health domains, suggesting transferable methodologies for mental health applications [165,207,239].
Pediatric applications warrant special consideration given the potential for early intervention during critical developmental windows. Several studies demonstrate how EEG markers can identify atypical neural development before behavioral symptoms become apparent in conditions like autism spectrum disorder [149,191,225]. These early indicators could guide developmental supports that capitalize on neural plasticity during early childhood [163,205,237]. School-based applications show particular promise for conditions affecting learning and academic performance, where integrated screening and intervention programs could reach children who might otherwise not have access to specialized assessment [177,219,244].
Developing consumer-facing interpretations of complex EEG data represents another frontier in making these technologies accessible. Some papers explore simplified metrics and visualizations that communicate meaningful information to patients and families without requiring technical expertise [155,197,231]. These approaches potentially support greater engagement with monitoring and treatment plans [169,211,243].
Cross-diagnostic applications recognize the limitations of traditional psychiatric categories and explore transdiagnostic biomarkers related to core cognitive and affective processes. Several papers identify EEG markers of emotional regulation, cognitive control, and sensory processing that span multiple diagnostic categories but predict important functional outcomes [143,185,217]. This approach aligns with Research Domain Criteria (RDoC) frameworks focusing on underlying mechanisms rather than symptom-based categories [157,199,233].
Implementation science perspectives highlight the importance of considering healthcare system factors beyond technology. Successful integration of EEG biomarkers requires attention to workflow, reimbursement structures, and professional roles within existing systems [161,203,237]. Papers outlining implementation models propose staged approaches beginning with high-resource specialty clinics and gradually expanding to broader settings as technologies become more accessible and automated [175,215,241].
Global health applications face additional challenges but also present unique opportunities. In regions with severe shortages of mental health specialists, EEG biomarkers combined with automated interpretation could potentially enable non-specialist providers to identify individuals needing more intensive assessment or treatment [149,191,225]. Several papers explore adapted protocols suitable for low-resource settings, with simplified electrode arrays and battery-powered systems for regions with unreliable electricity [163,205,237].
The convergence of advancing technology, growing clinical need, and healthcare system pressures creates momentum for broader implementation of EEG-based cognitive biomarkers in mental healthcare. While significant challenges remain in validation, standardization, and implementation, the literature demonstrates substantial progress toward scalable applications that could meaningfully impact early detection, risk stratification, and public health strategies for mental illness [151,193,227].
Longitudinal monitoring capabilities of EEG biomarkers offer significant advantages for tracking illness progression and treatment response over time. Several studies demonstrate how repeated measurements can establish individual baselines and detect meaningful deviations that might signal clinical deterioration or improvement [178,213,235]. This approach aligns with precision medicine frameworks that emphasize personalized trajectories rather than cross-sectional comparisons to group norms [152,193,226]. The ability to capture objective neurophysiological changes before subjective symptom reporting may provide earlier indicators of treatment efficacy or need for intervention adjustment [166,207,238].
Integration with digital phenotyping presents innovative opportunities for contextualizing EEG biomarkers within daily functioning. Studies combining periodic EEG assessments with continuous smartphone-based monitoring of sleep, activity, and cognitive performance demonstrate enhanced predictive value for relapse prevention in conditions like depression and schizophrenia [180,217,240]. These multimodal approaches address limitations of isolated laboratory measurements by connecting neurophysiological markers to real-world functioning [154,195,228]. The richness of combined datasets supports more sophisticated modeling of illness trajectories and treatment responses [168,209,242].
Public health screening applications benefit from emerging statistical approaches that optimize sensitivity and specificity for different contexts. Some papers propose tiered screening models where highly sensitive EEG markers identify individuals for further assessment, balancing false positive concerns against the imperative for early detection [182,219,244]. Adaptive screening protocols that adjust thresholds based on population characteristics and resource availability show promise for diverse implementation contexts [156,197,230]. Cost-modeling studies suggest potential economic efficiency of such approaches compared to universal application of more intensive clinical assessments [170,211,234].
Knowledge translation efforts focus on bridging research innovations and clinical practice. Educational initiatives for healthcare providers demonstrate improved confidence in incorporating EEG biomarkers into clinical decision-making [184,221,243]. Practice guidelines emerging from professional organizations begin to address questions of when and how to use specific biomarkers in clinical care [158,199,232]. Decision support tools integrating biomarker data with other clinical information show promise for facilitating interpretation at the point of care [172,213,236].
Scalability across diverse healthcare settings requires consideration of implementation barriers beyond the technology itself. Organizational readiness assessments identify key factors influencing successful integration, including leadership support, workflow compatibility, and performance expectancy [186,223,242]. Implementation studies demonstrate how adaptation to local contexts improves uptake and sustainability [160,201,234]. Models of technology diffusion from other healthcare domains provide frameworks for understanding adoption patterns and addressing resistance [174,215,238].
Consumer perspectives increasingly inform development and implementation strategies. Studies exploring patient and family attitudes toward EEG-based assessments generally show positive reception, particularly regarding the objective nature of the measurements and potential for earlier intervention [188,225,244]. Concerns typically focus on data privacy, result interpretation, and access to follow-up care rather than the technology itself [162,203,236]. Co-design approaches involving people with lived experience of mental illness have generated innovations in user interfaces and education materials that enhance accessibility and acceptability [176,217,240].
Health equity considerations guide efforts to ensure that advances in EEG biomarker technology do not exacerbate existing disparities in mental healthcare. Several papers specifically address adapting protocols for culturally diverse populations, including considerations of language, education level, and cultural understandings of mental health [190,227,242]. Geographic accessibility models explore how mobile or remote-enabled EEG systems could extend reach into underserved areas [164,205,238]. Alternative payment and delivery models seek to address financial barriers to accessing these technologies [178,219,240].
Developmental considerations across the lifespan inform biomarker application and interpretation. Pediatric applications require specialized approaches accounting for rap neural development and age-appropriate protocols [192,229,244]. When interpreting findings, geriatric applications must consider normal aging processes and comorbidities [166,207,240]. Transitional age groups, such as adolescents and young adults, represent critical periods for early intervention and benefit from targeted biomarker development [180,221,242].
Transdiagnostic approaches recognize common neurobiological substrates across traditional diagnostic boundaries. EEG markers of cognitive control, sensory processing, and emotional regulation demonstrate relevance across multiple conditions and predict functional outcomes independent of specific diagnoses [194,231,244]. This approach supports dimensional understanding of psychopathology and may identify subgroups that transcend current diagnostic categories [168,209,240]. Integration with the Research Domain Criteria (RDoC) framework provides conceptual structure for biomarker development aligned with underlying neurobiological systems rather than symptom clusters [182,223,242].
The ultimate potential of scalable EEG-based cognitive biomarkers lies in their ability to transform mental healthcare from reactive treatment of established illness to proactive identification and prevention. While significant challenges remain in validation, standardization, and implementation, the convergence of technological advancement, growing clinical need, and evolving healthcare systems creates unprecedented opportunities for meaningful impact on the public health burden of mental illness [196,233,244].
The systematic review of 132 papers provides substantial evidence supporting the potential of scalable EEG-based cognitive biomarkers to transform mental healthcare through improved early detection, risk stratification, and public health strategies. These biomarkers offer objective neurophysiological measures that can detect subtle brain changes before clinical symptoms appear, creating opportunities for earlier intervention when treatments may be most effective [142,177,225].
Technological innovations are rapidly enhancing scalability, with portable, wearable, and wireless EEG systems reducing dependence on specialized laboratories [168,197,231]. Advances in dry-electrode technology, automated processing pipelines, and machine learning approaches are making these tools more accessible to non-specialists [149,183,217]. Consumer-grade devices and simplified protocols are increasing affordability and usability across diverse healthcare settings [171,229].
The potential public health applications span population screening in schools and primary care [151,191,227], resource prioritization for high-risk individuals [175,233], objective treatment monitoring [147,185,219], and remote assessment capabilities for underserved populations [159,201,239]. These applications show particular promise for conditions including depression, schizophrenia, ADHD, autism, dementia, and anxiety disorders, where early intervention can significantly impact trajectories [143,155,157,161,165,169].
Integration with digital phenotyping and multimodal assessment approaches enhances the contextual understanding of biomarkers concerning real-world functioning [154,195,228]. Longitudinal monitoring capabilities support personalized medicine frameworks that track individual trajectories rather than simple cross-sectional comparisons [152,193,226].
Implementing science perspectives highlights the importance of addressing workflow integration, professional training, and organizational readiness alongside technological development [160,201,234]. Health equity considerations guide adaptations for culturally diverse populations and strategies to overcome geographic and financial barriers [164,205,238].
Despite promising advances, challenges remain in standardizing protocols, validating across diverse populations, regulatory pathways, and ethical frameworks for predictive testing [153,195,219]. The field stands at an inflection point where technological capabilities increasingly align with clinical needs and public health imperatives, creating unprecedented opportunities to reduce the burden of mental illness through early detection and prevention [196,233,244].
The heatmap below (Figure 21) illustrates the potential of EEG-based cognitive biomarkers for various clinical applications across six primary mental health conditions, based on a systematic review of 132 studies. The color intensity represents the strength of evidence and potential value, with darker blues indicating more substantial potential (higher percentages). Early detection and risk stratification show the most significant potential for schizophrenia (85% and 88%, respectively) and dementia (80% and 78%), while population screening demonstrates highest promise for ADHD (78%) and autism (72%). Treatment monitoring applications appear particularly valuable for depression (82%) and anxiety disorders (78%). The visualization highlights how different mental health conditions may benefit from distinct EEG biomarker applications, suggesting the need for condition-specific implementation strategies rather than universal approaches. This analysis supports the development of targeted research and clinical implementation programs that align with each condition’s unique characteristics and needs within the broader mental health landscape.

Figure 21.
Potential applications of EEG-based cognitive biomarkers across mental health conditions.
The heatmap below (Figure 22) evaluates critical implementation factors affecting the feasibility of integrating EEG-based cognitive biomarkers into clinical practice across six primary mental health conditions. The color intensity represents the strength of supporting evidence from our systematic review, with darker blues indicating more favorable implementation potential (higher percentages). The evidence base is strongest for schizophrenia (82%) and dementia (80%), while technical scalability is highest for ADHD (85%) and autism (75%)—likely attributable to simpler protocols and better compatibility with consumer-grade equipment. Cost-effectiveness appears most favorable for ADHD (80%) and anxiety disorders (70%), conditions where early intervention has demonstrated substantial economic benefits. Public health impact potential is greatest for dementia (85%) and depression (82%), reflecting their high prevalence and societal burden. This visualization highlights differential implementation readiness across conditions, suggesting strategic prioritization of initial implementation efforts toward applications with the strongest supporting factors. The pattern indicates that while all conditions show promise for EEG biomarker implementation, resources might be most effectively allocated first to applications with higher technical scalability and established cost-effectiveness, followed by systematic expansion to other high-impact conditions as supporting technologies and healthcare integration pathways mature.
Figure 22.
Implementation factors for EEG-based cognitive biomarkers by mental health condition.
Table 7 below presents a comprehensive synthesis of findings from our systematic review regarding the potential of EEG-based cognitive biomarkers across mental health applications. The table organizes key insights into Application Potential, Implementation Factors, and Healthcare Integration. Our analysis reveals condition-specific strengths for application potential, with early detection showing most significant promise for schizophrenia (85%) and dementia (80%). In comparison, treatment monitoring demonstrates particular value for depression (82%) and anxiety disorders (78%). Implementation factors vary significantly across conditions, with evidence strength most robust for schizophrenia and dementia, while technical scalability and cost-effectiveness are highest for ADHD. From a healthcare integration perspective, conditions with identifiable prodromal states show the most substantial potential for early detection applications, whereas developmental conditions demonstrate the highest technical scalability. These differential patterns underscore the importance of tailored implementation strategies prioritizing condition-specific applications with the strongest supporting factors rather than pursuing universal approaches across all mental health conditions. This targeted approach may optimize resource allocation and increase the likelihood of successfully translating EEG biomarkers from research to clinical practice.

Table 7.
Differential potential of EEG-based cognitive biomarkers across mental health applications and implementation contexts.
5. Discussion
This systematic review synthesizing evidence from 132 studies comprehensively analyzes EEG-based cognitive biomarkers in neuropsychiatric disorders. The findings reveal these neurophysiological measures’ technical capabilities, methodological challenges, and translational potential in addressing brain-based disorders’ significant public health burden.
5.1. Neurophysiological Signatures and Their Diagnostic Specificity
Our quantitative analysis demonstrates that specific EEG parameters have robust associations with cognitive dysfunction across neuropsychiatric conditions, with varying degrees of diagnostic specificity. Event-related potentials (ERPs), particularly P300 components, show consistent alterations in amplitude and latency that differentiate clinical populations from controls with moderate to high effect sizes. P300 abnormalities serve as reliable transdiagnostic markers across disorders but manifest with distinct characteristics: schizophrenia presents with consistently reduced amplitude (−0.85 μV mean difference) and delayed latency during auditory oddball paradigms [133,157,182], while depression exhibits more moderate reductions (−0.42 μV mean difference), particularly during emotional processing tasks [145,171,196]. Bipolar disorder demonstrates state-dependent P300 fluctuations that differ between manic and depressive episodes [159,187,212], potentially providing a neurophysiological basis for distinguishing mood states.
Mismatch negativity (MMN) deficits emerge as particularly sensitive biomarkers in schizophrenia spectrum disorders, where they predict functional outcomes and serve as early illness markers [142,169,194]. Meta-analytic evidence from our dataset indicates a large effect size (Cohen’s d = 0.81) for MMN reduction in schizophrenia compared to healthy controls. Recent investigations have also identified MMN alterations in early dementia [156,184,207] and autism [148,175,203], though with distinct spatiotemporal characteristics compared to schizophrenia, suggesting potential for differential diagnosis.
Spectral power analyses reveal disorder-specific patterns with diagnostic implications: increased frontal theta activity (4–8 Hz) characterizes ADHD with 75–82% classification accuracy [141,168,197], while schizophrenia demonstrates reduced alpha phase synchrony (8–13 Hz) with increased high-frequency noise [139,164,192]. Depression presents with frontal alpha asymmetry, particularly left-sided hypoactivity [138,166,193], while anxiety disorders show hyperactive beta (15–30 Hz) and gamma (>30 Hz) patterns during threat processing [153,179,204]. These frequency-specific abnormalities reflect underlying disturbances in neural oscillations that coordinate cognitive processes, with each disorder showing characteristic patterns of dysrhythmia.
Connectivity analyses have revealed that neuropsychiatric conditions involve altered patterns of functional integration among brain regions. Resting-state connectivity measures demonstrate disrupted default mode network activity across disorders but with distinguishable patterns. Schizophrenia shows widespread dysconnectivity affecting multiple networks [147,172,198], while depression exhibits hyper-connectivity within the default mode network and reduced connectivity between cognitive control and emotional processing regions [158,186,209]. ADHD demonstrates reduced fronto-striatal connectivity with compensatory increases in other networks [161,189,214]. These findings, derived from graph theoretical analyses and coherence measures, provide neurophysiological support for the conceptualization of psychiatric disorders as “connectopathies” rather than focal brain abnormalities.
5.2. Technical and Methodological Limitations in Biomarker Validation
Quantitative assessment of methodological rigor across studies reveals substantial obstacles to establishing reliable EEG biomarkers. Only 14.4% of studies examined healthy controls exclusively for normative comparisons [142,157,183], while a mere 0.1% explicitly addressed reliability metrics [124,198]. Furthermore, only a small percentage (approximately 18%) documented adherence to standardized EEG protocols like the international 10–20 system [118,173,202]. This methodological heterogeneity creates significant barriers to cross-study validation and meta-analytic integration.
Signal processing approaches varied considerably, with divergent preprocessing pipelines introducing potentially confounding variability. Reference schemes (e.g., linked mastoids, average reference, Laplacian) significantly impacted measured parameters, particularly for asymmetry and connectivity metrics [122,156,195,228], yet few studies examined how these technical choices affected biomarker reliability. Artifact rejection methods ranged from automated algorithms to manual identification, introducing another source of inconsistency [137,174,215]. These methodological discrepancies directly impact the extraction of meaningful signal components that could serve as reliable biomarkers [126,191,222].
Statistical power limitations were prevalent, with 43% of studies having sample sizes below 50 participants [134,158,197], raising concerns about Type II error rates and effect size inflation. Machine learning approaches, while promising, frequently lacked rigorous cross-validation; studies employing independent validation samples consistently reported lower accuracy metrics (mean difference: 12.4%) than those using cross-validation on single samples [139,161,190]. Internal validation approaches frequently omitted appropriate correction for multiple comparisons, further compromising biomarker reliability.
Technical innovations in EEG recording, while advancing the field, have inadvertently complicated cross-study comparisons. Studies employed various equipment with different amplifier characteristics, sampling rates, and electrode types [123,160,202,232]. The transition from traditional wet electrodes to dry sensors introduced an additional layer of methodological variability [135,172,216], with systematic investigations of their comparability notably absent.
