The Efficacy and Tolerability of Colistin Versus Non-Colistin Antimicrobial Regimens Among Hospitalized COVID-19 Patients with Multidrug-Resistant Bacterial Superinfection: An Observational Multicenter Study
Abstract
1. Introduction
2. Results
2.1. Baseline Study Population Characteristics
2.2. Assessment of Efficacy
2.3. Assessment of Systemic Toxicity
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Study Design and Target Population
4.3. Study Outcomes
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bazaid, A.S.; Barnawi, H.; Qanash, H.; Alsaif, G.; Aldarhami, A.; Gattan, H.; Alharbi, B.; Alrashidi, A.; Al-Soud, W.A.; Moussa, S. Bacterial coinfection and antibiotic resistance profiles among hospitalised COVID-19 patients. Microorganisms 2022, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.M.; Elgendy, M.O.; Fahmy, A.M.; El Gendy, S.O.; El-Gendy, A.O.; Abdelrahman, M.A.; El-Bahrawy, A.H.; Elsisi, A.M.M.; Shafiq, S.N. Relation between ABO and RhD and prevalence and severity of COVID-19 disease. Int. J. Clin. Med. Res. 2024, 2, 78–86. [Google Scholar] [CrossRef]
- Zawbaa, H.M.; El-Gendy, A.; Saeed, H.; Osama, H.; Ali, A.M.; Gomaa, D.; Abdelrahman, M.; Harb, H.S.; Madney, Y.M.; Abdelrahim, M.E. A study of the possible factors affecting COVID-19 spread, severity and mortality and the effect of social distancing on these factors: Machine learning forecasting model. Int. J. Clin. Pract. 2021, 75, e14116. [Google Scholar] [CrossRef]
- AbdelHalim, M.M.; El Sherbini, S.A.; Ahmed, E.S.S.; Gharib, H.A.A.; Elgendy, M.O.; Ibrahim, A.R.; Abdel Aziz, H.S. Management of Ventilator-Associated Pneumonia Caused by Pseudomonas and Acinetobacter Organisms in a Pediatric Center: A Randomized Controlled Study. Medicina 2024, 60, 2098. [Google Scholar] [CrossRef]
- Aziz, H.S.A.; Ismail, D.K.; Mohammed, N.S.A.; Elgendy, M.O.; Bassiouny, D.M. Distribution and antifungal susceptibility profiles of Candida species isolated from candidemia patients admitted to Egyptian tertiary hospitals: A cross-sectional study. BMC Infect. Dis. 2024, 24, 1177. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Bakr, R.B.; El-Gendy, A.O.; Kamel, G.M.; Azouz, A.A.; Bukhari, S.N.A. Discovery of a COX-2 selective inhibitor hit with anti-inflammatory activity and gastric ulcer protective effect. Future Med. Chem. 2017, 9, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elela, M.M.; ElKasabgy, N.A. COVID-19 a global crisis: Features, complications and suggested treatments. Int. J. Clin. Med. Res. 2023, 1, 43–55. