Can Antidromic and Orthodromic Stimulation Both Be Used for Correct Carpal Tunnel Syndrome Staging by J. D. Bland and L. Padua?
Abstract
1. Introduction
- Moderate: there is a slowing of median sensory conduction velocity (CV) and an abnormal median distal motor latency (DML).
- Severe: there is an unobtainable median sensory nerve action potential (SNAP) and an abnormal median DML [2].
- Moderate: sensory potential preserved with sensory slowing; DML to abductor pollicis brevis (APB) < 6.5 ms;
- Severe: sensory potentials absent but motor response preserved; DML to APB < 6.5 ms;
- Very severe: sensory potentials absent, but motor response preserved; DML to APB > 6.5 ms [3].
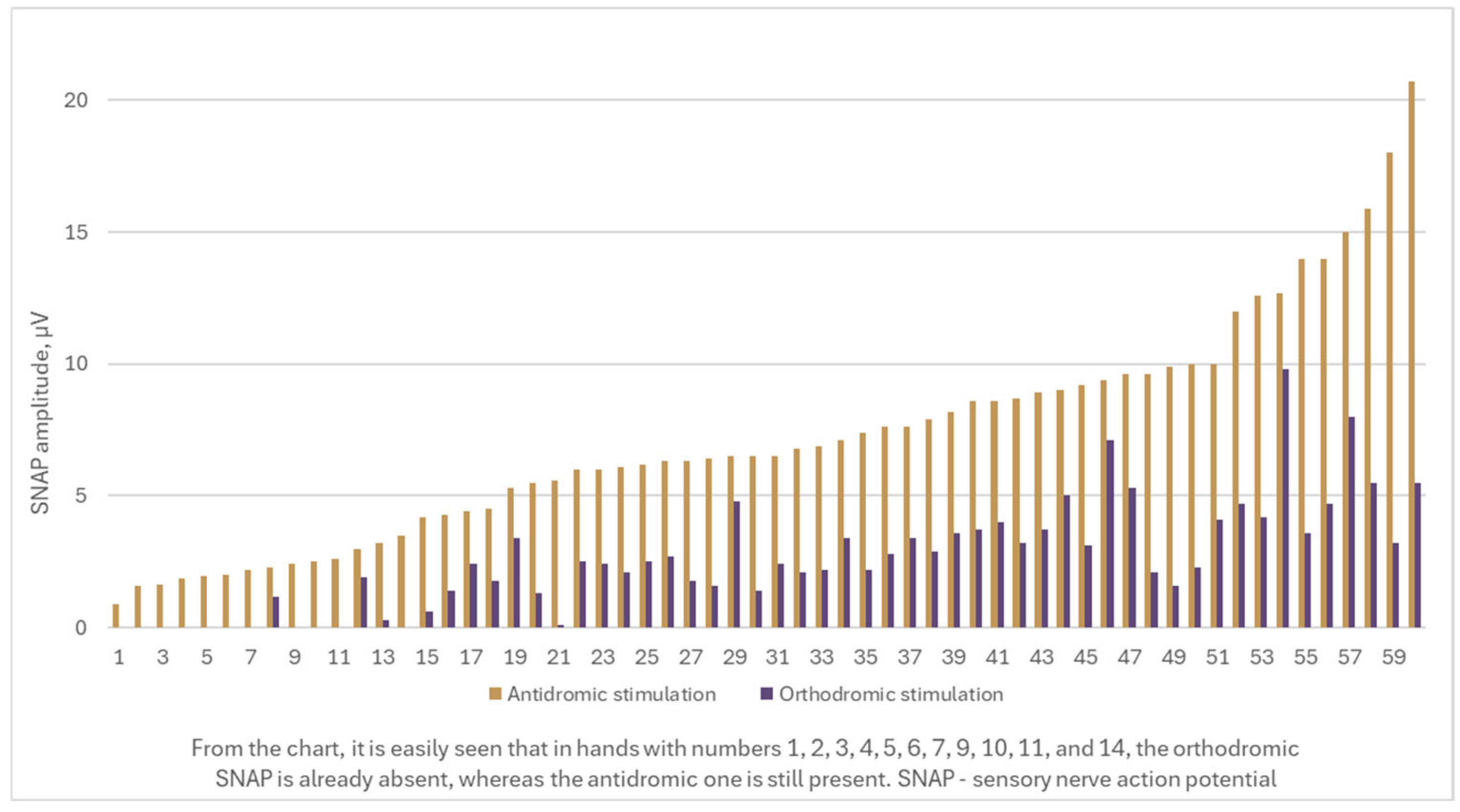
- A considerable number of cases exhibit a recordable antidromic SNAP, while the orthodromic SNAP is absent. Consequently, the severity of CTS, as determined by the Padua and Bland classifications, varies significantly depending on whether the orthodromic or antidromic technique is applied.