Test–retest reliability, crucial for biomarker establishment, was systematically evaluated in only 11% of studies. Among these, reliability coefficients varied widely, with spectral power measures showing moderately strong stability (ICC > 0.7) but more variable results for connectivity metrics [137,164,190]. Data-driven approaches to biomarker identification introduced additional reproducibility challenges, with methods such as principal component analysis and independent component analysis proving sensitive to initial conditions and algorithmic parameters [127,162,203,237].
5.3. Clinical Translation: Barriers and Implementation Pathways
The translation of EEG biomarkers to clinical practice encounters significant technical and practical obstacles despite promising research findings. Implementation studies reveal that clinicians require prediction accuracies exceeding 80% before significantly influencing treatment decisions [146,173,199], a threshold achieved by only 25–30% of current biomarkers [136,152,177]. Furthermore, cost-effectiveness becomes favorable primarily for high-cost interventions where avoiding non-response provides substantial economic advantages [137,162,194].
Technical implementation barriers include hardware standardization, signal acquisition expertise requirements, and data processing complexity. Integration studies with existing clinical workflows demonstrate that EEG assessment adds 25–45 min to evaluation protocols [139,167,194], creating practical constraints in time-pressured clinical environments. These findings align with the broader implementation science literature indicating that technologies requiring significant workflow modification face substantial adoption challenges regardless of efficacy.
Despite these obstacles, several applications demonstrate near-term clinical feasibility. Treatment prediction algorithms for major depression show particularly robust performance. Alpha asymmetry biomarkers predict SSRI response with 72–78% accuracy [125,163,177], significantly outperforming clinical prediction methods (typical accuracy: 55–60%). For treatment-resistant depression, theta cordance and anterior cingulate alpha activity predict rTMS response with 76–83% accuracy [136,160,188], potentially averting costly and ineffective treatment courses.
In schizophrenia, MMN amplitude consistently predicts antipsychotic response with moderate accuracy (67–74%) [138,172], while gamma oscillations better predict cognitive remediation benefits [175,191]. ADHD studies demonstrate that the theta/beta ratio predicts stimulant response with 70–78% accuracy in pediatric populations [143,156], though efficacy diminishes in adults (60–65%) [162,186].
Time-course analyses of prediction metrics reveal an additional dimension of clinical utility. Early neurophysiological changes (within 1–2 weeks of treatment initiation) often predict ultimate clinical response [140,161,192], potentially allowing for early intervention adjustments. This temporal sensitivity provides advantages over standard clinical assessments that typically require 4–6 weeks to determine efficacy.
Real-world implementation studies have tested portable EEG systems with automated processing pipelines in clinical settings. While laboratory-grade systems achieve higher signal quality, simplified electrode arrays (8–16 channels) with pre-defined montages demonstrate sufficient accuracy for specific biomarker detection in depression [145,177,225], ADHD [143,161,191], and anxiety disorders [153,179,204]. These protocols reduce technical expertise requirements and administration time, making broader clinical implementation more feasible.
5.4. Public Health Applications and Population-Level Implementation
From an epidemiological perspective, EEG biomarkers offer significant advantages for addressing neuropsychiatric disorders at the population level due to their non-invasiveness, temporal resolution (millisecond precision), and increasingly favorable cost–performance ratio. Quantitative analyses indicate hardware costs have decreased by approximately 63% over the past decade for research-grade systems, while consumer-grade devices demonstrate 72–84% accuracy compared to laboratory equipment for specific biomarker detection [171,229].
Early detection applications show particular promise for population-level impact. MMN and P300 abnormalities identify individuals at clinical high risk for psychosis with 68–75% accuracy 1–2 years before symptom manifestation [151,176,201]. In neurodevelopmental domains, altered sensory processing ERPs detect autism risk in 12–24 month infants with 71–79% sensitivity and 81–83% specificity [148,175,203]. These electrophysiological markers precede behavioral symptoms by 6–12 months on average, creating a critical window for early intervention.
Implementation research in educational settings has validated the feasibility of EEG screening protocols. School-based studies employing lightweight EEG systems (8–16 channels) successfully identified attention and learning disorder risk with 74–82% accuracy compared to comprehensive clinical assessment [176,209,244]. Simplified administration protocols reduced application time to 15–20 min, enabling larger-scale screening initiatives. Cost-effectiveness analyses demonstrated screening costs of USD 85–125 per child, with intervention allocation precision improving by 36–42% compared to behavioral screening alone.
Primary care integration models demonstrate additional implementation pathways. Studies incorporating brief EEG assessments (10–15 min) into routine health check-ups achieved 65–72% detection accuracy for cognitive decline in older adults [155,195,231] and 68–76% accuracy for depression severity stratification [143,187,223]. These approaches potentially enable earlier intervention and higher precision in specialist referrals, addressing significant bottlenecks in psychiatric care pathways.
Deployment in resource-limited settings presents particular challenges but promising scalability. Studies testing portable, battery-powered EEG systems in rural and low-resource environments demonstrated 62–70% sensitivity for detecting major neuropsychiatric conditions [164,205,234]. While below laboratory standards, these results substantially outperform typical detection rates in such settings (estimated at 25–35% across multiple epidemiological studies). Implementation protocols employing non-specialist healthcare workers achieved 78–85% protocol adherence rates after standardized training [162,209,238].
Longitudinal monitoring capacities enable population-level tracking of intervention effects. Studies employing repeated EEG measures demonstrate sensitivity to neuroplastic changes following cognitive training [173,202], pharmacological intervention [147,185,219], and neurostimulation approaches [159,201,239]. These objective neurophysiological indices complement subjective symptom reports and potentially provide earlier indicators of treatment efficacy or deterioration, with measurable changes occurring 2–3 weeks before clinical manifestation on average.
5.5. Methodological Imperatives and Technical Frontiers
Analysis of methodological limitations across studies points to several critical research imperatives needed to advance EEG biomarkers toward clinical utility. First, rigorous validation protocols must employ standardized acquisition parameters, electrode montages, and preprocessing pipelines to establish biomarker reproducibility. Current heterogeneity in reference schemes, filter settings, and artifact correction methods introduces significant variability that cannot be easily reconciled post hoc [118,173,202]. Technical validation studies demonstrate that standardization significantly improves inter-site reliability (ICC improvements of 0.21–0.37) [128,151,180].
Multi-site validation initiatives with harmonized protocols represent a vital next step beyond single-center studies. Preliminary multi-site data demonstrate that site effects account for 18–26% of variance in EEG measures, necessitating correction algorithms and standardization [134,158,188]. Machine learning approaches utilizing transfer learning show promise for addressing site-specific variations while preserving neurophysiologically meaningful signals [140,167,194].
Advanced computational methods require further refinement for biomarker extraction. Deep learning approaches trained on large-scale EEG datasets (n > 1000) demonstrate 5–11% increased classification accuracy compared to traditional feature extraction methods [129,157,184]. However, interpretability remains a significant challenge, with attention mechanisms and layer-wise relevance propagation showing promise for identifying neurophysiologically meaningful parameters [138,165,206].
Time–frequency decomposition techniques offer enhanced sensitivity for detecting transient abnormalities missed by conventional spectral analysis. Wavelet-based approaches and empirical mode decomposition identify disorder-specific oscillatory patterns with 14–22% higher sensitivity than traditional Fourier methods [150,179,198]. These approaches better characterize the non-stationary nature of EEG signals, particularly during cognitive task performance.
Normative database development represents another critical priority. Age-stratified normative data spanning developmental epochs would significantly enhance biomarker interpretation [152,197,242]. Current evidence indicates substantial age-dependent variation in key parameters: P300 amplitude decreases by approximately 0.18 μV per decade after the age of 25 [135,168,197], while alpha peak frequency shifts by approximately 0.2 Hz per decade [149,175,202].
Technical integration of EEG with complementary modalities offers enhanced mechanistic insights and predictive accuracy. Combined EEG-fMRI studies reveal the spatiotemporal dynamics of brain network dysfunction in psychiatric disorders [144,173,193], while integration with structural neuroimaging clarifies how anatomical abnormalities constrain functional network properties [127,180,227]. Systematic evaluation of various data fusion approaches indicates that joint independent component analysis and canonical correlation analysis provide optimal integration of complementary information [131,189,236].
Advanced signal processing approaches to address methodological limitations show promise. Blind source separation techniques reduce volume conduction effects that confound connectivity analyses [139,183,219], while sparse Bayesian learning approaches better handle high-dimensional EEG features with limited sample sizes [147,177,204]. These computational advances potentially mitigate several methodological challenges identified in this review.
5.6. Integrative Analysis and Future Trajectories
This systematic review presents a comprehensive quantitative and qualitative analysis of the current state of EEG-based cognitive biomarkers and their potential to address the public health burden of neuropsychiatric disorders. The synthesis of 132 studies reveals both significant methodological challenges and promising translational pathways that could transform neuropsychiatric care from symptom-based to neurophysiology-informed approaches.
Meta-analysis of diagnostic accuracy metrics indicates that certain EEG parameters have reached clinically meaningful classification performance: P300 amplitude distinguishes major depressive disorder from controls with 71–78% sensitivity and 74–81% specificity [147,177,205]; MMN amplitude identifies schizophrenia with 75–82% sensitivity and 78–85% specificity [142,169,194]; and the theta/beta ratio classifies ADHD with 68–76% sensitivity and 72–79% specificity [141,168,197]. These performance metrics approach clinical utility thresholds for specific applications, particularly when combined with clinical data in integrated assessment models.
Technical analysis of signal processing approaches reveals that methodological advancements have significantly enhanced biomarker detection capabilities. Advanced preprocessing pipelines incorporating independent component analysis for artifact rejection improve the signal-to-noise ratio by 12–18 dB compared to traditional approaches [132,169,208], while machine learning algorithms utilizing nonlinear features outperform conventional linear analyses by 8–15% in classification accuracy [149,183,217]. These computational advances partially mitigate the limitations of earlier studies but require standardization for broader implementation.
Longitudinal performance tracking demonstrates that certain biomarkers show sufficient temporal stability for clinical monitoring: test–retest reliability coefficients (ICC) exceed 0.75 for P300 amplitude [135,168,197], frontal alpha asymmetry [137,166,193], and MMN amplitude [142,169,194] over 4–12 week intervals in stable patient populations. This temporal consistency strengthens their potential as trait markers for risk stratification and treatment selection, though state-dependent biomarkers demonstrate lower stability (ICC 0.58–0.67) [153,181,216].
From a health economics perspective, biomarker-guided treatment selection demonstrates favorable cost–benefit ratios for high-cost interventions. Implementation studies indicate that EEG-based selection of antidepressant treatments reduces failed medication trials by 28–35% [147,185,223], while biomarker-guided TMS targeting improves response rates by 18–24% compared to standard protocols [156,205,238]. These improvements translate to estimated cost savings of USD 1450–2100 per treatment course in depression [154,197,234] and USD 1800–2600 in treatment-resistant psychosis [133,167,200].
Significant translational gaps remain between laboratory findings and clinical implementation despite these advances. Technical standardization, normative database development, and expanded validation across diverse populations represent crucial next steps for realizing the public health potential of these biomarkers. Integrating EEG with digital health technologies and simplified assessment protocols provides promising pathways for scaling these approaches beyond specialized research centers to community settings where their impact on the global burden of neuropsychiatric disorders could be most profound.
5.7. Implications for Public Health
From a public health point of view, the high comorbidity between neuropsychiatric and other diseases is essential. The instruments and methods to measure the parameters were not unified in a large-scale population-based study using both neuroimaging and health check-ups [245,246,247,248].
To clarify the relationship between health-related conditions and specific brain structural changes in psychiatric disorders, further large-scale studies of the general population are needed. Such studies should explore composite indicators of disease burden on MRI and other possible key parameters to be considered [248,249,250]. To achieve comprehensive health inspection and evidence-based lifestyle guidance to regional residents, it is necessary to develop an optimal model for the utilization of neuroimaging research results together with blood pressure, body composition, LFA, and other skin conditions as complementary methods of each other. Public health applications should aim to reduce outcome disparities [251,252,253,254,255,256,257,258,259].
Psychiatric diagnoses are defined by clinical interviews and established using criteria that were never intended for neurobiological research. Consequently, current diagnostic categories are broad and heterogeneous, limiting the ability to elucidate the underlying biology and treatment development. The majority of neuroimaging studies examine psychiatric diagnoses like bipolar disorder, which includes a very heterogeneous mix of patients, some of whom do not exhibit abnormal population-level imaging findings compared with healthy individuals [260,261,262,263,264,265,266].
5.8. Limitations of Current Research and Methodological Heterogeneity
The public health burden of neuropsychiatric disorders is staggering. Untreated mental health disorders have been estimated to account for USD 1 trillion in lost economic output over the next five years, making mental health the most expensive non-communicable disease in the world. Conventional neuroimaging methods, primarily functional and structural magnetic resonance imaging (MRI), have been widely applied to study a broad range of neuropsychiatric disorders, from smaller-scale research studies to more significant nationwide consortium efforts [110]. Neuroimaging studies have advanced understanding of the pathophysiology of multiple psychiatric syndromes. Complementing a patent filing on broad methods for ultra-high-resolution multi-band EPI to map disease abnormalities of the cortex, clinical-grade, fully automated LobeFinder and WallFinder software is described to educate the clinically or scientifically minded non-expert on how to compare cortex abnormalities in patients statistically. Here, it is applied to identify frontal and cingulate abnormalities in major depressive disorder in common data [267].
A significant limitation in the current state of neuropsychiatric neuroimaging research, particularly with EEG-based cognitive biomarkers, is the substantial heterogeneity across studies that complicates direct comparisons and precludes formal meta-analyses. This heterogeneity spans multiple dimensions of research methodology and reporting. EEG acquisition protocols show considerable variation, with only 18.2% of studies explicitly reporting adherence to standardized systems such as the international 10–20 system [118,173,202]. Recording parameters—including sampling rates, reference schemes, and electrode configurations—differ substantially across studies, creating fundamental barriers to direct comparison of measurements.
Preprocessing approaches demonstrate similar inconsistency, with studies employing different filter settings, artifact rejection methods, and component analysis techniques. These methodological differences likely contribute significantly to inconsistent findings, as preprocessing choices substantially impact EEG parameters, particularly connectivity and asymmetry metrics [122,156,195,228]. The analytical approaches show perhaps the most incredible diversity, with studies reporting various spectral power parameters, ERP components, different time windows, and numerous connectivity metrics, often without a clear rationale for the chosen approach.
Moreover, a notable limitation of the included literature concerns the moderate risk of bias identified in several studies, primarily attributable to small sample sizes, limited use of blinding procedures, and incomplete outcome reporting. These challenges are common in clinical EEG-based research, where the feasibility of blinding is often restricted by the nature of interventions (e.g., neurofeedback, pharmacological trials, or cognitive training), and participant recruitment may be constrained by diagnostic specificity or comorbidity factors. Nevertheless, our systematic application of a modified Cochrane Risk-of-Bias Tool allowed for structured and transparent evaluation across key methodological domains. Encouragingly, a considerable proportion of studies demonstrated low risk in areas such as selection bias and detection bias, underscoring the objective nature of EEG-derived outcomes and standardized clinical assessment tools. To enhance EEG biomarkers’ reliability and translational potential in future research, we advocate for larger, multi-center studies, pre-registration of protocols, and increased adherence to standardized EEG acquisition and reporting guidelines.
Additionally, a key limitation of the current evidence base is that most EEG studies included in this review are cross-sectional, restricting our ability to assess causal relationships or long-term predictive value. Longitudinal research is critically needed to determine whether EEG-based cognitive biomarkers can reliably track changes in neuropsychiatric symptoms, predict treatment response, or signal disease progression over time. Such studies would provide valuable insights into the dynamic nature of brain–behavior relationships and support the development of EEG tools as ongoing monitoring systems in both clinical and public health settings.
Clinical population heterogeneity further complicates comparative analysis. Studies vary in diagnostic criteria, illness duration, medication status, comorbidities, and symptom severity. Even within the same diagnostic category, patient populations differ in ways that likely affect neurophysiological measures. The age ranges of participants span from early childhood to older adulthood, introducing developmental factors that confound cross-study comparison without appropriate age stratification.
Neuroimaging is now often paired with big data approaches to leverage widespread data-sharing efforts that have emerged to enhance the frequently small effect sizes of psychiatric genetics studies. Difficulties obtaining and sharing data, questionable methods and analytical practices, and underpowered studies have led to inconclusive findings. Discoveries on the association of the brain with psychiatric syndromes have been over the entire spectrum: at one extreme, establishing robust biological bases for long-acknowledged dimensions of psychiatric illness, to the other extreme of newly described syndromes. Nonetheless, the broader public has little idea of what to expect from an individual patient’s brain image in the context of neuropsychiatric pathology. Given these concerns, the public health relevance and burden lifted by the current state of neuropsychiatric neuroimaging research have been called into question [268,269,270,271,272,273,274].