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Vaghasiya, D.D. SARS-CoV-2 variants and vulnerability at the global level. J. Med. Virol. 2022, 94, 2986–3005. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Peacock, S.J.; Barclay, W.S.; De Silva, T.I.; Towers, G.J.; Robertson, D.L. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Khalifa, R.; Ismail, B.; Al-Jahdali, H.; Alghamdi, H.; Joharjy, H.; Eibani, K.; Sabour, R.; Almalki, F.; Alfarhan, S.; Alatram, M. Secondary multidrug resistant bacterial pneumonia among adult COVID-19 patients: A molecular study. Microbes Infect. Dis. 2023, 4, 11–26. [Google Scholar] [CrossRef]
- Mutua, J.M.; Njeru, J.M.; Musyoki, A.M. Multidrug resistant bacterial infections in severely ill COVID-19 patients admitted in a national referral and teaching hospital, Kenya. BMC Infect. Dis. 2022, 22, 877. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; El-Gendy, A.O.; Khairalla, A.S. Microbial diversity of mer operon genes and their potential rules in mercury bioremediation and resistance. Open Biotechnol. J. 2018, 12, 56–77. [Google Scholar] [CrossRef]
- Sayed, A.M.; Alhadrami, H.A.; El-Gendy, A.O.; Shamikh, Y.I.; Belbahri, L.; Hassan, H.M.; Abdelmohsen, U.R.; Rateb, M.E. Microbial natural products as potential inhibitors of SARS-CoV-2 main protease (Mpro). Microorganisms 2020, 8, 970. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; El-Gendy, A.O.; Hamblin, M.R.; Mohamed, T. The effect of femtosecond laser irradiation on the growth kinetics of Staphylococcus aureus: An in vitro study. J. Photochem. Photobiol. B Biol. 2021, 221, 112240. [Google Scholar] [CrossRef]
- Behera, B.; Tripathy, S.; Venkateshan, M.; Mahapatra, A.; Mohanty, S.; Gupta, K.; Mishra, B.; Rao, P.B.; Mitra, J.K.; Mohapatra, P.R.; et al. Spectrum of Bacterial Pathogens in Critical COVID-19 Patients Admitted in Intensive Care Units of a Tertiary Care Hospital During the First and Second Wave of the Pandemic. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar]
- El-Gendy, A.O.; Saeed, H.; Ali, A.M.; Zawbaa, H.M.; Gomaa, D.; Harb, H.S.; Madney, Y.M.; Osama, H.; Abdelrahman, M.A.; Abdelrahim, M.E. Bacillus Calmette–Guérin vaccine, antimalarial, age and gender relation to COVID-19 spread and mortality. Vaccine 2020, 38, 5564–5568. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, K.-H.; Chen, W.; Yu, Y.; Feng, S.-F. Epidemiology and risk factors for nosocomial infection in the respiratory intensive care unit of a teaching hospital in China: A prospective surveillance during 2013 and 2015. BMC Infect. Dis. 2019, 19, 145. [Google Scholar] [CrossRef]