- There is an antidromic SNAP amplitude that serves as a cut-off value for undetectable orthodromic SNAP.
- There is a median nerve DML value that indicates when orthodromic SNAP registration is no longer possible, but antidromic SNAP is still present.
2. Materials and Methods
2.1. Equipment
2.2. Patients
2.3. NCS Protocol
3. Results
4. Discussion
- The first selection bias concerns the exclusion of patients with polyneuropathy. It is important to specify that this group of patients was not included in either the original Padua or Bland studies. Given the potential impact of polyneuropathy on nerve conduction parameters, this population should be studied separately. Interpreting nerve conduction results in polyneuropathic patients requires particular caution to ensure precise diagnosis.
- The second selection bias relates to the age distribution of our study participants.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| CMAP | compound muscle action potential |
| CTS | carpal tunnel syndrome |
| CV | conduction velocity |
| DML | distal motor latency |
| DSL | distal sensory latency |
| G1 | active recording electrode |
| G2 | reference electrode |
| NCS | nerve conduction study |
| ROC | receiver operating characteristic |
| SNAP | sensory nerve action potential |
| SS | stimulation site |
References
- Atroshi, I.; Gummesson, C.; Johnsson, R.; Ornstein, E.; Ranstam, J.; Rosén, I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999, 282, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Padua, L.; Monaco, M.L.; Padua, R.; Gregori, B.; Tonali, P. Neurophysiological classification of carpal tunnel syndrome: Assessment of 600 symptomatic hands. Ital. J. Neurol. Sci. 1997, 18, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.D. A neurophysiological grading scale for carpal tunnel syndrome. Muscle Nerve 2000, 23, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Valls-Sole, J.; Leote, J.; Pereira, P. Antidromic vs orthodromic sensory median nerve conduction studies. Clin. Neurophysiol. Pract. 2016, 1, 18–25. [Google Scholar] [CrossRef]
- Fujimaki, Y.; Kuwabara, S.; Sato, Y.; Isose, S.; Shibuya, K.; Sekiguchi, Y.; Nasu, S.; Noto, Y.; Taniguchi, J.; Misawa, S. The effects of age, gender, and body mass index on amplitude of sensory nerve action potentials: Multivariate analyses. Clin. Neurophysiol. 2009, 120, 1683–1686. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.C.; Shapiro, B.E. Electromyography and Neuromuscular Disorders Clinical-Electrophysiological Correlations, 3rd ed.; Saunders: Oxford, UK, 2012. [Google Scholar]