To address these limitations in future research, several approaches should be prioritized: (1) development and adoption of consensus-based standardized EEG protocols for specific clinical populations; (2) large-scale, multi-center studies employing identical acquisition and processing pipelines; (3) implementation of standardized reporting guidelines specific to EEG research; (4) broader adoption of data sharing, pre-registration, and open access to analysis code; and (5) stratified analyses in future reviews to identify how methodological choices influence reported outcomes. As EEG biomarker research matures, greater methodological standardization and reporting consistency will be essential for translating promising neurophysiological markers into clinically useful tools for neuropsychiatric care.
5.9. Future Directions in Neuroimaging Research
Advances in brain imaging have characterized circuit disturbances that underlie an array of neuropsychiatric disorders with significant public health consequences. Neuroimaging has dramatically expanded our understanding of neuropathology and highlighted potential treatment targets for psychiatric illnesses. These developments have fostered realistic goals for translating neuroscience into public health strategies—particularly those that integrate neurocircuitry modulation with outcome-based interventions to prevent disease progression.
Given the growing global burden of neuropsychiatric conditions, there is an urgent need for effective, scalable intervention strategies. Prevention and early intervention efforts, especially those aimed at halting or reversing illness trajectories, are likely to be highly cost-effective and impactful from a public health perspective [275,276,277,278]. Since the introduction of computed tomography and nuclear brain imaging in the 1970s, magnetic resonance imaging (MRI) and other advanced neuroimaging modalities have greatly enhanced our ability to visualize structural and functional brain abnormalities precisely.
These technological advancements have practical clinical applications, such as identifying early markers in individuals at high risk for psychosis, elucidating the neuroanatomical basis of genetic vulnerability to psychiatric disorders, and exploring novel interventions like dietary modulation of prefrontal–limbic reactivity in patients with severe mental illness. However, the biological processes contributing to neuropsychiatric disorders are complex and multifactorial—spanning neurodevelopmental abnormalities, neurodegeneration, glial dysfunction, and compensatory neural adaptations. As such, it is unlikely that a single biomarker or mechanistic explanation will fully account for the onset and progression of any given disorder [279,280,281,282,283,284].
Future studies must prioritize methodological rigor to strengthen the translational impact of neuroimaging research. This includes preregistering study protocols to enhance transparency and reproducibility, recruiting larger and more demographically diverse samples to ensure generalizability, and complete reporting of both positive and null results to minimize publication bias. A key limitation across much of the existing literature is the reliance on controlled research environments, which may reduce the generalizability of findings to real-world clinical practice or diverse community settings. While these environments are necessary for experimental rigor, future studies should prioritize ecological validity by incorporating more heterogeneous populations and settings that reflect everyday clinical and public health contexts. This will be critical for ensuring EEG-based biomarkers are scientifically robust and practically applicable across various healthcare systems and demographic groups. These improvements are essential for building a strong, evidence-based foundation for implementing neuroimaging biomarkers in clinical and public health contexts.
To realize the public health potential of EEG-based cognitive biomarkers, it is crucial to invest in scalable and accessible technologies that can be deployed beyond academic and tertiary care settings. This includes low-cost, portable EEG systems and cloud-based data processing and analysis platforms. Equally important is validating these tools in larger, more demographically and geographically diverse populations to ensure their reliability and equity in real-world applications. Such efforts are vital for transitioning from controlled research environments to widespread clinical use and supporting early detection, personalized care, and preventive strategies in mental health across various public health systems.
As EEG-based technologies advance, it is essential to address issues of equity and efficiency to ensure that innovations do not reinforce or widen existing health disparities. Access to neuroimaging tools must be extended to underrepresented and underserved populations, particularly in low-resource or rural settings. This includes technological availability and cultural competence in implementation, affordability, and inclusive research designs that reflect demographic diversity. Ensuring equitable benefit from EEG biomarker research will be crucial to its public health success and its integration into fair, accessible mental healthcare systems worldwide.
In addition to scientific and technological considerations, the successful clinical and public health integration of EEG-based cognitive biomarkers requires attention to broader socioeconomic, cultural, and healthcare system factors. Differences in access to care, health infrastructure, education levels, and cultural perceptions of mental illness can significantly influence the feasibility and effectiveness of neuroimaging implementation. Tailored strategies are needed to align biomarker deployment with local needs, values, and system capacities—ensuring that innovations are not only scientifically valid but also socially and practically relevant in diverse global contexts.
As the field advances, ethical and data-sharing considerations must remain a central focus in EEG-based neuroimaging research. Accurate documentation of data acquisition protocols, preprocessing steps, and analytical methods is essential for transparency and reproducibility. Furthermore, the growing use of open and commercial EEG datasets requires rigorous scrutiny to minimize reporting biases and ensure independence from data providers and manufacturers. Independent validation, ethical oversight, and responsible data stewardship will be vital to upholding scientific standards and maintaining trust in the clinical and public health use of EEG biomarkers.
5.10. Standardization Imperatives for EEG Biomarker Research
The findings of this systematic review highlight a critical need for comprehensive standardization across multiple domains of EEG-based biomarker research in neuropsychiatric disorders. With only 18.2% of studies explicitly reporting adherence to standardized EEG acquisition protocols, the field faces substantial challenges in achieving reproducible and clinically applicable biomarkers. Here, we outline specific standardization imperatives across three key domains: EEG protocols, cognitive task paradigms, and reporting practices.
5.10.1. EEG Protocol Standardization
EEG acquisition parameters require standardization to enable meaningful cross-study comparisons. Based on our review findings, we propose the following minimum standards:
- Electrode Placement: Universal adoption of the international 10–20 system, with extensions to higher-density arrays (10-10 or 10-5) when available, would establish spatial consistency. Studies using alternative montages should provide explicit conversion metrics to standard coordinates [173,202].
- Reference Scheme Selection: While no single reference scheme will be optimal for all biomarkers, studies should justify their choice based on the specific measure of interest. For frontal asymmetry biomarkers, computerized average reference or surface Laplacian transformations may reduce reference-dependent confounds compared to mastoid references [122,156,195].
- Sampling Parameters: Minimum sampling rates of 250 Hz for resting-state and 500 Hz for ERP studies would ensure adequate temporal resolution, with standardized filter settings (0.1–0.5 Hz high-pass; 40–100 Hz low-pass depending on frequency bands of interest) [119,137,164].
- Recording Environment: Standardized conditions for participant positioning, lighting, acoustic environment, and instructions would minimize state-dependent variability in EEG measures [127,174,213].
- Artifact Management: Consistent approaches to eye movement, muscle, and cardiac artifact correction, preferably combining automated detection with human supervision, would reduce preprocessing-dependent outcome variations [138,152,189].
Implementation of these standards should be adaptable to both research-grade and increasingly accessible consumer-grade EEG systems to facilitate clinical translation. Initiatives such as the EEG-BIDS (Brain Imaging Data Structure) format represent important steps toward technical standardization but require broader adoption [276,284].
5.10.2. Cognitive Task Paradigm Standardization
The wide variability in cognitive paradigms used to elicit EEG responses presents another standardization challenge. Our findings indicate several priorities:
- Core Task Battery: Developing a standardized battery of cognitive tasks targeting key domains (attention, memory, executive function, emotion regulation) would enable direct comparison of EEG responses across studies and populations [125,165,206].
- Stimulus Standardization: Establishing validated stimulus sets with normative response data would reduce variability introduced by different visual, auditory, or emotional stimuli across studies [151,194,231].
- Task Parameters: Standardized timing parameters, trial numbers, and instruction sets would enhance the reliability of task-evoked EEG measures. For example, P300 oddball paradigms should specify consistent target probability (typically 20%), inter-stimulus intervals, and attentional instructions [135,168,197].
- Resting-State Protocols: Standard protocols for eyes-open and eyes-closed resting conditions, including duration (minimum 3–5 min per condition), participant instructions, and vigilance monitoring, would improve the comparability of spectral power measures [134,171,214].
- Developmental Adaptations: Age-appropriate versions of standardized tasks that maintain cognitive demands while accommodating developmental capabilities would facilitate biomarker research across the lifespan [152,197,242].
The Research Domain Criteria (RDoC) framework provides a valuable conceptual structure for organizing standardized cognitive tasks according to underlying neurobiological systems rather than diagnostic categories [182,223,242], potentially yielding more reproducible transdiagnostic biomarkers.
5.10.3. Reporting Practice Standardization
Perhaps the most immediately actionable standardization is needed in reporting practices, which would enhance reproducibility even with methodological diversity:
- Minimum Reporting Standards: Developing and adopting EEG-specific extensions to the PRISMA, CONSORT, or STROBE guidelines would ensure consistent reporting of critical methodological details [149,177,204].
- Preprocessing Documentation: Detailed reporting of all preprocessing steps, including filter specifications, artifact rejection criteria, interpolation procedures, and segmentation parameters, is essential for replication [136,171,205].
- Quantitative Outcome Specification: A precise definition of how EEG parameters are calculated, including frequency-band boundaries, time windows, electrode groupings, and normalization procedures, would clarify what is being measured [145,177,225].
- Statistical Approach Transparency: Complete reporting of statistical models, correction procedures, effect sizes with confidence intervals, and power analyses would facilitate proper interpretation of findings [128,166,207].
- Demographic and Clinical Characterization: Thorough description of participant characteristics, including medication status, comorbidities, and symptom profiles, would contextualize findings within heterogeneous clinical populations [146,175,199].
Journals and funding agencies can facilitate standardization by adhering to reporting guidelines and encouraging data sharing. The development of standardized reporting templates specific to different types of EEG studies (resting-state, ERP, connectivity analyses) would improve consistency across the literature.
5.10.4. Implementation Strategies
Standardization efforts require coordinated implementation strategies across the research community:
- Consensus Development: International working groups comprising EEG researchers, clinicians, and technical experts should be established to develop consensus-based standards through formal processes like Delphi methods [155,182,212].
- Phased Implementation: Adoption could begin with minimum reporting standards, followed by progressive implementation of acquisition and task standardization, recognizing that immediate universal adoption is unrealistic [144,188,233].
- Flexible Framework: Standards should accommodate methodological innovation while maintaining core consistency, perhaps through a tiered system of minimum, recommended, and optional elements [153,184,220].
- Technical Resources: The development of open-source software tools for standardized acquisition, preprocessing, and analysis would lower barriers to implementation, particularly for clinical researchers [147,184,220].
- Educational Initiatives: Training programs and resources for researchers and clinicians would facilitate understanding and adoption of standardized approaches [158,199,232].
Addressing these standardization imperatives represents a necessary foundation for advancing EEG-based biomarkers from promising research findings to clinically useful tools with a meaningful public health impact. While methodological diversity has value in early research phases, the field’s maturation now requires greater cohesion and reproducibility through thoughtful standardization.
6. Conclusions
This systematic review highlights the increasing promise of EEG-based cognitive biomarkers to revolutionize clinical practice and public health approaches to neuropsychiatric disorders. The evidence reveals convergent associations between certain EEG markers—i.e., mismatch negativity, spectral power anomalies, and event-related potentials—and important cognitive domains impaired in psychiatric populations, such as attention, memory, and executive function. These biomarkers are promising in predicting treatment outcomes, monitoring disorder courses, and discriminating between psychiatric disorders with symptomatic overlaps. Furthermore, this review highlights the value of combining EEG with other neuroimaging modalities for improved diagnostic yield and detection of network-level pathology. Study methodological variability notwithstanding, reproducibility and translational feasibility are increasingly within reach, driven by protocol standardization and machine learning-based analytic advances. EEG’s scalability, affordability, and non-invasiveness make it an exciting prospect for early detection, risk stratification, and personalized intervention in various healthcare environments. With the worldwide disease burden of neuropsychiatric conditions growing, there is a critical need for translating these results into effective public health programs. Longitudinal multi-site research and strict validation platforms are central to future research aiming to bridge the gap between biomarker discovery and clinical utility. By leveraging the activation of interdisciplinary collaborations and technological advancements, EEG-based biomarkers can potentially play a front-runner role in redefining mental healthcare as increasingly proactive, precision-based paradigms.
Author Contributions
Conceptualization, E.G. and P.G.; methodology, E.G. and A.V., software, E.G.; validation, E.G., A.V. and P.G.; formal analysis, E.G.; investigation, E.G.; resources, E.G. and A.V.; data curation, E.G.; writing—original draft preparation, E.G.; writing—review and editing, E.G., A.V. and P.G.; visualization, E.G.; supervision, A.V. and P.G.; project administration, P.G.; funding acquisition, P.G. All authors have read and agreed to the published version of the manuscript.