- Sayed, A.M.; Sherif, N.H.; El-Gendy, A.O.; Shamikh, Y.I.; Ali, A.T.; Attia, E.Z.; El-Katatny, M.H.; Khalifa, B.A.; Hassan, H.M.; Abdelmohsen, U.R. Metabolomic profiling and antioxidant potential of three fungal endophytes derived from Artemisia annua and Medicago sativa. Nat. Prod. Res. 2022, 36, 2404–2408. [Google Scholar] [CrossRef]
- Abdelrahman, M.A.; Zaki, A.; Salem, S.A.; Salem, H.F.; Ibrahim, A.R.; Hassan, A.; Elgendy, M.O. The Impact of Cefepime and Ampicillin/Sulbactam on Preventing Post-Cesarean Surgical Site Infections, Randomized Controlled Trail. Antibiotics 2023, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, A.; Essam, T.; Amin, M.; Ahmed, S.; Nes, I. Clinical screening for bacteriocinogenic Enterococcus faecalis isolated from intensive care unit inpatient in Egypt. J. Microb. Biochem. Technol. 2013, 4, 161–167. [Google Scholar] [CrossRef]
- Babar, Z.U.; Dodani, S.K.; Nasim, A. Treatment outcome and adverse effects of colistin in adult patients with carbapenem-resistant gram-negative bacteremia from Pakistan. Int. J. Infect. Dis. 2021, 106, 171–175. [Google Scholar] [CrossRef]
- Alessio, B. COVID-19’s effects on undergraduate medical education. Int. J. Clin. Med. Res. 2024, 2, 60–61. [Google Scholar] [CrossRef]
- Audrick, J. Immediate and long-term effects of COVID-19’s on the pharmaceutical industry. Int. J. Clin. Med. Res. 2024, 2, 209–213. [Google Scholar] [CrossRef]
- Mahmoud Farhan, S.; Mahmoud Abd El-Baky, R.; Abdalla, M.; Osama El-Gendy, A.; Farag Azmy, A. Efficacy of Amikacin and Imipenem Against Multi-Drug Resistant Gram-Negative Bacteria Isolated from Wound Infections, Egypt. Iran. J. Med. Microbiol. 2023, 17, 218–229. [Google Scholar] [CrossRef]
- Farhan, S.M.; Raafat, M.; Abourehab, M.A.; Abd El-Baky, R.M.; Abdalla, S.; El-Gendy, A.O.; Azmy, A.F. Effect of imipenem and amikacin combination against multi-drug resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 1429. [Google Scholar] [CrossRef]
- Kengkla, K.; Kongpakwattana, K.; Saokaew, S.; Apisarnthanarak, A.; Chaiyakunapruk, N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: A systematic review and network meta-analysis. J. Antimicrob. Chemother. 2018, 73, 22–32. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, Z.; Chen, Y.; Xiao, Y.; Huang, X.; Fan, X.-G. Bacterial and fungal infections in COVID-19 patients: A matter of concern. Infect. Control Hosp. Epidemiol. 2020, 41, 1124–1125. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Salah, H.; Sinan, I.; Alsamani, O.; Abdelghani, L.S.; ElLithy, M.H.; Bukamal, N.; Jawad, H.; Hussein, R.R.S.; Elgendy, M.O.; Rabie, A.S.I.; et al. COVID-19 booster doses: A multi-center study reflecting healthcare providers’ perceptions. Vaccines 2023, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.A.E.G.; Saafan, A.E.; El-Gendy, A.O.; Hefzy, E.M.; AbdelGhani, S. Molecular Characterization of Quinolone Resistant Urinary Isolates of Escherichia coli. J. Pure Appl. Microbiol. 2020, 14, 1269–1278. [Google Scholar] [CrossRef]