- Jablecki, C.K.; Andary, M.T.; Floeter, M.K.; Miller, R.G.; Quartly, C.A.; Vennix, M.J.; Wilson, J.R. Practice parameter: Electrodiagnostic studies in carpal tunnel syndrome. Report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2002, 58, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Sandin, K.J.; Asch, S.M.; Jablecki, C.K.; Kilmer, D.D.; Nuckols, T.K. Clinical quality measures for electrodiagnosis in suspected carpal tunnel syndrome. Muscle Nerve 2010, 41, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Basiri, K.; Katirji, B. Practical approach to electrodiagnosis of the carpal tunnel syndrome: A review. Adv. Biomed. Res. 2015, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
| Total number of patients, N | 47 |
| Sex | |
| Males | 17 (36.2%) |
| Females | 30 (63.8%) |
| Age | |
| Mean age, years (–max) | 61 (42–84) |
| Males’ mean age, years (min–max) | 60 (44–83) |
| Females’ mean age, years (min–max) | 61 (42–84) |
| Nr | Sex | Age, years | DML, ms | SNAP Amplitude, µV | Onset DSL, ms | Sensory CV, m/s | CMAP Amplitude at the Distal Stimulation Site, mV | CMAP Amplitude at the Proximal Stimulation Site, mV | Motor CV, m/s | |
|---|---|---|---|---|---|---|---|---|---|---|
| Antidromic Stimulation | Orthodromic Stimulation | |||||||||
| 1 | male | 63 | 5.63 | 4.5 | 1.75 | 3.98 | 29.6 | 4.2 | 3.3 | 55.9 |
| 2 | male | 61 | 4.81 | 12.6 | 4.2 | 3.73 | 39.7 | 5.3 | 4.9 | 52.0 |
| 3 | male | 61 | 4.94 | 12.0 | 4.7 | 3.71 | 38.5 | 5.8 | 5.6 | 51.3 |
| 4 | female | 63 | 5.14 | 3.5 | 0.0 | 3.04 | 39.5 | 3.2 | 3.1 | 52.1 |
| 5 | male | 61 | 7.5 | 1.65 | 0.0 | 4.74 | 27.4 | 0.39 | 0.39 | 31.5 |
| 6 | male | 61 | 4.60 | 2.3 | 1.18 | 4.17 | 29.9 | 7.0 | 6.7 | 50.9 |
| 7 | female | 62 | 4.56 | 5.3 | 3.4 | 4.11 | 35.3 | 6.9 | 6.5 | 50.0 |
| 8 | female | 64 | 4.71 | 4.2 | 0.61 | 4.21 | 28.5 | 4.6 | 4.1 | 50.0 |
| 9 | male | 73 | 4.50 | 8.6 | 3.7 | 4.62 | 39.5 | 8.4 | 7.6 | 50.2 |
| 10 | male | 73 | 4.57 | 9.4 | 7.1 | 4.96 | 36.0 | 5.9 | 5.4 | 50.3 |
| 11 | female | 58 | 6.5 | 9.9 | 1.6 | 4.5 | 27.6 | 6.6 | 6.3 | 62.9 |
| 12 | female | 60 | 5.9 | 8.6 | 4.0 | 4.3 | 26.8 | 6.3 | 5.0 | 59.5 |
| 13 | female | 50 | 4.7 | 8.9 | 3.7 | 3.7 | 34.3 | 4.6 | 4.6 | 57.3 |
| 14 | male | 61 | 6.1 | 2.6 | 0.0 | 3.8 | 34.2 | 3.2 | 3.2 | 50.0 |
| 15 | female | 46 | 4.9 | 9.2 | 3.1 | 3.6 | 33.8 | 9.3 | 9.2 | 51.2 |
| 16 | female | 46 | 5.2 | 6.9 | 2.2 | 3.8 | 31.3 | 8.3 | 8.1 | 50.0 |
| 17 | female | 72 | 5.6 | 6.4 | 1.6 | 4.0 | 31.9 | 4.9 | 4.5 | 54.9 |
| 18 | female | 72 | 6.5 | 0.9 | 0.0 | 5.3 | 24.5 | 3.2 | 2.9 | 51.1 |
| 19 | female | 66 | 4.5 | 8.7 | 3.2 | 3.2 | 38.9 | 8.8 | 7.8 | 59.1 |
| 20 | female | 47 | 4.9 | 3.0 | 1.9 | 4.6 | 26.55 | 9.7 | 9.0 | 51.3 |
| 21 | male | 46 | 6.2 | 3.2 | 0.3 | 5.0 | 26.4 | 7.4 | 7.2 | 60.5 |
| 22 | male | 46 | 6.8 | 1.6 | 0.0 | 6.2 | 21.0 | 6.5 | 6.2 | 50.0 |
| 23 | male | 70 | 6.4 | 2.0 | 0.0 | 6.0 | 21.7 | 4.4 | 4.2 | 50.0 |