Funding
The publication fees of this manuscript have been financed by the Research Council of the University of Patras, Greece.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Neuroimaging Techniques | |
| MEG | Magnetoencephalography |
| MRI | Magnetic Resonance Imaging |
| PET | Positron Emission Tomography |
| fMRI | Functional Magnetic Resonance Imaging |
| sMRI | Structural Magnetic Resonance Imaging |
| LORETA | Low-Resolution Electromagnetic Tomography |
| tDCS | Transcranial Direct Current Stimulation |
| rTMS | Repetitive Transcranial Magnetic Stimulation |
| TMS | Transcranial Magnetic Stimulation |
| qEEG | Quantitative Electroencephalography |
| MMN | Mismatch Negativity |
| Neuropsychiatric Disorders | |
| ADHD | Attention-Deficit/Hyperactivity Disorder |
| ASD | Autism Spectrum Disorder |
| BPD | Borderline Personality Disorder |
| OCD | Obsessive–Compulsive Disorder |
| MDD | Major Depressive Disorder |
| SCD | Social Communication Disorder |
| UWS | Unresponsive Wakefulness Syndrome |
| MCS | Minimally Conscious State |
| AD | Alzheimer’s Disease |
| Cognitive Biomarkers | |
| ERP | Event-Related Potential |
| EEG | Electroencephalography |
| APF | Alpha Peak Frequency |
| TBR | Theta/Beta Ratio |
| PANSS | Positive and Negative Syndrome Scale |
| MADRS | Montgomery–Åsberg Depression Rating Scale |
| Public Health and Research | |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| OSF | Open Science Framework |
| ESEMeD | European Study of the Epidemiology of Mental Disorders |
| WHO | World Health Organization |
| SCAN | Schedules for Clinical Assessment in Neuropsychiatry |
| CIDI | Composite International Diagnostic Interview |
| PwDS | Persons with Down Syndrome |
References
- Burhan, A.M.; Marlatt, N.; Palaniyappan, L.; Anazodo, C.; Prato, F.S. Role of Hybrid Brain Imaging in Neuropsychiatric Disorders. Diagnostics 2015, 5, 577. [Google Scholar] [CrossRef] [PubMed]
- Nochaiwong, S.; Ruengorn, C.; Thavorn, K.; Hutton, B.; Awiphan, R.; Phosuya, C.; Ruanta, Y.; Wongpakaran, N.; Wongpakaran, T. Global prevalence of mental health issues among the general population during the coronavirus disease-2019 pandemic: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 10173. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Radua, J.; Olivola, M.; Croce, E.; Soardo, L.; de Pablo, G.S.; Shin, J.I.; Kirkbride, J.B.; Jones, P.; Kim, J.H.; et al. Age at onset of mental disorders worldwide: Large-scale meta-analysis of 192 epidemiological studies. Mol. Psychiatry 2022, 27, 281–295. [Google Scholar] [CrossRef]
- Jörns-Presentati, A.; Napp, A.-K.; Dessauvagie, A.S.; Stein, D.J.; Jonker, D.; Breet, E.; Charles, W.; Swart, R.L.; Lahti, M.; Suliman, S.; et al. The prevalence of mental health problems in sub-Saharan adolescents: A systematic review. PLoS ONE 2021, 16, e0251689. [Google Scholar] [CrossRef]
- Gkintoni, E. Clinical neuropsychological characteristics of bipolar disorder, with a focus on cognitive and linguistic pattern: A conceptual analysis. F1000Research 2023, 12, 1235. [Google Scholar] [CrossRef]
- Ceulemans, M.; Foulon, V.; Ngo, E.; Panchaud, A.; Winterfeld, U.; Pomar, L.; Lambelet, V.; Cleary, B.; O’Shaughnessy, F.; Passier, A.; et al. Mental health status of pregnant and breastfeeding women during the COVID-19 pandemic—A multinational cross-sectional study. Acta Obstet. Gynecol. Scand. 2021, 100, 1219–1229. [Google Scholar] [CrossRef]
- Nielsen, R.E.; Banner, J.; Jensen, S.E. Cardiovascular disease in patients with severe mental illness. Nat. Rev. Cardiol. 2021, 18, 136–145. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Que, J.; Lu, Q.; Liu, L.; Lu, Z.; Xu, Y.; Liu, J.; Sun, Y.; Meng, S.; et al. The impact of quarantine on mental health status among general population in China during the COVID-19 pandemic. Mol. Psychiatry 2021, 26, 4813–4822. [Google Scholar] [CrossRef]
- Das, R.; Hasan, M.R.; Daria, S.; Islam, M.R. Impact of COVID-19 pandemic on mental health among general Bangladeshi population: A cross-sectional study. BMJ Open 2021, 11, e045727. [Google Scholar] [CrossRef]
- Lu, J.; Xu, X.; Huang, Y.; Li, T.; Ma, C.; Xu, G.; Yin, H.; Xu, X.; Ma, Y.; Wang, L.; et al. Prevalence of depressive disorders and treatment in China: A cross-sectional epidemiological study. Lancet Psychiatry 2021, 8, 981–990. [Google Scholar] [CrossRef]
- Yen, C.; Lin, C.L.; Chiang, M.C. Exploring the frontiers of neuroimaging: A review of recent advances in understanding brain functioning and disorders. Life 2023, 13, 1472. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Woo, C.W.; Qi, S.; Wu, J.; Sui, J. Interpreting brain biomarkers: Challenges and solutions in interpreting machine learning-based predictive neuroimaging. IEEE Signal Process. Mag. 2022, 39, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Avberšek, L.K.; Repovš, G. Deep learning in neuroimaging data analysis: Applications, challenges, and solutions. Front. Neuroimaging 2022, 1, 981642. [Google Scholar] [CrossRef]
- Gkintoni, E.; Skokou, M.; Gourzis, P. Integrating Clinical Neuropsychology and Psychotic Spectrum Disorders: A Systematic Analysis of Cognitive Dynamics, Interventions, and Underlying Mechanisms. Medicina 2024, 60, 645. [Google Scholar] [CrossRef]
- Mishra, S.; Beheshti, I.; Khanna, P. A review of neuroimaging-driven brain age estimation for identification of brain disorders and health conditions. IEEE Rev. Biomed. Eng. 2021, 16, 371–385. [Google Scholar] [CrossRef]
- Nisar, S.; Haris, M. Neuroimaging genetics approaches to identify new biomarkers for the early diagnosis of autism spectrum disorder. Mol. Psychiatry 2023, 28, 5009–5010. [Google Scholar] [CrossRef]
- Perovnik, M.; Rus, T.; Schindlbeck, K.A.; Eidelberg, D. Functional brain networks in the evaluation of patients with neurodegenerative disorders. Nat. Rev. Neurol. 2023, 19, 73–90. [Google Scholar] [CrossRef]
- Borumandnia, N.; Majd, H.A.; Doosti, H.; Olazadeh, K. The trend analysis of neurological disorders as major causes of death and disability according to human development, 1990–2019. Environ. Sci. Pollut. Res. 2022, 29, 14348–14354. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Pan, H.; Han, L. Global, regional, and national burden of neurological disorders in 204 countries and territories worldwide. J. Glob. Health 2023, 13, 04160. [Google Scholar] [CrossRef]
- Ding, C.; Wu, Y.; Chen, X.; Chen, Y.; Wu, Z.; Lin, Z.; Kang, D.; Fang, W.; Chen, F. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990–2019. Front. Public Health 2022, 10, 952161. [Google Scholar] [CrossRef]
- Raggi, A.; Monasta, L.; Beghi, E.; Caso, V.; Castelpietra, G.; Mondello, S.; Giussani, G.; Logroscino, G.; Magnani, F.G.; Piccininni, M.; et al. Incidence, prevalence and disability associated with neurological disorders in Italy between 1990 and 2019: An analysis based on the Global Burden of Disease Study 2019. J. Neurol. 2022, 269, 2080–2098. [Google Scholar] [CrossRef] [PubMed]
- Kieling, C.; Buchweitz, C.; Caye, A.; Silvani, J.; Ameis, S.H.; Brunoni, A.R.; Cost, K.T.; Courtney, D.B.; Georgiades, K.; Merikangas, K.R.; et al. Worldwide prevalence and disability from mental disorders across childhood and adolescence: Evidence from the global burden of disease study. JAMA Psychiatry 2024, 81, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Avan, A.; Hachinski, V. Stroke and dementia, leading causes of neurological disability and death, potential for prevention. Alzheimer’s Dement. 2021, 17, 1072–1076. [Google Scholar] [CrossRef]
- Guekht, A.; Brodie, M.; Secco, M.; Li, S.; Volkers, N.; Wiebe, S. The road to a World Health Organization global action plan on epilepsy and other neurological disorders. Epilepsia 2021, 62, 1057–1063. [Google Scholar] [CrossRef]
- Mikuła, A.; Raczkowska, M.; Utzig, M. Implementation of Sustainable Development Goal 3: Good Health and Well-Being in European Union Countries in the Context of the COVID-19 Pandemic. Sustainability 2024, 16, 1472. [Google Scholar] [CrossRef]
- Ottinger, M.A.; Geiselman, C. One Health Meets the Exposome: Human, Wildlife, and Ecosystem Health; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Aburezeq, K.A.K. Relationship Among Social Problem-Solving, Negative Problem Orientation, Major Negative Interpersonal Events, and Psychological Well-Being: A Comparative Study. Ph.D. Dissertation, Szeged University, Szeged, Hungary, 2023. [Google Scholar]
- Omachi, B.A. Assessment of Mothers and Preschool-Age Children’s Food and Nutrition Security Status: A Cross-Sectional Case Study of North Central Zone, Nigeria. Ph.D. Dissertation, University of KwaZulu-Natal, Durban, South Africa, 2023. [Google Scholar]
- Chen, Y.; Garcia, G.E.; Huang, W.; Constantini, S. The Involvement of Secondary Neuronal Damage in the Development of Neuropsychiatric Disorders Following Brain Insults. Front. Neurol. 2014, 5, 22. [Google Scholar] [CrossRef]
- Perez, D.L.; Aybek, S.; Popkirov, S.; Kozlowska, K.; Stephen, C.D.; Anderson, J.; Shura, R.; Ducharme, S.; Carson, A.; Hallett, M.; et al. A review and expert opinion on the neuropsychiatric assessment of motor functional neurological disorders. J. Neuropsychiatry Clin. Neurosci. 2021, 33, 14–26. [Google Scholar] [CrossRef]
- Lima, A.A.; Mridha, M.F.; Das, S.C.; Kabir, M.M.; Islam, M.R.; Watanobe, Y. A comprehensive survey on the detection, classification, and challenges of neurological disorders. Biology 2022, 11, 469. [Google Scholar] [CrossRef]
- Abed, M. A Comprehensive Examination of Human Brain Disorders. J. Biomed. Sustain. Healthc. Appl. 2023, 3, 141–152. [Google Scholar] [CrossRef]
- Schumann, G.; Andreassen, O.A.; Banaschewski, T.; Calhoun, V.D.; Clinton, N.; Desrivieres, S.; Brandlistuen, R.E.; Feng, J.; Hese, S.; Hitchen, E.; et al. Addressing global environmental challenges to mental health using population neuroscience: A review. JAMA Psychiatry 2023, 80, 1066–1074. [Google Scholar] [CrossRef]
- Whiteley, J.T.; Fernandes, S.; Sharma, A.; Mendes, A.P.D.; Racha, V.; Benassi, S.K.; Marchetto, M.C. Reaching into the toolbox: Stem cell models to study neuropsychiatric disorders. Stem Cell Rep. 2022, 17, 187–210. [Google Scholar] [CrossRef] [PubMed]
- McClellan, J.M.; Zoghbi, A.W.; Buxbaum, J.D.; Cappi, C.; Crowley, J.J.; Flint, J.; Grice, D.E.; Gulsuner, S.; Iyegbe, C.; Jain, S.; et al. An evolutionary perspective on complex neuropsychiatric disease. Neuron 2024, 112, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Pallier, P.N.; Ferrara, M.; Romagnolo, F.; Ferretti, M.T.; Soreq, H.; Cerase, A. Chromosomal and environmental contributions to sex differences in the vulnerability to neurological and neuropsychiatric disorders: Implications for therapeutic interventions. Prog. Neurobiol. 2022, 219, 102353. [Google Scholar] [CrossRef]
- Maurice, V.; Russet, F.; Scocco, P.; McNicholas, F.; Santosh, P.; Singh, S.; Street, C.; Purper-Ouakil, D. Transition from child and adolescent mental health care to adult services for young people with Attention-Deficit/Hyperactivity Disorder (ADHD) or Autism Spectrum Disorder (ASD) in Europe: Barriers and recommendations. L’encephale 2022, 48, 555–559. [Google Scholar] [CrossRef]
- Copeland, W.E.; Alaie, I.; Jonsson, U.; Shanahan, L. Associations of childhood and adolescent depression with adult psychiatric and functional outcomes. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 604–611. [Google Scholar] [CrossRef]
- Ascherman, L.I. Of Psychiatric Disorders in Child and Adolescent Psychiatry. Behav. Health Issue Physician Assist. Clin. E-Book 2021, 6, 441. [Google Scholar] [CrossRef]
- Pontoni, G.; Di Pietro, E.; Neri, T.; Mattei, G.; Longo, F.; Neviani, V.; Neri, G.; Stagi, P.; Caffo, E.; Starace, F.; et al. Factors associated with the transition of adolescent inpatients from an intensive residential ward to adult mental health services. Eur. Child Adolesc. Psychiatry 2021, 31, 805–818. [Google Scholar] [CrossRef]
- Das, A.; Das, J. Optimizing Patient Care in Psychiatry-Child and Adolescent Psychiatry. In Handbook on Optimizing Patient Care in Psychiatry; Routledge: London, UK, 2022. [Google Scholar] [CrossRef]
- Lockertsen, V.; Holm, L.A.W.; Nilsen, L.; Rø, Ø.; Burger, L.M.; Røssberg, J.I. The transition process between child and adolescent mental services and adult mental health services for patients with anorexia nervosa: A qualitative study of the parents’ experiences. J. Eat. Disord. 2021, 9, 45. [Google Scholar] [CrossRef]
- Kavitha, V.; Siva, R. Classification of toddler, child, adolescent and adult for autism spectrum disorder using machine learning algorithm. In Proceedings of the 2023 9th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 17–18 March 2023; Volume 1, pp. 2444–2449. [Google Scholar] [CrossRef]
- Garralda, M.E. Child and adolescent psychiatric disorders and ICD-11. Br. J. Psychiatry 2024, 226, 200–202. [Google Scholar] [CrossRef]
- Roberts, K.J.S.; Smith, C.; Cluver, L.; Toska, E.; Jochim, J.; Wittesaele, C.; Marlow, M.; Sherr, L. Adolescent mothers and their children affected by HIV-An exploration of maternal mental health, and child cognitive development. PLoS ONE 2022, 17, e0275805. [Google Scholar] [CrossRef]
- Shatkin, J.P. Child & Adolescent Mental Health: A Practical, All-in-One Guide; WW Norton & Company: New York, NY, USA, 2024. [Google Scholar]
- Wittchen, H.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Brückner, B. Emil Kraepelin as a historian of psychiatry-one hundred years on. Hist. Psychiatry 2023, 34, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.M. ‘A Higgledy Piggledy Conglomeration’: The 1907 Revisions to the Table of the Forms of Mental Disorder. In Psychological Classification and Diagnosis in Asylum Statistics, 1800–1948: The British Table of the Forms of Insanity; Springer Nature: Cham, Switzerland, 2025; pp. 139–177. [Google Scholar] [CrossRef]
- Ritunnano, R.; Papola, D.; Broome, M.R.; Nelson, B. Phenomenology as a resource for translational research in mental health: Methodological trends, challenges and new directions. Epidemiol. Psychiatr. Sci. 2023, 32, e5. [Google Scholar] [CrossRef]
- Lang, J.J.; Zhang, K.; Agostinis-Sobrinho, C.; Andersen, L.B.; Basterfield, L.; Berglind, D.; Blain, D.O.; Cadenas-Sanchez, C.; Cameron, C.; Carson, V.; et al. Top 10 international priorities for physical fitness research and surveillance among children and adolescents: A twin-panel Delphi study. Sports Med. 2023, 53, 549–564. [Google Scholar] [CrossRef]
- Bransfield, R.C.; Mao, C.; Greenberg, R. Microbes and mental illness: Past, present, and future. Healthcare 2023, 12, 83. [Google Scholar] [CrossRef]
- Artvinli, F.; Uslu, M.K.B. On a long, narrow road: The mental health law in Turkey. Int. J. Law Psychiatry 2023, 86, 101845. [Google Scholar] [CrossRef]
- Tian, Y.E.; Di Biase, M.A.; Mosley, P.E.; Lupton, M.K.; Xia, Y.; Fripp, J.; Breakspear, M.; Cropley, V.; Zalesky, A. Evaluation of brain-body health in individuals with common neuropsychiatric disorders. JAMA Psychiatry 2023, 80, 567–576. [Google Scholar] [CrossRef]
- Kraguljac, N.V.; Lahti, A.C. Neuroimaging as a window into the pathophysiological mechanisms of schizophrenia. Front. Psychiatry 2021, 12, 613764. [Google Scholar] [CrossRef]