- Lee, S.H.; Ruan, S.-Y.; Pan, S.-C.; Lee, T.-F.; Chien, J.-Y.; Hsueh, P.-R. Performance of a multiplex PCR pneumonia panel for the identification of respiratory pathogens and the main determinants of resistance from the lower respiratory tract specimens of adult patients in intensive care units. J. Microbiol. Immunol. Infect. 2019, 52, 920–928. [Google Scholar] [CrossRef]
- Molham, F.; Khairalla, A.S.; Azmy, A.F.; El-Gebaly, E.; El-Gendy, A.O.; AbdelGhani, S. Anti-proliferative and anti-biofilm potentials of bacteriocins produced by non-pathogenic Enterococcus sp. Probiotics Antimicrob. Proteins 2021, 13, 571–585. [Google Scholar] [CrossRef]
- Gu, W.-J.; Wang, F.; Tang, L.; Bakker, J.; Liu, J.-C. Colistin for the treatment of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2014, 44, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Almangour, T.A.; Alruwaili, A.; Almutairi, R.; Alrasheed, A.; Alhifany, A.A.; Eljaaly, K.; Alkofide, H.; Alhammad, A.M.; Ghonem, L.; Alsharidi, A. Aerosolized plus intravenous colistin vs intravenous colistin alone for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria: A retrospective cohort study. Int. J. Infect. Dis. 2021, 108, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Khalil, E.M.; Madney, Y.M.; Hassan, M.; Fahmy, A.M.; Alshammari, S.O.; Alshammari, Q.A.; Abou-Taleb, H.A.; Taha, A.A.; Elgendy, M.O.; Ali, H.A. Maternal and Fetal Outcome of COVID-19 Infection among Pregnant Women. Medicina 2024, 60, 1676. [Google Scholar] [CrossRef]
- Ismael, M.S.; Elgendy, M.O.; Binsaleh, A.Y.; Saleh, A.; Abdelrahim, M.E.; Osama, H. Impulsivity and Its Association with Depression and Anxiety in the Normal Egyptian Population Post COVID-19 Pandemic. Medicina 2024, 60, 1367. [Google Scholar] [CrossRef]
- Lim, S.-K.; Lee, S.-O.; Choi, S.-H.; Choi, J.-P.; Kim, S.-H.; Jeong, J.-Y.; Choi, S.-H.; Woo, J.H.; Kim, Y.S. The outcomes of using colistin for treating multidrug resistant Acinetobacter species bloodstream infections. J. Korean Med. Sci. 2011, 26, 325–331. [Google Scholar] [CrossRef]