| 24 | female | 65 | 4.6 | 6.5 | 4.8 | 4.2 | 31.4 | 6.0 | 5.2 | 50.0 |
| 25 | female | 55 | 5.2 | 9.6 | 5.3 | 3.7 | 32.85 | 6.7 | 6.3 | 51.1 |
| 26 | male | 62 | 4.7 | 5.5 | 1.3 | 4.2 | 32.5 | 10.1 | 8.9 | 50.0 |
| 27 | female | 75 | 5.2 | 6.5 | 1.4 | 4.2 | 27.4 | 6.2 | 5.5 | 50.0 |
| 28 | female | 42 | 5.4 | 5.6 | 0.1 | 4.3 | 28.5 | 8.6 | 8.6 | 52.3 |
| 29 | male | 52 | 4.9 | 6.2 | 2.5 | 3.6 | 36.1 | 5.2 | 4.9 | 57.1 |
| 30 | male | 44 | 4.8 | 14.0 | 3.6 | 3.8 | 34.0 | 9.2 | 8.7 | 57.3 |
| 31 | male | 44 | 5.1 | 7.4 | 2.2 | 4.0 | 31.7 | 10.0 | 8.9 | 57.3 |
| 32 | female | 54 | 4.65 | 15.9 | 5.5 | 3.8 | 39.2 | 5.8 | 5.5 | 53.8 |
| 33 | female | 60 | 5.15 | 1.96 | 0.0 | 4.68 | 26.7 | 2.2 | 1.36 | 53.9 |
| 34 | male | 55 | 5.28 | 4.4 | 2.4 | 4.20 | 33.3 | 6.9 | 6.9 | 53.8 |
| 35 | female | 68 | 6.48 | 2.5 | 0.0 | 4.71 | 27.6 | 4.6 | 4.6 | 58.7 |
| 36 | female | 68 | 5.52 | 7.6 | 2.8 | 3.78 | 34.4 | 3.8 | 3.8 | 54.6 |
| 37 | male | 59 | 4.5 | 10.0 | 2.3 | 3.3 | 39.0 | 6.9 | 6.4 | 55.6 |
| 38 | male | 56 | 4.6 | 14.0 | 4.7 | 3.6 | 38.9 | 6.2 | 6.0 | 59.5 |
| 39 | male | 56 | 5.2 | 7.6 | 3.4 | 4.1 | 34.1 | 5.3 | 5.2 | 55.3 |
| 40 | female | 78 | 4.6 | 15.0 | 8.0 | 3.3 | 39.4 | 4.7 | 4.3 | 57.1 |
| 41 | female | 53 | 5.3 | 6.8 | 2.1 | 4.2 | 31.0 | 4.7 | 4.6 | 54.8 |
| 42 | female | 66 | 4.5 | 6.5 | 2.4 | 3.4 | 39.0 | 6.7 | 6.5 | 58.8 |
| 43 | male | 63 | 4.5 | 9.0 | 5.0 | 3.8 | 36.8 | 5.3 | 5.3 | 57.1 |
| 44 | male | 55 | 4.51 | 20.7 | 5.5 | 3.00 | 39.4 | 4.3 | 4.3 | 55.3 |
| 45 | male | 59 | 4.8 | 18.0 | 3.2 | 3.6 | 38.9 | 8.0 | 7.6 | 55.3 |
| 46 | female | 78 | 4.5 | 10.0 | 4.1 | 3.2 | 38.2 | 6.3 | 6.0 | 55.6 |
| 47 | female | 78 | 4.8 | 7.9 | 2.9 | 3.4 | 38.2 | 4.5 | 4.0 | 57.1 |
| 48 | male | 83 | 4.6 | 6.3 | 2.7 | 3.6 | 38.9 | 3.7 | 3.7 | 46.7 |
| 49 | female | 78 | 5.3 | 2.2 | 0.0 | 4.2 | 33.3 | 5.0 | 5.0 | 54.5 |
| 50 | female | 65 | 5.2 | 6.0 | 2.5 | 4.5 | 31.1 | 5.2 | 5.2 | 58.5 |
| 51 | female | 84 | 5.1 | 4.3 | 1.4 | 4.0 | 29.8 | 7.1 | 6.6 | 52.6 |
| 52 | female | 73 | 7.06 | 2.4 | 0.0 | 5.19 | 25.0 | 6.4 | 6.3 | 58.2 |
| 53 | female | 73 | 6.98 | 1.85 | 0.0 | 5.11 | 25.4 | 6.3 | 6.0 | 61.5 |
| 54 | female | 60 | 4.60 | 12.7 | 9.8 | 3.21 | 38.9 | 7.6 | 7.1 | 59.4 |
| 55 | female | 60 | 5.99 | 6.0 | 2.4 | 4.43 | 27.1 | 4.3 | 4.1 | 50.6 |
| 56 | female | 52 | 5.07 | 6.1 | 2.1 | 3.96 | 31.6 | 5.1 | 4.9 | 59.2 |
| 57 | female | 48 | 4.59 | 9.6 | 2.1 | 3.84 | 36.45 | 4.0 | 3.8 | 59.1 |
| 58 | female | 51 | 4.50 | 8.2 | 3.6 | 3.87 | 36.1 | 5.0 | 5.0 | 58.5 |
| 59 | female | 50 | 5.13 | 6.3 | 1.78 | 4.69 | 29.9 | 5.5 | 5.2 | 57.5 |
| 60 | female | 50 | 5.05 | 7.1 | 3.4 | 3.92 | 35.75 | 2.8 | 2.8 | 52.6 |