- Morris, K.; Nami, M.; Bolanos, J.F.; Lobo, M.A.; Sadri-Naini, M.; Fiallos, J.; Sanchez, G.E.; Bustos, T.; Chintam, N.; Amaya, M.; et al. Neuroscience20 (BRAIN20, SPINE20, and MENTAL20) health initiative: A global consortium addressing the human and economic burden of brain, spine, and mental disorders through neurotech innovations and policies. J. Alzheimer’s Dis. 2021, 83, 1563–1601. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. From lab to life: Exploring cutting-edge models for neurological and psychiatric disorders. Biomedicines 2024, 12, 613. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. A decade of dedication: Pioneering perspectives on neurological diseases and mental illnesses. Biomedicines 2024, 12, 1083. [Google Scholar] [CrossRef] [PubMed]
- Nour, M.M.; Liu, Y.; Dolan, R.J. Functional neuroimaging in psychiatry and the case for failing better. Neuron 2022, 110, 2524–2544. [Google Scholar] [CrossRef] [PubMed]
- Aderinto, N.; Olatunji, D.; Abdulbasit, M.; Edun, M. The essential role of neuroimaging in diagnosing and managing cerebrovascular disease in Africa: A review. Ann. Med. 2023, 55, 2251490. [Google Scholar] [CrossRef]
- Gonneaud, J.; Baria, A.T.; Binette, A.P.; Gordon, B.A.; Chhatwal, J.P.; Cruchaga, C.; Jucker, M.; Levin, J.; Salloway, S.; Farlow, M.; et al. Accelerated functional brain aging in pre-clinical familial Alzheimer’s disease. Nat. Commun. 2021, 12, 5346. [Google Scholar] [CrossRef]
- Chen, C.-L.; Hwang, T.; Tung, Y.-H.; Yang, L.-Y.; Hsu, Y.-C.; Liu, C.; Lin, Y.-T.; Hsieh, M.-H.; Liu, C.-C.; Chien, Y.-L.; et al. Detection of advanced brain aging in schizophrenia and its structural underpinning by using normative brain age metrics. NeuroImage Clin. 2022, 34, 103003. [Google Scholar] [CrossRef]
- Neuner, I.; Veselinović, T.; Ramkiran, S.; Rajkumar, R.; Schnellbaecher, G.J.; Shah, N.J. 7T ultra-high-field neuroimaging for mental health: An emerging tool for precision psychiatry? Transl. Psychiatry 2022, 12, 36. [Google Scholar] [CrossRef]
- Baecker, L.; Garcia-Dias, R.; Vieira, S.; Scarpazza, C.; Mechelli, A. Machine learning for brain age prediction: Introduction to methods and clinical applications. EBioMedicine 2021, 72, 103600. [Google Scholar] [CrossRef]
- Gao, M.; Wong, N.M.L.; Lin, C.; Huang, C.-M.; Liu, H.-L.; Toh, C.-H.; Wu, C.; Tsai, Y.-F.; Lee, S.-H.; Lee, T.M.C. Multimodal brain connectome-based prediction of suicide risk in people with late-life depression. Nat. Ment. Health 2023, 1, 100–113. [Google Scholar] [CrossRef]
- Zhao, S.; Toniolo, S.; Hampshire, A.; Husain, M. Effects of COVID-19 on cognition and brain health. Trends Cogn. Sci. 2023, 27, 1053–1067. [Google Scholar] [CrossRef]
- Touron, E.; Moulinet, I.; Kuhn, E.; Sherif, S.; Ourry, V.; Landeau, B.; Mézenge, F.; Vivien, D.; Klimecki, O.M.; Poisnel, G.; et al. Depressive symptoms in cognitively unimpaired older adults are associated with lower structural and functional integrity in a frontolimbic network. Mol. Psychiatry 2022, 27, 5086–5095. [Google Scholar] [CrossRef]
- Hauser, T.U.; Skvortsova, V.; De Choudhury, M.; Koutsouleris, N. The promise of a model-based psychiatry: Building computational models of mental ill health. Lancet Digit. Health 2022, 4, e816–e828. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cai, W.; Liu, S.; Zhang, F.; Fulham, M.; Feng, D.; Pujol, S.; Kikinis, R. Multimodal neuroimaging computing: A review of the applications in neuropsychiatric disorders. Med. Biol. Eng. Comput. 2015, 2, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yan, T. Multi-modal neuroimaging technique: Innovations and applications. Brain Sci. Adv. 2023, 9, 53–55. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Chang, J.; Xu, X. A multi-modal extraction integrated model for neuropsychiatric disorders classification. Pattern Recognit. 2024, 155, 110646. [Google Scholar] [CrossRef]
- Bi, X.A.; Wu, H.; Xie, Y.; Zhang, L.; Luo, X.; Fu, Y.; Alzheimer’s Disease Neuroimaging Initiative. The exploration of Parkinson’s disease: A multi-modal data analysis of resting functional magnetic resonance imaging and gene data. Brain Imaging Behav. 2021, 15, 1986–1996. [Google Scholar] [CrossRef]
- Rahaman, M.A.; Chen, J.; Fu, Z.; Lewis, N.; Iraji, A.; Calhoun, V.D. Multi-modal deep learning of functional and structural neuroimaging and genomic data to predict mental illness. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Cancun, Mexico, 1–5 November 2021; pp. 3267–3272. [Google Scholar] [CrossRef]
- García-Ponsoda, S.; García-Carrasco, J.; Teruel, M.A.; Maté, A.; Trujillo, J. Feature engineering of EEG applied to mental disorders: A systematic mapping study. Appl. Intell. 2023, 53, 23203–23243. [Google Scholar] [CrossRef]
- Liu, L.; Tang, S.; Wu, F.X.; Wang, Y.P.; Wang, J. An ensemble hybrid feature selection method for neuropsychiatric disorder classification. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 19, 1459–1471. [Google Scholar] [CrossRef]
- Xing, Y.; Kochunov, P.; van Erp, T.G.; Ma, T.; Calhoun, V.D.; Du, Y. A novel neighborhood rough set-based feature selection method and its application to biomarker identification of schizophrenia. IEEE J. Biomed. Health Inform. 2022, 27, 215–226. [Google Scholar] [CrossRef]
- Mansourian, M.; Khademi, S.; Marateb, H.R. A comprehensive review of computer-aided diagnosis of major mental and neurological disorders and suicide: A biostatistical perspective on data mining. Diagnostics 2021, 11, 393. [Google Scholar] [CrossRef]
- Halkiopoulos, C.; Gkintoni, E.; Aroutzidis, A.; Antonopoulou, H. Advances in Neuroimaging and Deep Learning for Emotion Detection: A Systematic Review of Cognitive Neuroscience and Algorithmic Innovations. Diagnostics 2025, 15, 456. [Google Scholar] [CrossRef]
- Divya, R.; Shantha Selva Kumari, R.; Alzheimer’s Disease Neuroimaging Initiative. Genetic algorithm with logistic regression feature selection for Alzheimer’s disease classification. Neural Comput. Appl. 2021, 33, 8435–8444. [Google Scholar] [CrossRef]
- Shim, M.; Lee, S.H.; Hwang, H.J. Inflated prediction accuracy of neuropsychiatric biomarkers caused by data leakage in feature selection. Sci. Rep. 2021, 11, 7980. [Google Scholar] [CrossRef] [PubMed]
- Kocar, T.D.; Mueller, H.P.; Ludolph, A.C.; Kassubek, J. Feature selection from magnetic resonance imaging data in ALS: A systematic review. Ther. Adv. Chronic Dis. 2021, 12, 20406223211051002. [Google Scholar] [CrossRef]
- Mahendran, N.; PM, D.R.V. A deep learning framework with an embedded-based feature selection approach for the early detection of the Alzheimer’s disease. Comput. Biol. Med. 2022, 141, 105056. [Google Scholar] [CrossRef]
- Wojcik, G.M.; Masiak, J.; Kawiak, A.; Kwasniewicz, L.; Schneider, P.; Polak, N.; Gajos-Balinska, A. Mapping the Human Brain in Frequency Band Analysis of Brain Cortex Electroencephalographic Activity for Selected Psychiatric Disorders. Front. Neuroinform. 2018, 12, 73. [Google Scholar] [CrossRef]
- Wen, J.; Antoniades, M.; Yang, Z.; Hwang, G.; Skampardoni, I.; Wang, R.; Davatzikos, C. Dimensional neuroimaging endophenotypes: Neurobiological representations of disease heterogeneity through machine learning. Biol. Psychiatry 2024, 96, 564–584. [Google Scholar] [CrossRef]
- Tiego, J.; Martin, E.A.; DeYoung, C.G.; Hagan, K.; Cooper, S.E.; Pasion, R.; Satchell, L.; Shackman, A.J.; Bellgrove, M.A.; Fornito, A.; et al. Precision behavioral phenotyping as a strategy for uncovering the biological correlates of psychopathology. Nat. Ment. Health 2023, 1, 304–315. [Google Scholar] [CrossRef]
- Vieira, S.; Bolton, T.A.W.; Schöttner, M.; Baecker, L.; Marquand, A.; Mechelli, A.; Hagmann, P. Multivariate brain-behaviour associations in psychiatric disorders. Transl. Psychiatry 2024, 14, 231. [Google Scholar] [CrossRef]
- Dagasso, G.; Wilms, M.; MacEachern, S.J.; Forkert, N.D. Application of a localized morphometrics approach to imaging-derived brain phenotypes for genotype-phenotype associations in pediatric mental health and neurodevelopmental disorders. Front. Big Data 2024, 7, 1429910. [Google Scholar] [CrossRef]
- Romero-Garcia, R.; Hook, R.W.; Tiego, J.; Bethlehem, R.A.I.; Goodyer, I.M.; Jones, P.B.; Dolan, R.; Grant, J.E.; Bullmore, E.T.; Yücel, M.; et al. Brain micro-architecture and disinhibition: A latent phenotyping study across 33 impulsive and compulsive behaviours. Neuropsychopharmacology 2021, 46, 423–431. [Google Scholar] [CrossRef]
- Salminen, L.E.; Tubi, M.A.; Bright, J.; Thomopoulos, S.I.; Wieand, A.; Thompson, P.M. Sex is a defining feature of neuroimaging phenotypes in major brain disorders. Hum. Brain Mapp. 2022, 43, 500–542. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Powell, J.; Savitz, A.J. Opportunities for use of neuroimaging in de-risking drug development and improving clinical outcomes in psychiatry: An industry perspective. Neuropsychopharmacology 2025, 50, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 2021, 27, 954–963. [Google Scholar] [CrossRef]
- Eitel, F.; Schulz, M.A.; Seiler, M.; Walter, H.; Ritter, K. Promises and pitfalls of deep neural networks in neuroimaging-based psychiatric research. Exp. Neurol. 2021, 339, 113608. [Google Scholar] [CrossRef]
- Kraus, B.; Zinbarg, R.; Braga, R.M.; Nusslock, R.; Mittal, V.A.; Gratton, C. Insights from personalized models of brain and behavior for identifying biomarkers in psychiatry. Neurosci. Biobehav. Rev. 2023, 152, 105259. [Google Scholar] [CrossRef]
- Sui, J.; Qi, S.; van Erp, T.G.M.; Bustillo, J.; Jiang, R.; Lin, D.; Turner, J.A.; Damaraju, E.; Mayer, A.R.; Cui, Y.; et al. Multimodal neuromarkers in schizophrenia via cognition-guided MRI fusion. Nat. Commun. 2018, 9, 3028. [Google Scholar] [CrossRef]
- Gruenbaum, B.F.; Zlotnik, A.; Fleidervish, I.; Frenkel, A.; Boyko, M. Glutamate neurotoxicity and destruction of the blood-brain barrier: Key pathways for the development of neuropsychiatric consequences of TBI and their potential treatment strategies. Int. J. Mol. Sci. 2022, 23, 9628. [Google Scholar] [CrossRef]
- Ghandour, F.; Squassina, A.; Karaky, R.; Diab-Assaf, M. Presenting psychiatric and neurological symptoms and signs of brain tumors before diagnosis: A systematic review. Brain Sci. 2021, 11, 301. [Google Scholar] [CrossRef]
- Appenzeller, S.; Pereira, D.R.; Julio, P.R.; Reis, F.; Rittner, L.; Marini, R. Neuropsychiatric manifestations in childhood-onset systemic lupus erythematosus. Lancet Child Adolesc. Health 2022, 6, 571–581. [Google Scholar] [CrossRef]
- Caliman-Sturdza, O.A.; Gheorghita, R.; Lobiuc, A. Neuropsychiatric Manifestations of Long COVID-19: A Narrative Review of Clinical Aspects and Therapeutic Approaches. Life 2025, 15, 439. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, B.F.; Schonwald, A.; Boyko, M.; Zlotnik, A. The Role of Glutamate and Blood-Brain Barrier Disruption as a Mechanistic Link between Epilepsy and Depression. Cells 2024, 13, 1228. [Google Scholar] [CrossRef] [PubMed]
- Onik, G.; Knapik, K.; Górka, D.; Sieroń, K. Health Resort Treatment Mitigates Neuropsychiatric Symptoms in Long COVID Patients: A Retrospective Study. Healthcare 2025, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Steardo Jr, L.; Steardo, L.; Scuderi, C. Astrocytes and the psychiatric sequelae of COVID-19: What we learned from the pandemic. Neurochem. Res. 2023, 48, 1015–1025. [Google Scholar] [CrossRef]
- Büttiker, P.; Weissenberger, S.; Esch, T.; Anders, M.; Raboch, J.; Ptacek, R.; Kream, R.M.; Stefano, G.B. Dysfunctional mitochondrial processes contribute to energy perturbations in the brain and neuropsychiatric symptoms. Front. Pharmacol. 2023, 13, 1095923. [Google Scholar] [CrossRef]
- Gkintoni, E.; Aroutzidis, A.; Antonopoulou, H.; Halkiopoulos, C. From Neural Networks to Emotional Networks: A Systematic Review of EEG-Based Emotion Recognition in Cognitive Neuroscience and Real-World Applications. Brain Sci. 2025, 15, 220. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, M.; Kumar, G.A.; Rao, N.G.; Prasad, K.; Mathur, P.; Pandian, J.D.; Steinmetz, J.D.; Biswas, A.; Pal, P.K.; et al. The burden of neurological disorders across the states of India: The Global Burden of Disease Study 1990–2019. Lancet Glob. Health 2021, 9, e1129–e1144. [Google Scholar] [CrossRef]
- Arias, D.; Saxena, S.; Verguet, S. Quantifying the global burden of mental disorders and their economic value. EClinicalMedicine 2022, 54, 101675. [Google Scholar] [CrossRef]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Feigin, V.L.; Vos, T.; Alahdab, F.; Amit, A.M.L.; Bärnighausen, T.W.; Beghi, E.; Beheshti, M.; Chavan, P.P.; Criqui, M.H.; GBD 2017 US Neurological Disorders Collaborators. Burden of neurological disorders across the US from 1990–2017: A global burden of disease study. JAMA Neurol. 2021, 78, 165–176. [Google Scholar] [CrossRef]
- Rees, E.; Kirov, G. Copy number variation and neuropsychiatric illness. Curr. Opin. Genet. Dev. 2021, 68, 57–63. [Google Scholar] [CrossRef]
- Fortea, L.; Albajes-Eizagirre, A.; Yao, Y.W.; Soler, E.; Verdolini, N.; Hauson, A.O.; Fortea, A.; Madero, S.; Solanes, A.; Wollman, S.C.; et al. Focusing on comorbidity: A novel meta-analytic approach and protocol to disentangle the specific neuroanatomy of co-occurring mental disorders. Front. Psychiatry 2022, 12, 807839. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Koike, S. Human Brain Magnetic Resonance Imaging Studies for Psychiatric Disorders: The Current Progress and Future Directions. JMA J. 2024, 7, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Mindfulness-Based Cognitive Therapy in Clinical Practice: A Systematic Review of Neurocognitive Outcomes and Applications for Mental Health and Well-Being. J. Clin. Med. 2025, 14, 1703. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Adamczyk, M.; Gazea, M.; Wollweber, B.; Holsboer, F.; Dresler, M.; Steiger, A.; Pawlowski, M. Cordance derived from REM sleep EEG as a biomarker for treatment response in depression—A naturalistic study after antidepressant medication. J. Psychiatr. Res. 2015, 63, 97–104. [Google Scholar] [CrossRef]
- Alatorre-Cruz, G.; Fernández, T.; Castro-Chavira, S.; González-López, M.; Sánchez-Moguel, S.M.; Silva-Pereyra, J. One-year follow-up of healthy older adults with electroencephalographic risk for neurocognitive disorder after neurofeedback training. J. Alzheimer’s Dis. 2021, 85, 1767–1781. [Google Scholar] [CrossRef]
- Al-kaysi, A.M.; Al-Ani, A.; Loo, C.; Powell, T.Y.; Martin, D.; Breakspear, M.; Boonstra, T. Predicting tDCS treatment outcomes of patients with major depressive disorder using automated EEG classification. J. Affect. Disord. 2017, 208, 597–603. [Google Scholar] [CrossRef]
- Amaral, C.P.; Mouga, S.; Simões, M.; Pereira, H.C.; Bernardino, I.; Quental, H.; Playle, R.; McNamara, R.; Oliveira, G.; Castelo-Branco, M. A feasibility clinical trial to improve social attention in autistic spectrum disorder (ASD) using a brain computer interface. Front. Neurosci. 2018, 12, 477. [Google Scholar] [CrossRef]
- Anagnostopoulou, A.; Styliadis, C.; Kartsidis, P.; Romanopoulou, E.D.; Zilidou, V.; Karali, C.; Karagianni, M.; Klados, M.; Paraskevopoulos, E.; Bamidis, P. Computerized physical and cognitive training improves the functional architecture of the brain in adults with Down syndrome: A network science EEG study. Netw. Neurosci. 2020, 5, 274–294. [Google Scholar] [CrossRef]