- Balkan, I.I.; Batirel, A.; Karabay, O.; Agalar, C.; Akalin, S.; Alici, O.; Alp, E.; Altay, F.A.; Altin, N.; Arslan, F. Comparison of colistin monotherapy and non-colistin combinations in the treatment of multi-drug resistant Acinetobacter spp. bloodstream infections: A Multicenter retrospective analysis. Indian J. Pharmacol. 2015, 47, 95–100. [Google Scholar] [CrossRef]
- Said, K.B.; Alsolami, A.; Moussa, S.; Alfouzan, F.; Bashir, A.I.; Rashidi, M.; Aborans, R.; Taha, T.E.; Almansour, H.; Alazmi, M. COVID-19 clinical profiles and fatality rates in hospitalized patients reveal case aggravation and selective co-infection by limited gram-negative bacteria. Int. J. Environ. Res. Public Health 2022, 19, 5270. [Google Scholar] [CrossRef]
- Abbas, A.M.; Hamed, R.M.R.; Elgendy, M.O.; Abdel Aziz, H.S. Prevalence and Antimicrobial Susceptibility Profile of Gram-negative and Gram-positive Bacteria in a Tertiary Hospital: A Retrospective Study. Egypt. J. Med. Microbiol. 2025, 34, 89–104. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef]
- Branco, C.G.; Duarte, I.; Gameiro, J.; Costa, C.; Marques, F.; Oliveira, J.; Bernardo, J.; Fonseca, J.N.; Carreiro, C.; Braz, S. Presentation and outcomes of chronic kidney disease patients with COVID-19. Braz. J. Nephrol. 2021, 44, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Chung, E.K.; Jun, M.S.; Son, E.S.; Rhie, S.J. Differences in colistin administration and bacterial and treatment outcomes in critically ill patients. Sci. Rep. 2019, 9, 8781. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, M.O.; Khalaf, A.M.; El-Gendy, A.O.; Abdelrahman, M.A.; El Gendy, S.O.; Hamied, A.M.A.; Essam, O.; Al Amir, K.; Yousry, E.M.; Abdelrahim, M.E. An observational study on the management of COVID-19 patients in limited-resource hospitals. J. Clin. Nurs. Res. 2022, 6, 43–53. [Google Scholar] [CrossRef]
- Yarijani, Z.M.; Najafi, H. Kidney injury in COVID-19 patients, drug development and their renal complications: Review study. Biomed. Pharmacother. 2021, 142, 111966. [Google Scholar]
- Elgendy, M.O.; El-Gendy, A.O.; Elgendy, S.O.; Abdelaty, L.N.; Abdelrahim, M.E.; Abdelrahman, M.A. Perceptions, Knowledge, and Experiences of Using Face Masks among Egyptian Healthcare Workers during the COVID-19 Pandemic: A Cross-Sectional Study. Healthcare 2023, 11, 838. [Google Scholar] [CrossRef]
- Rocco, M.; Montini, L.; Alessandri, E.; Venditti, M.; Laderchi, A.; De Gennaro, P.; Raponi, G.; Vitale, M.; Pietropaoli, P.; Antonelli, M. Risk factors for acute kidney injury in critically ill patients receiving high intravenous doses of colistin methanesulfonate and/or other nephrotoxic antibiotics: A retrospective cohort study. Crit. Care 2013, 17, R174. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Wang, L.; Zhu, X.; Zhang, M.; Zheng, J. Acute kidney injury and drugs prescribed for COVID-19 in diabetes patients: A real-world disproportionality analysis. Front. Pharmacol. 2022, 13, 833679. [Google Scholar] [CrossRef]