| Variables | AUC | Standard Error | p Value | Confidence Interval | Youden Index | Optimal Cut-Off Value | Sensitivity | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Antidromic SNAP amplitude | 0.989 | 0.010 | <0.001 | 0.969 | 1.000 | 0.939 | 3.9 µV | 100% |
| DML | 0.920 | 0.041 | <0.001 | 0.840 | 1.000 | 0.694 | 5.14 ms | 100% |
| DSL | 0.819 | 0.094 | 0.001 | 0.635 | 1.000 | 0.666 | 4.65 ms | 72.7% |
| Sensory CV | 0.803 | 0.093 | 0.002 | 0.621 | 0.985 | 0.605 | 28.05 m/s | 72.7% |
| Investigated Arms, Nr | DML, ms | SNAP Amplitude, µV | CTS Staging Using Orthodromic Stimulation | CTS Staging Using Antidromic Stimulation | CTS Staging Using Antidromic SNAP Amplitude Cut-Off Value of ≤3.85 µV | |
|---|---|---|---|---|---|---|
| Orthodromic Stimulation | Antidromic Stimulation | |||||
| 1 | 5.63 | 1.75 | 4.5 | moderate | moderate | moderate |
| 2 | 4.81 | 4.2 | 12.6 | moderate | moderate | moderate |
| 3 | 4.94 | 4.7 | 12.0 | moderate | moderate | moderate |
| 4 | 5.14 | 0.0 | 3.5 | severe | moderate | severe |
| 5 | 7.5 | 0.0 | 1.65 | severe (very severe) ^ | moderate (cannot classify) ^ | severe (very severe) ^ |
| 6 | 4.60 | 1.18 | 2.3 | moderate | moderate | severe |
| 7 | 4.56 | 3.4 | 5.3 | moderate | moderate | moderate |
| 8 | 4.71 | 0.61 | 4.2 | moderate | moderate | moderate |
| 9 | 4.50 | 3.7 | 8.6 | moderate | moderate | moderate |
| 10 | 4.57 | 7.1 | 9.4 | moderate | moderate | moderate |
| 11 | 6.5 | 1.6 | 9.9 | moderate | moderate | moderate |
| 12 | 5.9 | 4.0 | 8.6 | moderate | moderate | moderate |
| 13 | 4.7 | 3.7 | 8.9 | moderate | moderate | moderate |
| 14 | 6.1 | 0.0 | 2.6 | severe | moderate | severe |
| 15 | 4.9 | 3.1 | 9.2 | moderate | moderate | moderate |
| 16 | 5.2 | 2.2 | 6.9 | moderate | moderate | moderate |
| 17 | 5.6 | 1.6 | 6.4 | moderate | moderate | moderate |
| 18 | 6.5 | 0.0 | 0.9 | severe | moderate | severe |
| 19 | 4.5 | 3.2 | 8.7 | moderate | moderate | moderate |
| 20 | 4.9 | 1.9 | 3.0 | moderate | moderate | severe |
| 21 | 6.2 | 0.3 | 3.2 | moderate | moderate | severe |
| 22 | 6.8 | 0.0 | 1.6 | severe (very severe) ^ | moderate (cannot classify) | severe (very severe) |
| 23 | 6.4 | 0.0 | 2.0 | severe | moderate | severe |
| 24 | 4.6 | 4.8 | 6.5 | moderate | moderate | moderate |
| 25 | 5.2 | 5.3 | 9.6 | moderate | moderate | moderate |
| 26 | 4.7 | 1.3 | 5.5 | moderate | moderate | moderate |
| 27 | 5.2 | 1.4 | 6.5 | moderate | moderate | moderate |
| 28 | 5.4 | 0.1 | 5.6 | moderate | moderate | moderate |
| 29 | 4.9 | 2.5 | 6.2 | moderate | moderate | moderate |
| 30 | 4.8 | 3.6 | 14.0 | moderate | moderate | moderate |
| 31 | 5.1 | 2.2 | 7.4 | moderate | moderate | moderate |
| 32 | 4.65 | 5.5 | 15.9 | moderate | moderate | moderate |
| 33 | 5.15 | 0.0 | 1.96 | severe | moderate | severe |
| 34 | 5.28 | 2.4 | 4.4 | moderate | moderate | moderate |