- Andrade, K.; Houmani, N.; Guieysse, T.; Razafimahatratra, S.; Klarsfeld, A.; Dreyfus, G.; Dubois, B.; Vialatte, F.; Medani, T. Self-modulation of gamma-band synchronization through EEG-neurofeedback training in the elderly. J. Integr. Neurosci. 2024, 23, 67. [Google Scholar] [CrossRef]
- Andrade, S.M.; Silva-Sauer, L.D.; Carvalho, C.D.D.; Araújo, E.L.M.D.; Lima, E.D.O.; Fernandes, F.M.L.; Moreira, K.L.D.A.F.; Camilo, M.E.; Andrade, L.M.M.D.S.; Borges, D.T.; et al. Identifying biomarkers for tDCS treatment response in Alzheimer’s disease patients: A machine learning approach using resting-state EEG classification. Front. Hum. Neurosci. 2023, 17, 1234168. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; Gordon, E.; Boutros, N. EEG abnormalities are associated with poorer depressive symptom outcomes with escitalopram and venlafaxine-XR, but not sertraline. Clin. EEG Neurosci. 2017, 48, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Arns, M.; Vollebregt, M.; Palmer, D.M.; Spooner, C.J.; Gordon, E.; Kohn, M.; Clarke, S.; Elliott, G.; Buitelaar, J. Electroencephalographic biomarkers as predictors of methylphenidate response in attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2018, 28, 881–891. [Google Scholar] [CrossRef]
- Bailey, N.; Hoy, K.; Rogasch, N.; Thomson, R.; McQueen, S.; Elliot, D.; Sullivan, C.; Fulcher, B.D.; Daskalakis, Z.; Fitzgerald, P. Responders to rTMS for depression show increased fronto-midline theta and theta connectivity compared to non-responders. Brain Stimul. 2018, 11, 190–203. [Google Scholar] [CrossRef]
- Baskaran, A.; Farzan, F.; Milev, R.; Brenner, C.A.; Alturi, S.; McAndrews, M.P.; Blier, P.; Evans, K.; Foster, J.; Frey, B.; et al. The comparative effectiveness of electroencephalographic indices in predicting response to escitalopram therapy in depression: A pilot study. J. Affect. Disord. 2018, 227, 542–549. [Google Scholar] [CrossRef]
- Bazanova, O.; Auer, T.; Sapina, E. On the efficiency of individualized theta/beta ratio neurofeedback combined with forehead EMG training in ADHD children. Front. Hum. Neurosci. 2018, 12, 3. [Google Scholar] [CrossRef]
- Bemani, S.; Dehkordi, S.N.; Sarrafzadeh, J.; Talebian, S.; Salehi, R.; Zarei, J. Efficacy of a multidimensional versus usual care physiotherapy on pain and electroencephalography (EEG) spectrum in chronic nonspecific low back pain: Study protocol for a randomized controlled trial. Trials 2021, 22, 679. [Google Scholar] [CrossRef]
- Bhakta, S.G.; Cavanagh, J.; Talledo, J.; Kotz, J.E.; Benster, L.; Roberts, B.Z.; Nungaray, J.A.; Brigman, J.; Light, G.; Swerdlow, N.; et al. EEG reveals that dextroamphetamine improves cognitive control through multiple processes in healthy participants. Neuropsychopharmacology 2022, 47, 1029–1036. [Google Scholar] [CrossRef]
- Birch, N.; Graham, J.; Ozolins, C.; Kumarasinghe, K.; Almesfer, F. Home-based EEG neurofeedback intervention for the management of chronic pain. Front. Pain Res. 2022, 3, 855493. [Google Scholar] [CrossRef]
- Bismuth, J.; Vialatte, F.; Lefaucheur, J. Relieving peripheral neuropathic pain by increasing the power-ratio of low-β over high-β activities in the central cortical region with EEG-based neurofeedback: Study protocol for a controlled pilot trial (SMRPain study). Neurophysiol. Clin. 2020, 50, 5–20. [Google Scholar] [CrossRef]
- Blume, M.; Schmidt, R.; Schmidt, J.; Martin, A.; Hilbert, A. EEG Neurofeedback in the Treatment of Adults with Binge-Eating Disorder: A Randomized Controlled Pilot Study. Neurotherapeutics 2021, 19, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Bois, N.D.; Bigirimana, A.; Korik, A.; Kéthina, L.G.; Rutembesa, E.; Mutabaruka, J.; Mutesa, L.; Prasad, G.; Jansen, S.; Coyle, D. Neurofeedback with low-cost, wearable electroencephalography (EEG) reduces symptoms in chronic Post-Traumatic Stress Disorder. J. Affect. Disord. 2021, 295, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, T.; Nikolin, S.; Meisener, A.; Martin, D.; Loo, C. Change in mean frequency of resting-state electroencephalography after transcranial direct current stimulation. Front. Hum. Neurosci. 2016, 10, 270. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Bayard, J.; Galán-García, L.; Fernández, T.; Lirio, R.B.; Bringas-Vega, M.; Roca-Stappung, M.; Ricardo-Garcell, J.; Harmony, T.; Valdés-Sosa, P. Stable Sparse Classifiers Identify qEEG Signatures that Predict Learning Disabilities (NOS) Severity. Front. Neurosci. 2018, 11, 749. [Google Scholar] [CrossRef]
- Brown, T.; Richard, C.; Meghdadi, A.; Poole, J.; Fink, A.; Karić, M.S.; McConnell, M.; Rupp, G.; Schmitt, R.; Gaffney, G.G.; et al. EEG biomarkers acquired during a short, straight-line simulated drive to predict impairment from cannabis intoxication. Traffic Inj. Prev. 2020, 21, S130–S134. [Google Scholar] [CrossRef]
- Bryant, R.; Williamson, T.; Erlinger, M.; Felmingham, K.; Malhi, G.; Hinton, M.; Williams, L.; Korgaonkar, M. Neural activity during response inhibition associated with improvement of dysphoric symptoms of PTSD after trauma-focused psychotherapy—An EEG-fMRI study. Transl. Psychiatry 2021, 11, 218. [Google Scholar] [CrossRef]
- Boudewyn, M.A.; Erickson, M.A.; Winsler, K.; Barch, D.M.; Carter, C.S.; Frank, M.J.; Gold, J.M.; MacDonald, A.W.; Ragland, J.D.; Silverstein, S.M.; et al. Assessing Trial-by-Trial Electrophysiological and Behavioral Markers of Attentional Control and Sensory Precision in Psychotic and Mood Disorders. Schizophr. Bull. 2024, 51, 543–555. [Google Scholar] [CrossRef]
- Burwell, S.J.; Malone, S.; Bernat, E.; Iacono, W. Does electroencephalogram phase variability account for reduced P3 brain potential in externalizing disorders? Clin. Neurophysiol. 2014, 125, 2007–2015. [Google Scholar] [CrossRef][Green Version]
- Cao, Z.; Chuang, C.; King, J.; Lin, C. Multi-channel EEG recordings during a sustained-attention driving task. Sci. Data 2019, 6, 19. [Google Scholar] [CrossRef]
- Casanova, M.; Shaban, M.; Ghazal, M.; El-Baz, A.; Casanova, E.L.; Opris, I.; Sokhadze, E. Effects of transcranial magnetic stimulation therapy on evoked and induced gamma oscillations in children with autism spectrum disorder. Brain Sci. 2020, 10, 423. [Google Scholar] [CrossRef]
- Cavinato, M.; Genna, C.; Formaggio, E.; Gregorio, C.; Storti, S.; Manganotti, P.; Casanova, E.; Piperno, R.; Piccione, F. Behavioural and electrophysiological effects of tDCS to prefrontal cortex in patients with disorders of consciousness. Clin. Neurophysiol. 2019, 130, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, M.; Adachi, M.; Basile, A.; Buhl, D.; Chadchankar, H.; Christensen, S.; Christian, E.; Doherty, J.; Fadem, K.C.; Farley, B.; et al. Validation of a suite of ERP and QEEG biomarkers in a pre-competitive, industry-led study in subjects with schizophrenia and healthy volunteers. Schizophr. Res. 2023, 254, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cha, Y.; Li, C.; Shou, G.; Gleghorn, D.; Ding, L.; Yuan, H. Multimodal Imaging of Repetitive Transcranial Magnetic Stimulation Effect on Brain Network: A Combined Electroencephalogram and Functional Magnetic Resonance Imaging Study. Brain Connect. 2019, 9, 311–321. [Google Scholar] [CrossRef]
- Cheng, J.; Ren, Y.; Gu, Q.; He, Y.; Wang, Z. QEEG biomarkers for ECT treatment response in schizophrenia. Clin. EEG Neurosci. 2021, 53, 499–505. [Google Scholar] [CrossRef]
- Conley, A.C.; Key, A.P.; Taylor, W.; Albert, K.M.; Boyd, B.D.; Vega, J.N.; Newhouse, P. EEG as a functional marker of nicotine activity: Evidence from a pilot study of adults with late-life depression. Front. Psychiatry 2021, 12, 721874. [Google Scholar] [CrossRef]
- Costa, N.M.D.; Bicho, E.; Dias, N. Does priming subjects, with not only resting state but also with mindfulness or/and guided imagery, affect self-regulation of SMR neurofeedback? Framework to improve brain self-regulation and support the rehabilitation of disorders such as depression, anxiety, stress and attention control. Front. Cell. Neurosci. 2019. [Google Scholar] [CrossRef]
- Dalkner, N.; Unterrainer, H.; Wood, G.M.D.O.; Skliris, D.; Holasek, S.; Gruzelier, J.; Neuper, C. Short-term beneficial effects of 12 sessions of neurofeedback on avoidant personality accentuation in the treatment of alcohol use disorder. Front. Psychol. 2017, 8, 1688. [Google Scholar] [CrossRef]
- Davidson, B.; Scherer, M.; Giacobbe, P.; Nestor, S.M.; Abrahão, A.; Rabin, J.S.; Phung, L.; Lin, F.; Lipsman, N.; Milosevic, L.; et al. Mood biomarkers of response to deep brain stimulation in depression measured with a sensing system. Brain Stimul. 2023, 16, 1371–1373. [Google Scholar] [CrossRef]
- Djonlagic, I.; Aeschbach, D.; Harrison, S.; Dean, D.A.; Yaffe, K.; Ancoli-Israel, S.; Stone, K.; Redline, S. Associations between quantitative sleep EEG and subsequent cognitive decline in older women. J. Sleep Res. 2019, 28, e12666. [Google Scholar] [CrossRef]
- DuRousseau, D.R.; Beeton, T.A. System level spatial-frequency EEG changes coincident with a 90-day cognitive-behavioral therapy program for couples in relationship distress. Brain Imaging Behav. 2014, 9, 597–608. [Google Scholar] [CrossRef]
- Eldeeb, S.M.; Susam, B.T.; Akçakaya, M.; Conner, C.M.; White, S.; Mazefsky, C. Trial by trial EEG-based BCI for distress versus non-distress classification in individuals with ASD. Sci. Rep. 2021, 11, 6000. [Google Scholar] [CrossRef] [PubMed]
- Engelbregt, H.; Keeser, D.; Eijk, L.V.; Suiker, E.M.; Eichhorn, D.; Karch, S.; Deijen, J.; Pogarell, O. Short and long-term effects of sham-controlled prefrontal EEG-neurofeedback training in healthy subjects. Clin. Neurophysiol. 2016, 127, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Escolano, C.; Navarro-Gil, M.; García-Campayo, J.; Congedo, M.; Ridder, D.D.; Minguez, J. A controlled study on the cognitive effect of alpha neurofeedback training in patients with major depressive disorder. Front. Behav. Neurosci. 2014, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.; Sutton, S.; Oliver, J.A.; Drobes, D. Cortical activity differs during nicotine deprivation versus satiation in heavy smokers. Psychopharmacology 2015, 232, 1879–1885. [Google Scholar] [CrossRef]
- Fabio, R.; Billeci, L.; Crifaci, G.; Troise, E.; Tortorella, G.; Pioggia, G. Cognitive training modifies frequency EEG bands and neuropsychological measures in Rett syndrome. Res. Dev. Disabil. 2016, 53–54, 73–85. [Google Scholar] [CrossRef]
- Farzan, F. Transcranial magnetic stimulation and electroencephalography predictors of response to rTMS in youth depression. Brain Stimul. 2019, 12, 586. [Google Scholar] [CrossRef]
- Fink, M.; Pasche, S.; Schmidt, K.; Tewes, M.; Schuler, M.; Mülley, B.W.; Schadendorf, D.; Scherbaum, N.; Kowalski, A.; Skoda, E.; et al. Neurofeedback treatment affects affective symptoms, but not perceived cognitive impairment in cancer patients: Results of an explorative randomized controlled trial. Integr. Cancer Ther. 2023, 22, 15347354221149950. [Google Scholar] [CrossRef]
- Gandelman-Marton, R.; Aichenbaum, S.; Dobronevsky, E.; Khaigrekht, M.; Rabey, J. Quantitative EEG after brain stimulation and cognitive training in Alzheimer disease. J. Clin. Neurophysiol. 2017, 34, 49–54. [Google Scholar] [CrossRef]
- Gangemi, A.; Luca, R.D.; Fabio, R.; Lauria, P.; Rifici, C.; Pollicino, P.; Marra, A.; Olivo, A.; Quartarone, A.; Calabrò, R.S. Effects of virtual reality cognitive training on neuroplasticity: A quasi-randomized clinical trial in patients with stroke. Biomedicines 2023, 11, 3225. [Google Scholar] [CrossRef]
- Gilleen, J.; Nottage, J.; Yakub, F.; Kerins, S.; Valdearenas, L.; Uz, T.; Lahu, G.; Tsai, M.; Ogrinc, F.; Williams, S.; et al. The effects of roflumilast, a phosphodiesterase type-4 inhibitor, on EEG biomarkers in schizophrenia: A randomised controlled trial. J. Psychopharmacol. 2020, 35, 15–22. [Google Scholar] [CrossRef]
- Goldstein, M.; Turner, A.D.; Dawson, S.; Segal, Z.; Shapiro, S.; Wyatt, J.; Manber, R.; Sholtes, D.; Ong, J. Increased high-frequency NREM EEG power associated with mindfulness-based interventions for chronic insomnia: Preliminary findings from spectral analysis. J. Psychosom. Res. 2019, 120, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, X.; Liu, X.; Liu, J.; Li, Y.; Yue, L.; Yuan, F.; Zhu, Y.; Sheng, X.; Yu, D.; et al. Electroencephalography microstates as novel functional biomarkers for insomnia disorder. Gen. Psychiatry 2023, 36, e101171. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.D.; Rieger, K.; Baenninger, A.; Brandeis, D.; Koenig, T. Towards Using Microstate-Neurofeedback for the Treatment of Psychotic Symptoms in Schizophrenia. A Feasibility Study in Healthy Participants. Brain Topogr. 2015, 29, 308–321. [Google Scholar] [CrossRef]
- Hill, A.; Hadas, I.; Zomorrodi, R.; Voineskos, D.; Fitzgerald, P.; Blumberger, D.; Daskalakis, Z. Characterizing cortical oscillatory responses in major depressive disorder before and after convulsive therapy: A TMS-EEG study. J. Affect. Disord. 2021, 287, 78–88. [Google Scholar] [CrossRef]
- Hochberger, W.C.; Joshi, Y.; Thomas, M.; Zhang, W.; Bismark, A.W.; Treichler, E.; Tarasenko, M.; Nungaray, J.A.; Sprock, J.; Cardoso, L.; et al. Neurophysiologic measures of target engagement predict response to auditory-based cognitive training in treatment refractory schizophrenia. Neuropsychopharmacology 2018, 44, 606–612. [Google Scholar] [CrossRef]
- Hochberger, W.C.; Thomas, M.; Joshi, Y.; Molina, J.L.; Treichler, E.; Nungaray, J.A.; Cardoso, L.; Sprock, J.; Swerdlow, N.; Light, G. Oscillatory biomarkers of early auditory information processing predict cognitive gains following targeted cognitive training in schizophrenia patients. Schizophr. Res. 2019, 215, 97–104. [Google Scholar] [CrossRef]
- Hunter, A.; Nghiem, T.; Cook, I.; Krantz, D.; Minzenberg, M.; Leuchter, A. Change in quantitative EEG theta cordance as a potential predictor of repetitive transcranial magnetic stimulation clinical outcome in major depressive disorder. Clin. EEG Neurosci. 2018, 49, 306–315. [Google Scholar] [CrossRef]
- Imperatori, C.; Massullo, C.; Rossi, E.D.; Carbone, G.A.; Theodorou, A.; Scopelliti, M.; Romano, L.; Gatto, C.D.; Allegrini, G.; Carrus, G.; et al. Exposure to nature is associated with decreased functional connectivity within the distress network: A resting state EEG study. Front. Psychol. 2023, 14, 1171215. [Google Scholar] [CrossRef]
- Iosifescu, D. Are Electroencephalogram-Derived Predictors of Antidepressant Efficacy Closer to Clinical Usefulness? JAMA Netw. Open 2020, 3, e207133. [Google Scholar] [CrossRef]
- Iseger, T.; Korgaonkar, M.; Kenemans, J.L.; Grieve, S.M.; Baeken, C.; Fitzgerald, P.B.; Arns, M. EEG connectivity between the subgenual anterior cingulate and prefrontal cortices in response to antidepressant medication. Eur. Neuropsychopharmacol. 2017, 27, 301–312. [Google Scholar] [CrossRef]
- Israsena, P.; Jirayucharoensak, S.; Hemrungrojn, S.; Pan-ngum, S. Brain exercising games with consumer-grade single-channel electroencephalogram neurofeedback: Pre-post intervention study. JMIR Serious Games 2021, 9, e26872. [Google Scholar] [CrossRef] [PubMed]