- Binois, Y.; Hachad, H.; Salem, J.-E.; Charpentier, J.; Lebrun-Vignes, B.; Pène, F.; Cariou, A.; Chiche, J.-D.; Mira, J.-P.; Nguyen, L.S. Acute kidney injury associated with lopinavir/ritonavir combined therapy in patients with COVID-19. Kidney Int. Rep. 2020, 5, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Kalin, G.; Alp, E.; Demiraslan, H.; Doganay, M.; Coskun, R.; Gündogan, K. Use of high-dose IV and aerosolized colistin for the treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia: Do we really need this treatment? J. Infect. Chemother. 2012, 18, 872–877. [Google Scholar] [CrossRef]
- Ahmed, E.; El-Gendy, A.O.; Moniem Radi, N.A.; Mohamed, T. The bactericidal efficacy of femtosecond laser-based therapy on the most common infectious bacterial pathogens in chronic wounds: An in vitro study. Lasers Med. Sci. 2021, 36, 641–647. [Google Scholar] [CrossRef]
- Lu, Q.; Luo, R.; Bodin, L.; Yang, J.; Zahr, N.; Aubry, A.; Golmard, J.-L.; Rouby, J.-J. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Surv. Anesthesiol. 2013, 57, 222–223. [Google Scholar] [CrossRef]
- Boisson, M.; Grégoire, N.; Cormier, M.; Gobin, P.; Marchand, S.; Couet, W.; Mimoz, O. Pharmacokinetics of nebulized colistin methanesulfonate in critically ill patients. J. Antimicrob. Chemother. 2017, 72, 2607–2612. [Google Scholar] [CrossRef] [PubMed]
- Karami, H.; Derakhshani, A.; Ghasemigol, M.; Fereidouni, M.; Miri-Moghaddam, E.; Baradaran, B.; Tabrizi, N.J.; Najafi, S.; Solimando, A.G.; Marsh, L.M.; et al. Weighted gene co-expression network analysis combined with machine learning validation to identify key modules and hub genes associated with SARS-CoV-2 infection. J. Clin. Med. 2021, 10, 3567. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Care for Severe Acute Respiratory Infection: Toolkit: COVID-19 Adaptation; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Zaki, A.; Elgendy, M.O.; Abdelrahman, M.A.; Ali, H.; Khalil, E.M.; Hassan, M.; Fahmy, A.M.; Gad, R.A.; Salem, H.F. The efficacy of using different antibiotics to prevent maternal surgical site infections in COVID-19-infected cases. Eur. Chem. Bull. 2023, 6, 1342–1348. [Google Scholar]