| 35 | 6.48 | 0.0 | 2.5 | severe | moderate | severe |
| 36 | 5.52 | 2.8 | 7.6 | moderate | moderate | moderate |
| 37 | 4.5 | 2.3 | 10.0 | moderate | moderate | moderate |
| 38 | 4.6 | 4.7 | 14.0 | moderate | moderate | moderate |
| 39 | 5.2 | 3.4 | 7.6 | moderate | moderate | moderate |
| 40 | 4.6 | 8.0 | 15.0 | moderate | moderate | moderate |
| 41 | 5.3 | 2.1 | 6.8 | moderate | moderate | moderate |
| 42 | 4.5 | 2.4 | 6.5 | moderate | moderate | moderate |
| 43 | 4.5 | 5.0 | 9.0 | moderate | moderate | moderate |
| 44 | 4.51 | 5.5 | 20.7 | moderate | moderate | moderate |
| 45 | 4.8 | 3.2 | 18.0 | moderate | moderate | moderate |
| 46 | 4.5 | 4.1 | 10.0 | moderate | moderate | moderate |
| 47 | 4.8 | 2.9 | 7.9 | moderate | moderate | moderate |
| 48 | 4.6 | 2.7 | 6.3 | moderate | moderate | moderate |
| 49 | 5.3 | 0.0 | 2.2 | severe | moderate | severe |
| 50 | 5.2 | 2.5 | 6.0 | moderate | moderate | moderate |
| 51 | 5.1 | 1.4 | 4.3 | moderate | moderate | moderate |
| 52 | 7.06 | 0.0 | 2.4 | severe (very severe) ^ | moderate (cannot classify) ^ | severe (very severe) ^ |
| 53 | 6.98 | 0.0 | 1.85 | severe (very severe) ^ | moderate (cannot classify) ^ | severe (very severe) ^ |
| 54 | 4.60 | 9.8 | 12.7 | moderate | moderate | moderate |
| 55 | 5.99 | 2.4 | 6.0 | moderate | moderate | moderate |
| 56 | 5.07 | 2.1 | 6.1 | moderate | moderate | moderate |
| 57 | 4.59 | 2.1 | 9.6 | moderate | moderate | moderate |
| 58 | 4.50 | 3.6 | 8.2 | moderate | moderate | moderate |
| 59 | 5.13 | 1.78 | 6.3 | moderate | moderate | moderate |
| 60 | 5.05 | 3.4 | 7.1 | moderate | moderate | moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meļņikova, V.; Timčenko, M.; Bērziņa, S.; Karelis, G. Can Antidromic and Orthodromic Stimulation Both Be Used for Correct Carpal Tunnel Syndrome Staging by J. D. Bland and L. Padua? Medicina 2025, 61, 938. https://doi.org/10.3390/medicina61050938
Meļņikova V, Timčenko M, Bērziņa S, Karelis G. Can Antidromic and Orthodromic Stimulation Both Be Used for Correct Carpal Tunnel Syndrome Staging by J. D. Bland and L. Padua? Medicina. 2025; 61(5):938. https://doi.org/10.3390/medicina61050938
Chicago/Turabian StyleMeļņikova, Vlada, Maksims Timčenko, Solvita Bērziņa, and Guntis Karelis. 2025. "Can Antidromic and Orthodromic Stimulation Both Be Used for Correct Carpal Tunnel Syndrome Staging by J. D. Bland and L. Padua?" Medicina 61, no. 5: 938. https://doi.org/10.3390/medicina61050938
APA StyleMeļņikova, V., Timčenko, M., Bērziņa, S., & Karelis, G. (2025). Can Antidromic and Orthodromic Stimulation Both Be Used for Correct Carpal Tunnel Syndrome Staging by J. D. Bland and L. Padua? Medicina, 61(5), 938. https://doi.org/10.3390/medicina61050938