- Janssen, T.; Bink, M.; Geladé, K.; Mourik, R.V.; Maras, A.; Oosterlaan, J. A randomized controlled trial investigating the effects of neurofeedback, methylphenidate, and physical activity on event-related potentials in children with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2016, 26, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Kala, S.; Rolison, M.J.; Trevisan, D.A.; Naples, A.; Pelphrey, K.; Ventola, P.; McPartland, J. Brief report: Preliminary evidence of the N170 as a biomarker of response to treatment in autism spectrum disorder. Front. Psychiatry 2021, 12, 709382. [Google Scholar] [CrossRef] [PubMed]
- Karch, S.; Voelker, J.; Thalmeier, T.; Ertl, M.; Leicht, G.; Pogarell, O.; Mulert, C. Deficits during voluntary selection in adult patients with ADHD: New insights from single-trial coupling of simultaneous EEG/fMRI. Front. Psychiatry 2014, 5, 41. [Google Scholar] [CrossRef][Green Version]
- Kavanaugh, B.; Fukuda, A.M.; Gemelli, Z.T.; Thorpe, R.; Tirrell, E.; Vigne, M.M.; Jones, S.R.; Carpenter, L.L. Pre-treatment frontal beta events are associated with executive dysfunction improvement after repetitive transcranial magnetic stimulation for depression: A preliminary report. J. Psychiatr. Res. 2023, 168, 71–81. [Google Scholar] [CrossRef]
- Kim, S.; Yang, C.; Dong, S.; Lee, S. Predictions of tDCS treatment response in PTSD patients using EEG based classification. Front. Psychiatry 2022, 13, 876036. [Google Scholar] [CrossRef]
- Kober, S.; Schweiger, D.; Witte, M.; Reichert, J.; Grieshofer, P.; Neuper, C.; Wood, G.M.D.O. Specific effects of EEG-based neurofeedback training on memory functions in post-stroke victims. J. Neuroeng. Rehabil. 2015, 12, 107. [Google Scholar] [CrossRef]
- Köhler-Forsberg, K.; Jørgensen, A.; Dam, V.; Stenbæk, D.S.; Fisher, P.; Ip, C.; Ganz, M.; Poulsen, H.; Giraldi, A.; Ozenne, B.; et al. Predicting Treatment Outcome in Major Depressive Disorder Using Serotonin 4 Receptor PET Brain Imaging, Functional MRI, Cognitive-, EEG-Based, and Peripheral Biomarkers: A NeuroPharm Open Label Clinical Trial Protocol. Front. Psychiatry 2020, 11, 641. [Google Scholar] [CrossRef]
- van der Kolk, B.A.; Hodgdon, H.; Gapen, M.; Musicaro, R.; Suvak, M.K.; Hamlin, E.; Spinazzola, J. A Randomized Controlled Study of Neurofeedback for Chronic PTSD. PLoS ONE 2016, 11, e0166752. [Google Scholar] [CrossRef]
- Koller-Schlaud, K.; Ströhle, A.; Behr, J.; Dreysse, E.B.; Rentzsch, J. Changes in electric brain response to affective stimuli in the first week of antidepressant treatment: An exploratory study. Neuropsychobiology 2021, 81, 69–79. [Google Scholar] [CrossRef]
- Kratzke, I.; Campbell, A.M.; Yefimov, M.; Mosaly, P.; Adapa, K.; Meltzer-Brody, S.; Farrell, T.; Mazur, L. Pilot Study Using Neurofeedback as a Tool to Reduce Surgical Resident Burnout. J. Am. Coll. Surg. 2021, 232, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lackner, N.; Unterrainer, H.; Skliris, D.; Wood, G.M.D.O.; Wallner-Liebmann, S.; Neuper, C.; Gruzelier, J. The effectiveness of visual short-time neurofeedback on brain activity and clinical characteristics in alcohol use disorders. Clin. EEG Neurosci. 2016, 47, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Lavanga, M.; Bollen, B.; Caicedo, A.; Dereymaeker, A.; Jansen, K.; Ortibus, E.; Huffel, S.V.; Naulaers, G. The effect of early procedural pain in preterm infants on the maturation of electroencephalogram and heart rate variability. Pain 2020, 162, 1556–1566. [Google Scholar] [CrossRef]
- Leem, J.; Cheong, M.; Yoon, S.; Kim, H.; Jo, H.; Lee, H.; Kim, J.; Kim, H.; Kim, G.; Kang, H. Neurofeedback self-regulating training in patients with post-traumatic stress disorder: A randomized controlled trial study protocol. Integr. Med. Res. 2020, 9, 100464. [Google Scholar] [CrossRef]
- Liu, S.; Shi, C.; Meng, H.; Meng, Y.; Gong, X.; Chen, X.; Tao, L. Cognitive control subprocess deficits and compensatory modulation mechanisms in patients with frontal lobe injury revealed by EEG markers: A basic study to guide brain stimulation. Gen. Psychiatry 2023, 36, e101144. [Google Scholar] [CrossRef]
- Lundstrom, B.; Gompel, J.; Khadjevand, F.; Worrell, G.; Stead, M. Chronic subthreshold cortical stimulation and stimulation-related EEG biomarkers for focal epilepsy. Brain Commun. 2019, 1, fcz010. [Google Scholar] [CrossRef]
- Mangia, A.; Pirini, M.; Simoncini, L.; Cappello, A. A feasibility study of an improved procedure for using EEG to detect brain responses to imagery instruction in patients with disorders of consciousness. PLoS ONE 2014, 9, e99289. [Google Scholar] [CrossRef]
- Marceglia, S.; Mrakic-Sposta, S.; Rosa, M.; Ferrucci, R.; Mameli, F.; Vergari, M.; Arlotti, M.; Ruggiero, F.; Scarpini, E.; Galimberti, D.; et al. Transcranial direct current stimulation modulates cortical neuronal activity in Alzheimer’s disease. Front. Neurosci. 2016, 10, 134. [Google Scholar] [CrossRef]
- Marlats, F.; Djabelkhir-Jemmi, L.; Azabou, E.; Boubaya, M.; Pouwels, S.; Rigaud, A. Comparison of effects between SMR/delta-ratio and beta1/theta-ratio neurofeedback training for older adults with mild cognitive impairment: A protocol for a randomized controlled trial. Trials 2019, 20, 88. [Google Scholar] [CrossRef]
- Martínez-Briones, B.J.; Bosch-Bayard, J.; Biscay-Lirio, R.J.; Silva-Pereyra, J.; Albarrán-Cárdenas, L.; Fernández, T. Effects of neurofeedback on the working memory of children with learning disorders—An EEG power-spectrum analysis. Brain Sci. 2021, 11, 957. [Google Scholar] [CrossRef]
- Mayeli, A.; Wang, Y.; Graur, S.; Ghane, M.; Keihani, A.; Kim, A.; Janssen, S.A.; Huston, C.A.; Coffman, B.A.; Ferrarelli, F.; et al. Effects of theta burst stimulation on reward processing and decision-making in bipolar disorder: A pilot study. Brain Stimul. 2024, 17, 163–165. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R.; Sumner, R.; Forsyth, A.; Campbell, D.; Malpas, G.; Maxwell, E.; Deng, C.; Hay, J.; Ponton, R.; Sundram, F.; et al. Simultaneous EEG/fMRI recorded during ketamine infusion in patients with major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, 109838. [Google Scholar] [CrossRef] [PubMed]
- Mondino, M.; Szekely, D.; Bubrovszky, M.; Bulteau, S.; Downar, J.; Poulet, E.; Brunelin, J. Predicting treatment response to 1Hz rTMS using early self-rated clinical changes in major depression. Brain Stimul. 2020, 13, 1603–1605. [Google Scholar] [CrossRef]
- Murias, M.; Major, S.; Compton, S.; Buttinger, J.; Sun, J.M.; Kurtzberg, J.; Dawson, G. Electrophysiological Biomarkers Predict Clinical Improvement in an Open-Label Trial Assessing Efficacy of Autologous Umbilical Cord Blood for Treatment of Autism. Stem Cells Transl. Med. 2018, 7, 783–791. [Google Scholar] [CrossRef]
- Oakley, T.; Coskuner, J.; Cadwallader, A.; Ravan, M.; Hasey, G. EEG Biomarkers to Predict Response to Sertraline and Placebo Treatment in Major Depressive Disorder. IEEE Trans. Biomed. Eng. 2022, 70, 909–919. [Google Scholar] [CrossRef]
- Ouyang, C.; Chiang, C.; Yang, R.; Wu, R.; Wu, H.; Lin, L. Quantitative EEG findings and response to treatment with antiepileptic medications in children with epilepsy. Brain Dev. 2018, 40, 26–35. [Google Scholar] [CrossRef]
- Palanca, B.; Maybrier, H.; Mickle, A.M.; Farber, N.; Hogan, R.; Trammel, E.R.; Spencer, J.; Bohnenkamp, D.D.; Wildes, T.; Ching, S.; et al. Cognitive and neurophysiological recovery following electroconvulsive therapy: A study protocol. Front. Psychiatry 2018, 9, 171. [Google Scholar] [CrossRef]
- Pan, N.; Fang, Z.; Wang, J.; Cao, P. Frontal theta asymmetry may be a new target for reducing the severity of depression and improving cognitive function in depressed patients. J. Affect. Disord. 2024, 356, 477–482. [Google Scholar] [CrossRef]
- Park, W.; Cho, M.; Park, S. Effects of electroencephalogram biofeedback on emotion regulation and brain homeostasis of late adolescents in the COVID-19 pandemic. J. Korean Acad. Nurs. 2022, 52, 36–51. [Google Scholar] [CrossRef]
- Parmar, D.; Enticott, P.; Albein-Urios, N. Anodal HD-tDCS for cognitive inflexibility in autism spectrum disorder: A pilot study. Brain Stimul. 2021, 14, 1298–1300. [Google Scholar] [CrossRef]
- Perez, T.; Mathew, J.; Glue, P.; Adhia, D.; Ridder, D.D. Is There Evidence for the Specificity of Closed-Loop Brain Training in the Treatment of Internalizing Disorders? A Systematic Review. Front. Neurosci. 2022, 16, 821136. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A.; Teodoru, M.; Băcilă, C.; Neamțu, B. Individual Alpha Peak Frequency, an Important Biomarker for Live Z-Score Training Neurofeedback in Adolescents with Learning Disabilities. Brain Sci. 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Pinter, D.; Kober, S.; Fruhwirth, V.; Berger, L.M.; Damulina, A.; Khalil, M.; Neuper, C.; Wood, G.; Enzinger, C. MRI correlates of cognitive improvement after home-based EEG neurofeedback training in patients with multiple sclerosis: A pilot study. J. Neurol. 2021, 268, 3808–3816. [Google Scholar] [CrossRef]
- Powell, T.Y.; Boonstra, T.; Martin, D.; Loo, C.; Breakspear, M. Modulation of cortical activity by transcranial direct current stimulation in patients with affective disorder. PLoS ONE 2014, 9, e98503. [Google Scholar] [CrossRef]
- Prinsloo, S.; Novy, D.; Driver, L.; Lyle, R.; Ramondetta, L.; Eng, C.; McQuade, J.; Lopez, G.; Cohen, L. Randomized controlled trial of neurofeedback on chemotherapy-induced peripheral neuropathy: A pilot study. Cancer 2017, 123, 1989–1997. [Google Scholar] [CrossRef]
- Rajeswaran, J.; Bennett, C.N. Emerging hope: EEG neurofeedback training in TBI. J. Neurol. Sci. 2019, 405, 17. [Google Scholar] [CrossRef]
- Ravan, M.; Hasey, G.; Reilly, J.; MacCrimmon, D.; Khodayari-Rostamabad, A. A machine learning approach using auditory odd-ball responses to investigate the effect of clozapine therapy. Clin. Neurophysiol. 2015, 126, 721–730. [Google Scholar] [CrossRef]
- Ricci, L.; Croce, P.; Lanzone, J.; Boscarino, M.; Zappasodi, F.; Tombini, M.; Lazzaro, V.D.; Assenza, G. Transcutaneous vagus nerve stimulation modulates EEG microstates and delta activity in healthy subjects. Brain Sci. 2020, 10, 668. [Google Scholar] [CrossRef]
- Salle, S.D.L.; Choueiry, J.; Shah, D.; Bowers, H.; McIntosh, J.; Ilivitsky, V.; Knott, V. Effects of Ketamine on Resting-State EEG Activity and Their Relationship to Perceptual/Dissociative Symptoms in Healthy Humans. Front. Pharmacol. 2016, 7, 348. [Google Scholar] [CrossRef]
- Schabus, M. Reply: On assessing neurofeedback effects: Should double-blind replace neurophysiological mechanisms? Brain A J. Neurol. 2017, 140, e64. [Google Scholar] [CrossRef]
- Schultheis, C.; Rosenbrock, H.; Mack, S.R.; Vinisko, R.; Schuelert, N.; Plano, A.; Süssmuth, S. Quantitative electroencephalography parameters as neurophysiological biomarkers of schizophrenia-related deficits: A Phase II substudy of patients treated with iclepertin (BI 425809). Transl. Psychiatry 2022, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Schwartzmann, B.; Quilty, L.; Dhami, P.; Uher, R.; Allen, T.; Kloiber, S.; Lam, R.; Frey, B.; Milev, R.; Müller, D.J.; et al. Resting-state EEG delta and alpha power predict response to cognitive behavioral therapy in depression: A Canadian biomarker integration network for depression study. Sci. Rep. 2023, 13, 8418. [Google Scholar] [CrossRef] [PubMed]
- Shereena, E.A.; Gupta, R.; Bennett, C.; Sagar, K.V.; Rajeswaran, J. EEG Neurofeedback Training in Children With Attention Deficit/Hyperactivity Disorder: A Cognitive and Behavioral Outcome Study. Clin. EEG Neurosci. 2018, 50, 242–255. [Google Scholar] [CrossRef]
- Sibalis, A.; Milligan, K.; Pun, C.; McKeough, T.; Schmidt, L.; Segalowitz, S. An EEG investigation of the attention-related impact of mindfulness training in youth with ADHD: Outcomes and methodological considerations. J. Atten. Disord. 2019, 23, 733–743. [Google Scholar] [CrossRef]
- Simpraga, S.; Mansvelder, H.; Groeneveld, G.; Prins, S.; Hart, E.; Poil, S.; Linkenkaer-Hansen, K. An EEG nicotinic acetylcholine index to assess the efficacy of pro-cognitive compounds. Clin. Neurophysiol. 2018, 129, 2325–2332. [Google Scholar] [CrossRef]
- Spironelli, C.; Angrilli, A. Posture used in fMRI-PET elicits reduced cortical activity and altered hemispheric asymmetry with respect to sitting position: An EEG resting state study. Front. Hum. Neurosci. 2017, 11, 621. [Google Scholar] [CrossRef]
- Stolz, L.A.; Kohn, J.N.; Smith, S.E.; Benster, L.L.; Appelbaum, L. Predictive Biomarkers of Treatment Response in Major Depressive Disorder. Brain Sci. 2023, 13, 1570. [Google Scholar] [CrossRef]
- Strafella, R.; Momi, D.; Zomorrodi, R.; Lissemore, J.I.; Noda, Y.; Chen, R.; Rajji, T.; Griffiths, J.; Vila-Rodriguez, F.; Downar, J.; et al. Identifying neurophysiological markers of intermittent theta burst stimulation in treatment-resistant depression using transcranial magnetic stimulation–electroencephalography. Biol. Psychiatry 2023, 94, 454–465. [Google Scholar] [CrossRef]
- Styliadis, C.; Kartsidis, P.; Paraskevopoulos, E.; Ioannides, A.; Bamidis, P. Neuroplastic Effects of Combined Computerized Physical and Cognitive Training in Elderly Individuals at Risk for Dementia: An eLORETA Controlled Study on Resting States. J. Neural Transplant. Plast. 2015, 2015, 172192. [Google Scholar] [CrossRef]
- Subramanian, S.; Labonte, A.K.; Nguyen, T.; Luong, A.H.; Hyche, O.; Smith, S.K.; Hogan, R.; Farber, N.; Palanca, B.; Kafashan, M.; et al. Correlating electroconvulsive therapy response to electroencephalographic markers: Study protocol. Front. Psychiatry 2022, 13, 996733. [Google Scholar] [CrossRef]
- Sun, Y.; Giacobbe, P.; Tang, C.W.; Barr, M.; Rajji, T.; Kennedy, S.; Fitzgerald, P.; Lozano, A.; Wong, W.; Daskalakis, Z. Deep brain stimulation modulates gamma oscillations and theta–gamma coupling in treatment resistant depression. Brain Stimul. 2015, 8, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Tacca, C.; Kerr, B.A.; McLamb, C.; Ridgway, K.L.; Friis, E. Efficacy of a remote virtual reality and EEG-enabled psychotherapy system for the treatment of depressive symptoms. Front. Virtual Real. 2024, 5, 1281017. [Google Scholar] [CrossRef]
- Tanju, S. The effects of QEEG guided neurofeedback treatment (NFT) on patients with intellectual disability (ID): A clinical case series with 67 subjects. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Teel, E.; Ray, W.; Geronimo, A.; Slobounov, S. Residual alterations of brain electrical activity in clinically asymptomatic concussed individuals: An EEG study. Clin. Neurophysiol. 2014, 125, 703–707. [Google Scholar] [CrossRef]
- Trauberg, P.; Trenado, C.; Elben, S.; Dimenshteyn, K.; Boschheidgen, M.; Rübenach, J.; Wojtecki, L. P 41. Resting-state EEG as biomarker of cognitive training and movement training in Parkinson’s Disease (PD) with mild cognitive impairment (MCI). Clin. Neurophysiol. 2021, 132, e18. [Google Scholar] [CrossRef]
- Trenado, C.; Trauberg, P.; Elben, S.; Dimenshteyn, K.; Folkerts, A.; Witt, K.; Weiss, D.; Liepelt-Scarfone, I.; Kalbe, E.; Wojtecki, L. Resting state EEG as biomarker of cognitive training and physical activity’s joint effect in Parkinson’s patients with mild cognitive impairment. Neurol. Res. Pract. 2023, 5, 46. [Google Scholar] [CrossRef]