- Shaban, M.; Elgendy, M.O.; Fahmy, A.M.; Khalil, D.M.; El-Gendy, A.O.; Mahmoud, T.M.; Abdelrahim, M.E. The Outcomes of COVID-19 Patients with Spontaneous Intracerebral Hemorrhage Comorbidity and the Efficacy of Enoxaparin in Decreasing the Mortality Rate in Them: Single Egyptian Center Report. J. Pers. Med. 2022, 12, 1822. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Torres, A.; Rinaudo, M.; Terraneo, S.; de Rosa, F.; Ramirez, P.; Diaz, E.; Fernández-Barat, L.; Ferrer, M. Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J. Infect. 2015, 70, 213–222. [Google Scholar]
- Kellum, J.A.; Lameire, N.; Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef]
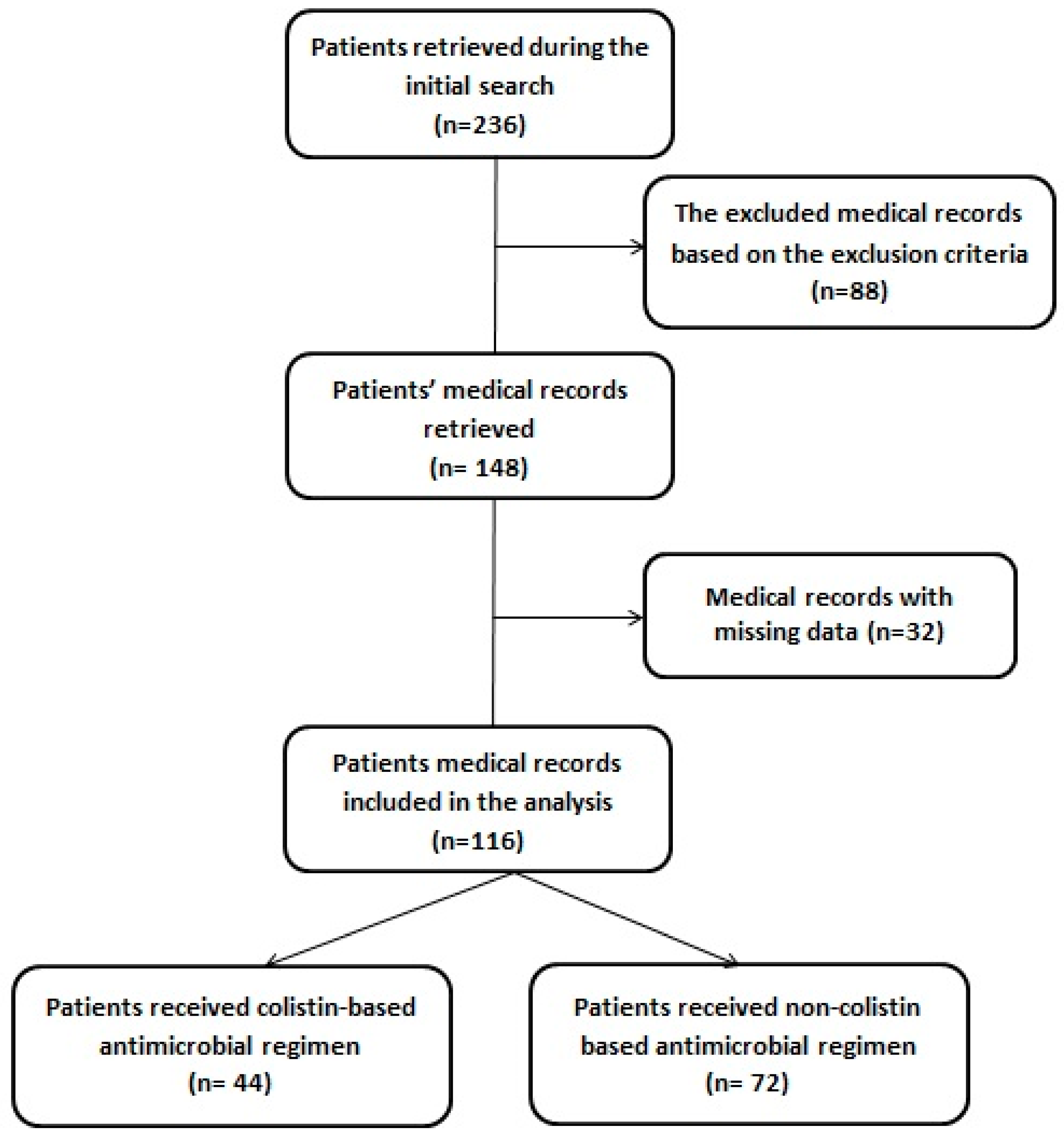

| Parameter | Intravenous Colistin Group (n = 44) | Non-Colistin (Control Group) (n = 72) | p-Value |
|---|---|---|---|
| Age | 58.9 ± 9.6 | 57.3 ± 8.2 | 0.316 |
| Gender (male, %) | 18 (40.9%) | 39 (54.2) | 0.166 |
| Co-administered antibiotic therapy (n, %) | |||
| Carbapenem | 21 (47.7) | 29 (40.3) | 0.432 |
| Piperacillin–tazobactam | 12 (27.3) | 16 (22.2) | 0.537 |
| Macrolides | 10 (22.7) | 18 (25) | 0.781 |
| Cephalosporins | 4 (9.1) | 11 (15.3) | 0.335 |
| Fluoroquinolones | 3 (6.8) | 7 (9.7) | 0.589 |
| Comorbidities (n, %) | |||
| Obesity | 12 (27.3) | 15 (20.8) | 0.426 |
| Chronic respiratory diseases | 3 (6.8) | 2 (2.8) | 0.298 |
| Diabetes mellitus | 16 (36.4) | 23 (31.9) | 0.625 |
| Hypertension | 10 (22.7) | 13 (18.1) | 0.540 |
| Laboratory findings (mean ± SD) | |||
| Hemoglobin (g/dL) | 12.6 ± 1.37 | 12.4 ± 1.34 | 0.310 |
| Platelet (cells/mm3) | 349.6 ± 49.7 | 331.8 ± 69.8 | 0.132 |
| Lymphocytes (%) | 19.3 ± 6.2 | 18.9 ± 6.7 | 0.716 |
| Serum urea (mg/dL) | 34.3 ± 7.9 | 36.2 ± 8.1 | 0.217 |
| Serum creatinine (mg/dL) | 0.86 ± 0.16 | 0.91 ± 0.17 | 0.071 |
| CRP (mg/L) | 91.6 ± 28.4 | 94.5 ± 31.4 | 0.616 |
| D-dimer (ng/mL) | 683 ± 541.1 | 882 ± 598.3 | 0.232 |
| AST (U/L) | 48.7 ± 16.5 | 50.9 ± 19.7 | 0.532 |
| ALT (U/L) | 42.6 ± 18.4 | 46.1 ± 17.1 | 0.298 |
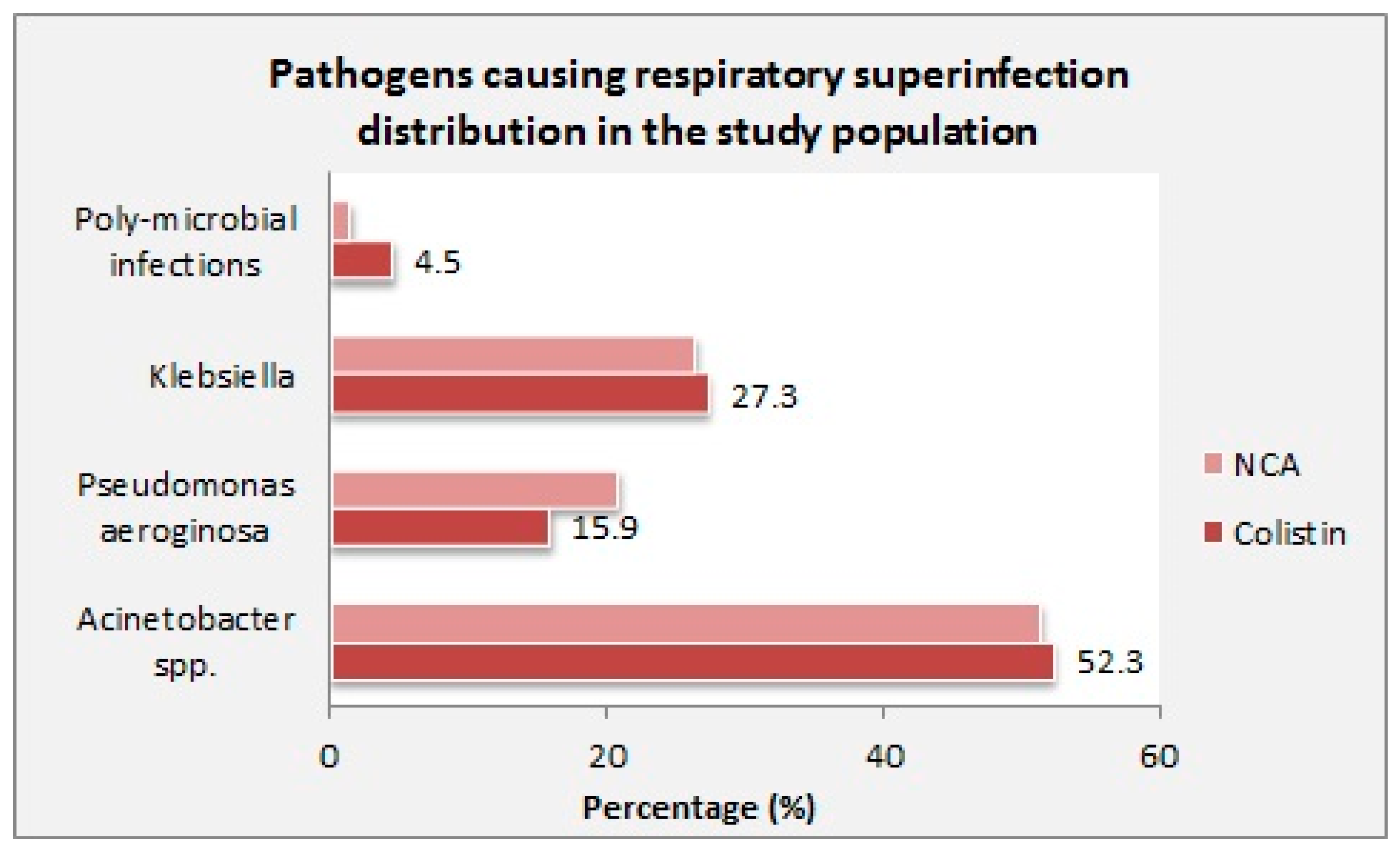
| Pathogen causing super-infection (n, %) | |||
| Acinetobacter spp. | 23 (52.3) | 37 (51.4) | 0.926 |
| Pseudomonas aeruginosa | 7 (15.9) | 15 (20.8) | 0.512 |
| Klebsiella | 12 (27.3) | 19 (26.4) | 0.917 |
| Poly-microbial infections | 2 (4.5) | 1 (1.4) | 0.299 |
| Smoking status (yes, n %) | 23 (52.3) | 30 (41.7) | 0.266 |
| Special therapy for COVID-19 treatment (n, %) | |||
| Steroids | 29 (65.9) | 38 (52.8) | 0.165 |
| Lopinavir/ritonavir | 6 (13.6) | 9 (12.5) | 0.860 |
| Remdesivir | 8 (18.2) | 21 (29.2) | 0.185 |
| Tocilizumab | 6 (13.6) | 17 (23.6) | 0.191 |
| Outcome | Colistin Group (n = 44) | Non-Colistin (Control Group) (n = 72) | p-Value |
|---|---|---|---|
| Hospitalization duration (days) mean | 11.3 ± 3.8 | 12.8 ± 4.7 | 0.073 |