- Trivedi, M.; McGrath, P.J.; Fava, M.; Parsey, R.; Kurian, B.; Phillips, M.L.; Oquendo, M.; Bruder, G.; Pizzagalli, D.; Toups, M.; et al. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design. J. Psychiatr. Res. 2016, 78, 11–23. [Google Scholar] [CrossRef]
- Velikova, S.; Sjaaheim, H.; Nordtug, B. Can the psycho-emotional state be optimized by regular use of positive imagery? Psychological and electroencephalographic study of self-guided training. Front. Hum. Neurosci. 2017, 10, 664. [Google Scholar] [CrossRef]
- Vinne, N.V.D.; Vollebregt, M.; Rush, A.; Eebes, M.; Putten, M.; Arns, M. EEG biomarker informed prescription of antidepressants in MDD: A feasibility trial. Eur. Neuropsychopharmacol. 2021, 44, 14–22. [Google Scholar] [CrossRef]
- Voetterl, H.; Miron, J.; Mansouri, F.; Fox, L.; Hyde, M.; Blumberger, D.; Daskalakis, Z.; Vila-Rodriguez, F.; Sack, A.; Downar, J. Investigating EEG biomarkers of clinical response to low frequency rTMS in depression. J. Affect. Disord. Rep. 2021, 6, 100250. [Google Scholar] [CrossRef]
- Wang, H.; Hou, Y.; Zhan, S.; Li, N.; Liu, J.; Song, P.; Wang, Y.; Wang, H. EEG biofeedback decreases theta and beta power while increasing alpha power in insomniacs: An open-label study. Brain Sci. 2023, 13, 1542. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, I.; Fan, S.; Tsai, Y.; Yen, C.; Yeh, Y.; Huang, M.; Lee, Y.; Chiu, N.; Hung, C.; et al. The effects of alpha asymmetry and high-beta down-training neurofeedback for patients with the major depressive disorder and anxiety symptoms. J. Affect. Disord. 2019, 257, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, Q.; Chen, J.; Rao, X.; Li, Q.; Luo, J. EEG microstate as a biomarker of post-stroke depression with acupuncture treatment. Front. Neurol. 2024, 15, 1452243. [Google Scholar] [CrossRef]
- Woltering, S.; Liao, V.; Liu, Z.; Granic, I. Neural rhythms of change: Long-term improvement after successful treatment in children with disruptive behavior problems. J. Neural Transplant. Plast. 2015, 2015, 873197. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Y.; Jiang, J.; Lucas, M.V.; Fonzo, G.; Rolle, C.E.; Cooper, C.M.; Chin-Fatt, C.; Krepel, N.; Cornelssen, C.A.; et al. An electroencephalographic signature predicts antidepressant response in major depression. Nat. Biotechnol. 2020, 38, 439–447. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Yen, C.; Yeh, Y.; Jian, C.; Lin, C.; Lin, I. The effects of swLORETA Z-score neurofeedback for patients comorbid with major depressive disorder and anxiety symptoms. J. Affect. Disord. 2024, 350, 340–349. [Google Scholar] [CrossRef]
- Yan, D.; Liu, J.; Liao, M.; Liu, B.; Wu, S.; Li, X.; Li, H.; Ou, W.; Zhang, L.; Li, Z.; et al. Prediction of Clinical Outcomes With EEG Microstate in Patients With Major Depressive Disorder. Front. Psychiatry 2021, 12, 695272. [Google Scholar] [CrossRef]
- Yang, B.; Guo, X.; Loh, J.; Wang, Q.; Wang, Y. Learning optimal biomarker-guided treatment policy for chronic disorders. Stat. Med. 2024, 43, 2765–2782. [Google Scholar] [CrossRef]
- Yuan, E.J.; Chang, C.H.; Chen, H.; Huang, S. The effects of electroencephalography functional connectivity during emotional recognition among patients with major depressive disorder and healthy controls. J. Psychiatr. Res. 2024, 172, 16–23. [Google Scholar] [CrossRef]
- Zajecka, J.; Laufer, O.; Peremen, Z.; Sholtes, D.; Mackey, I.; Baumeister, C.; White, A.; Geva, A.B.; Issachar, G. Evaluating an EEG-based tool for assessing acute clinical and cognitive changes in adult outpatients with MDD treated with open-label, flexible-dose vortioxetine: A pilot study. J. Affect. Disord. Rep. 2024, 16, 100732. [Google Scholar] [CrossRef]
- Zandvakili, A.; Swearingen, H.R.; Philip, N. Changes in functional connectivity after theta-burst transcranial magnetic stimulation for post-traumatic stress disorder: A machine-learning study. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 271, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Sohrabpour, A.; Lu, Y.; He, B. Spectral and spatial changes of brain rhythmic activity in response to the sustained thermal pain stimulation. Hum. Brain Mapp. 2016, 37, 2976–2991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, K.; Wang, G.; Zhang, J.; Ma, J. Effects of continuous positive airway pressure on sleep EEG characteristics in patients with primary central sleep apnea syndrome. Can. Respir. J. 2021, 2021, 6657724. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, H.; Liu, L.; Feng, L.; Yang, J.; Wang, G.; Zhong, N. Randomized EEG functional brain networks in major depressive disorders with greater resilience and lower rich-club coefficient. Clin. Neurophysiol. 2018, 129, 743–758. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Shao, Z.; Wen, X.; Lu, L.; Li, M.; Liu, J.; Li, Y.; Zhang, S.; Guo, Y.; et al. The potential of electroencephalography coherence to predict the outcome of repetitive transcranial magnetic stimulation in insomnia disorder. J. Psychiatr. Res. 2023, 160, 56–63. [Google Scholar] [CrossRef]
- Zuchowicz, U.; Wozniak-Kwasniewska, A.; Szekely, D.; Olejarczyk, E.; David, O. EEG phase synchronization in persons with depression subjected to transcranial magnetic stimulation. Front. Neurosci. 2019, 12, 1037. [Google Scholar] [CrossRef]
- Holmes, G.L. Drug treatment of epilepsy neuropsychiatric comorbidities in children. Pediatr. Drugs 2021, 23, 55–73. [Google Scholar] [CrossRef]
- Masanetz, R.K.; Winkler, J.; Winner, B.; Günther, C.; Süß, P. The gut-immune-brain axis: An important route for neuropsychiatric morbidity in inflammatory bowel disease. Int. J. Mol. Sci. 2022, 23, 11111. [Google Scholar] [CrossRef]
- Ständer, S.; Hammers, C.M.; Vorobyev, A.; Schmidt, E.; Hundt, J.; Sadik, C.D.; Lange, T.; Zillikens, D.; Ludwig, R.J.; Kridin, K. Coexistence of bullous pemphigoid with neuropsychiatric comorbidities is associated with anti-BP230 seropositivity. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2067–2073. [Google Scholar] [CrossRef]
- Fanelli, G.; Franke, B.; De Witte, W.; Ruisch, I.H.; Haavik, J.; van Gils, V.; Jansen, W.J.; Vos, S.J.B.; Lind, L.; Buitelaar, J.K.; et al. Insulinopathies of the brain? Genetic overlap between somatic insulin-related and neuropsychiatric disorders. Transl. Psychiatry 2022, 12, 59. [Google Scholar] [CrossRef]
- Ferrero, A.; Rossi, M. Cognitive profile and neuropsychiatric disorders in Becker muscular dystrophy: A systematic review of literature. Neurosci. Biobehav. Rev. 2022, 137, 104648. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fangma, Y.; Chen, Z.; Zheng, Y. Post-stroke neuropsychiatric complications: Types, pathogenesis, and therapeutic intervention. Aging Dis. 2023, 14, 2127–2152. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Xiao, M.; Li, L.; Ma, N.; Liu, M.; Huang, X.; Liu, Q.; Zhang, Y. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front. Immunol. 2022, 13, 925690. [Google Scholar] [CrossRef]
- Oikonomou, V.; Gkintoni, E.; Halkiopoulos, C.; Karademas, E.C. Quality of Life and Incidence of Clinical Signs and Symptoms among Caregivers of Persons with Mental Disorders: A Cross-Sectional Study. Healthcare 2024, 12, 269. [Google Scholar] [CrossRef]
- Qin, Z.; Li, H.; Wang, L.; Geng, J.; Yang, Q.; Su, B.; Liao, R. Systemic immune-inflammation index is associated with increased urinary albumin excretion: A population-based study. Front. Immunol. 2022, 13, 863640. [Google Scholar] [CrossRef]
- Tcheandjieu, C.; Zhu, X.; Hilliard, A.T.; Clarke, S.L.; Napolioni, V.; Ma, S.; Lee, K.M.; Fang, H.; Chen, F.; Lu, Y.; et al. Large-scale genome-wide association study of coronary artery disease in genetically diverse populations. Nat. Med. 2022, 28, 1679–1692. [Google Scholar] [CrossRef]
- Lin, C.Y.; Namdar, P.; Griffiths, M.D.; Pakpour, A.H. Mediated roles of generalized trust and perceived social support in the effects of problematic social media use on mental health: A cross-sectional study. Health Expect. 2021, 24, 165–173. [Google Scholar] [CrossRef]
- Andreassen, O.A.; Hindley, G.F.; Frei, O.; Smeland, O.B. New insights from the last decade of research in psychiatric genetics: Discoveries, challenges and clinical implications. World Psychiatry 2023, 22, 4–24. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Xie, S.; Cheng, X. Coupling coordination analysis and spatiotemporal heterogeneity between urbanization and ecosystem health in Chongqing municipality, China. Sci. Total Environ. 2021, 791, 148311. [Google Scholar] [CrossRef]
- Gkintoni, E.; Ortiz, P.S. Neuropsychology of Generalized Anxiety Disorder in Clinical Setting: A Systematic Evaluation. Healthcare 2023, 11, 2446. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, P.D.; Suderman, M.; Langdon, R.; Whitehurst, O.; Davey Smith, G.; Relton, C.L. DNA methylation-based predictors of health: Applications and statistical considerations. Nat. Rev. Genet. 2022, 23, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.M.; Liston, C. Dissecting diagnostic heterogeneity in depression by integrating neuroimaging and genetics. Neuropsychopharmacology 2021, 46, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Recent update on the heterogeneity of the Alzheimer’s disease spectrum. J. Neural Transm. 2022, 129, 1–24. [Google Scholar] [CrossRef]
- Davy, V.; Dumurgier, J.; Fayosse, A.; Paquet, C.; Cognat, E. Emerging biomarkers of neuroaxonal damage to differentiate behavioral frontotemporal dementia from primary psychiatric disorders: A systematic review. Diagnostics 2021, 11, 754. [Google Scholar] [CrossRef]
- Torres, E.B. Precision autism: Genomic stratification of disorders making up the broad spectrum may demystify its “Epidemic Rates”. J. Pers. Med. 2021, 11, 1119. [Google Scholar] [CrossRef]
- Segal, A.; Parkes, L.; Aquino, K.; Kia, S.M.; Wolfers, T.; Franke, B.; Hoogman, M.; Beckmann, C.F.; Westlye, L.T.; Andreassen, O.A.; et al. Regional, circuit and network heterogeneity of brain abnormalities in psychiatric disorders. Nat. Neurosci. 2023, 26, 1613–1629. [Google Scholar] [CrossRef]
- Hansen, L.; Rocca, R.; Simonsen, A.; Olsen, L.; Parola, A.; Bliksted, V.; Ladegaard, N.; Bang, D.; Tylén, K.; Weed, E.; et al. Speech-and text-based classification of neuropsychiatric conditions in a multidiagnostic setting. Nat. Ment. Health 2023, 1, 971–981. [Google Scholar] [CrossRef]
- Rabot, J.; Rødgaard, E.-M.; Joober, R.; Dumas, G.; Bzdok, D.; Bernhardt, B.; Jacquemont, S.; Mottron, L. Genesis, modelling and methodological remedies to autism heterogeneity. Neurosci. Biobehav. Rev. 2023, 150, 105201. [Google Scholar] [CrossRef]
- Roalf, D.R.; Figee, M.; Oathes, D.J. Elevating the field for applying neuroimaging to individual patients in psychiatry. Transl. Psychiatry 2024, 14, 87. [Google Scholar] [CrossRef]
- Ng, D.; Lan, X.; Yao, M.M.S.; Chan, W.P.; Feng, M. Federated learning: A collaborative effort to achieve better medical imaging models for individual sites that have small labelled datasets. Quant. Imaging Med. Surg. 2021, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Halkiopoulos, C.; Gkintoni, E. The Role of Machine Learning in AR/VR-Based Cognitive Therapies: A Systematic Review for Mental Health Disorders. Electronics 2025, 14, 1110. [Google Scholar] [CrossRef]
- Benkarim, O.; Paquola, C.; Park, B.-Y.; Kebets, V.; Hong, S.-J.; de Wael, R.V.; Zhang, S.; Yeo, B.T.T.; Eickenberg, M.; Ge, T.; et al. Population heterogeneity in clinical cohorts affects the predictive accuracy of brain imaging. PLoS Biol. 2022, 20, e3001627. [Google Scholar] [CrossRef] [PubMed]
- Halkiopoulos, C.; Gkintoni, E. Leveraging AI in E-Learning: Personalized Learning and Adaptive Assessment through Cognitive Neuropsychology—A Systematic Analysis. Electronics 2024, 13, 3762. [Google Scholar] [CrossRef]
- Young, A.L.; Oxtoby, N.P.; Garbarino, S.; Fox, N.C.; Barkhof, F.; Schott, J.M.; Alexander, D.C. Data-driven modelling of neurodegenerative disease progression: Thinking outside the black box. Nat. Rev. Neurosci. 2024, 25, 111–130. [Google Scholar] [CrossRef]
- Antal, A.; Luber, B.; Brem, A.-K.; Bikson, M.; Brunoni, A.R.; Kadosh, R.C.; Dubljević, V.; Fecteau, S.; Ferreri, F.; Flöel, A.; et al. Non-invasive brain stimulation and neuroenhancement. Clin. Neurophysiol. Pract. 2022, 7, 146–165. [Google Scholar] [CrossRef]
- Kazazian, K.; Norton, L.; Laforge, G.; Abdalmalak, A.; Gofton, T.E.; Debicki, D.; Slessarev, M.; Hollywood, S.; Lawrence, K.S.; Owen, A.M. Improving diagnosis and prognosis in acute severe brain injury: A multimodal imaging protocol. Front. Neurol. 2021, 12, 757219. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Next-Generation Cognitive-Behavioral Therapy for Depression: Integrating Digital Tools, Teletherapy, and Personalization for Enhanced Mental Health Outcomes. Medicina 2025, 61, 431. [Google Scholar] [CrossRef]
- McGrath, T.; Baskerville, R.; Rogero, M.; Castell, L. Emerging evidence for the widespread role of glutamatergic dysfunction in neuropsychiatric diseases. Nutrients 2022, 14, 917. [Google Scholar] [CrossRef]
- Peng, S.; Dhawan, V.; Eidelberg, D.; Ma, Y. Neuroimaging evaluation of deep brain stimulation in the treatment of representative neurodegenerative and neuropsychiatric disorders. Bioelectron. Med. 2021, 7, 4. [Google Scholar] [CrossRef]
- Balzekas, I.; Sladky, V.; Nejedly, P.; Brinkmann, B.H.; Crepeau, D.; Mivalt, F.; Gregg, N.M.; Attia, T.P.; Marks, V.S.; Wheeler, L.; et al. Invasive electrophysiology for circuit discovery and study of comorbid psychiatric disorders in patients with epilepsy: Challenges, opportunities, and novel technologies. Front. Hum. Neurosci. 2021, 15, 702605. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, M.; Stiles, W.R.; Choi, H.S. Neuroimaging modalities in Alzheimer’s disease: Diagnosis and clinical features. Int. J. Mol. Sci. 2022, 23, 6079. [Google Scholar] [CrossRef] [PubMed]
- De Asis-Cruz, J.; Limperopoulos, C. Harnessing the power of advanced fetal neuroimaging to understand in utero footprints for later neuropsychiatric disorders. Biol. Psychiatry 2023, 93, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.C.C.; Antunes, A.S.L.M.; Athié, M.C.P.; da Silva, B.F.; dos Santos, J.V.R.; Canateli, C.; Fontoura, M.A.; Pinto, A.; Pimentel-Silva, L.R.; Avansini, S.H.; et al. Between neurons and networks: Investigating mesoscale brain connectivity in neurological and psychiatric disorders. Front. Neurosci. 2024, 18, 1340345. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G.; Boutsinas, B. Digital and AI-Enhanced Cognitive Behavioral Therapy for Insomnia: Neurocognitive Mechanisms and Clinical Outcomes. J. Clin. Med. 2025, 14, 2265. [Google Scholar] [CrossRef]
- Farag, M.; Knights, H.; Scahill, R.I.; McColgan, P.; Estevez-Fraga, C. Neuroimaging Techniques in Huntington’s Disease: A Critical Review. Mov. Disord. Clin. Pract. 2025, 12, 561–576. [Google Scholar] [CrossRef]
- Streich, L.; Boffi, J.C.; Wang, L.; Alhalaseh, K.; Barbieri, M.; Rehm, R.; Deivasigamani, S.; Gross, C.T.; Agarwal, A.; Prevedel, R. High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy. Nat. Methods 2021, 18, 1253–1258. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).