| Admission for ICU (yes, %) | 14 (31.8) | 31 (43.1) | 0.228 |
| Duration of ICU stay (days) | 9.3 ± 3.1 | 10.4 ± 3.8 | 0.108 |
| Mechanical ventilation (yes, %) | 10 (22.7) | 17 (23.6) | 0.913 |
| All-cause-mortality at 30 days (n, %) | 14 (31.8) | 17 (23.6) | 0.332 |
| Microbiological eradication rate (n, %) | 35 (79.5) | 52 (72.2) | 0.377 |
| Parameter | Odds Ratio | 95% Confidence Interval (CI) |
|---|---|---|
| Age (>60) | 1.3 | 0.74–2.5 |
| Chronic respiratory diseases | 1.9 | 1.02–3.9 |
| Colistin use | 1.5 | 0.7–3.5 |
| Nephrotoxicity | 2.6 | 1.1–6.2 |
| Main pathogen | ||
| Acinetobacter baumannii | 0.76 | 0.48–1.2 |
| Pseudomonas aeruginosa | 1.4 | 0.49–3.7 |
| Klebsiella pneumonia | 1.7 | 0.96–3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahmy, A.M.; Elgendy, M.O.; Mohamed, A.A.; Imam, M.S.; Alharbi, A.N.; Al-Anezi, M.H.; Aldhafeeri, O.M.; Aldhafeeri, S.M.; Ajeebi, J.A.; Kamal, M.; et al. The Efficacy and Tolerability of Colistin Versus Non-Colistin Antimicrobial Regimens Among Hospitalized COVID-19 Patients with Multidrug-Resistant Bacterial Superinfection: An Observational Multicenter Study. Medicina 2025, 61, 884. https://doi.org/10.3390/medicina61050884
Fahmy AM, Elgendy MO, Mohamed AA, Imam MS, Alharbi AN, Al-Anezi MH, Aldhafeeri OM, Aldhafeeri SM, Ajeebi JA, Kamal M, et al. The Efficacy and Tolerability of Colistin Versus Non-Colistin Antimicrobial Regimens Among Hospitalized COVID-19 Patients with Multidrug-Resistant Bacterial Superinfection: An Observational Multicenter Study. Medicina. 2025; 61(5):884. https://doi.org/10.3390/medicina61050884
Chicago/Turabian StyleFahmy, Alzahraa M., Marwa O. Elgendy, Alaa Aboud Mohamed, Mohamed S. Imam, Abdullah Nasser Alharbi, Muhammad Husayn Al-Anezi, Omar Mana Aldhafeeri, Saif Mamdouh Aldhafeeri, Jawaher A. Ajeebi, Marwa Kamal, and et al. 2025. "The Efficacy and Tolerability of Colistin Versus Non-Colistin Antimicrobial Regimens Among Hospitalized COVID-19 Patients with Multidrug-Resistant Bacterial Superinfection: An Observational Multicenter Study" Medicina 61, no. 5: 884. https://doi.org/10.3390/medicina61050884
APA StyleFahmy, A. M., Elgendy, M. O., Mohamed, A. A., Imam, M. S., Alharbi, A. N., Al-Anezi, M. H., Aldhafeeri, O. M., Aldhafeeri, S. M., Ajeebi, J. A., Kamal, M., & Osama, H. (2025). The Efficacy and Tolerability of Colistin Versus Non-Colistin Antimicrobial Regimens Among Hospitalized COVID-19 Patients with Multidrug-Resistant Bacterial Superinfection: An Observational Multicenter Study. Medicina, 61(5), 884. https://doi.org/10.3390/medicina61050884







