Impact of Dexamethasone on Three-Dimensional Stem Cell Spheroids: Morphology, Viability, Osteogenic Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Cell Spheroids Using Gingiva-Derived Mesenchymal Stem Cells
2.2. The Assessment of Cellular Viability
2.3. Quantification of Alkaline Phosphatase Activity and Calcium Deposition
2.4. Total RNA Extraction and Real-Time Polymerase Chain Reaction Quantification
2.5. Statistical Analysis
3. Results
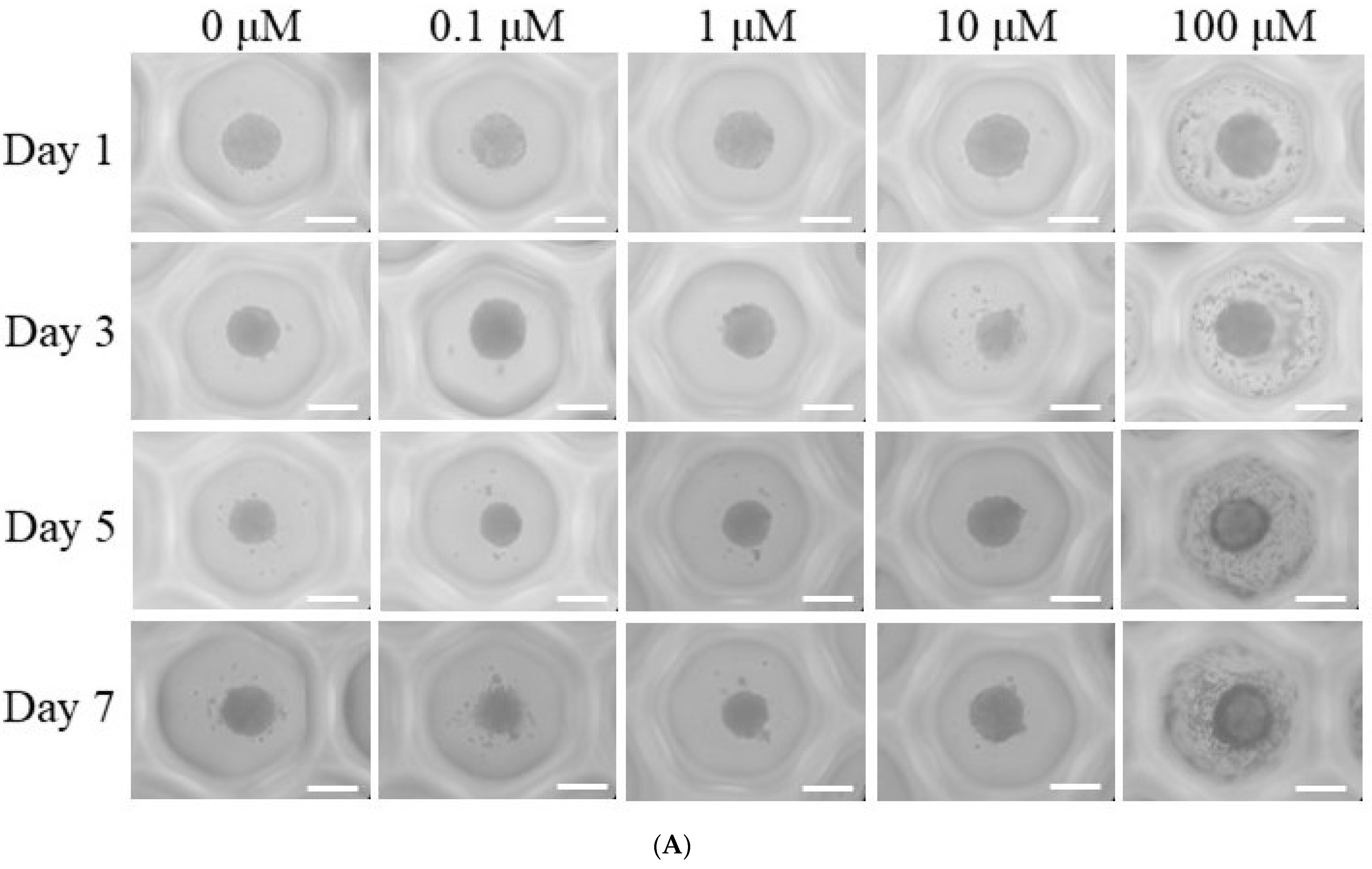
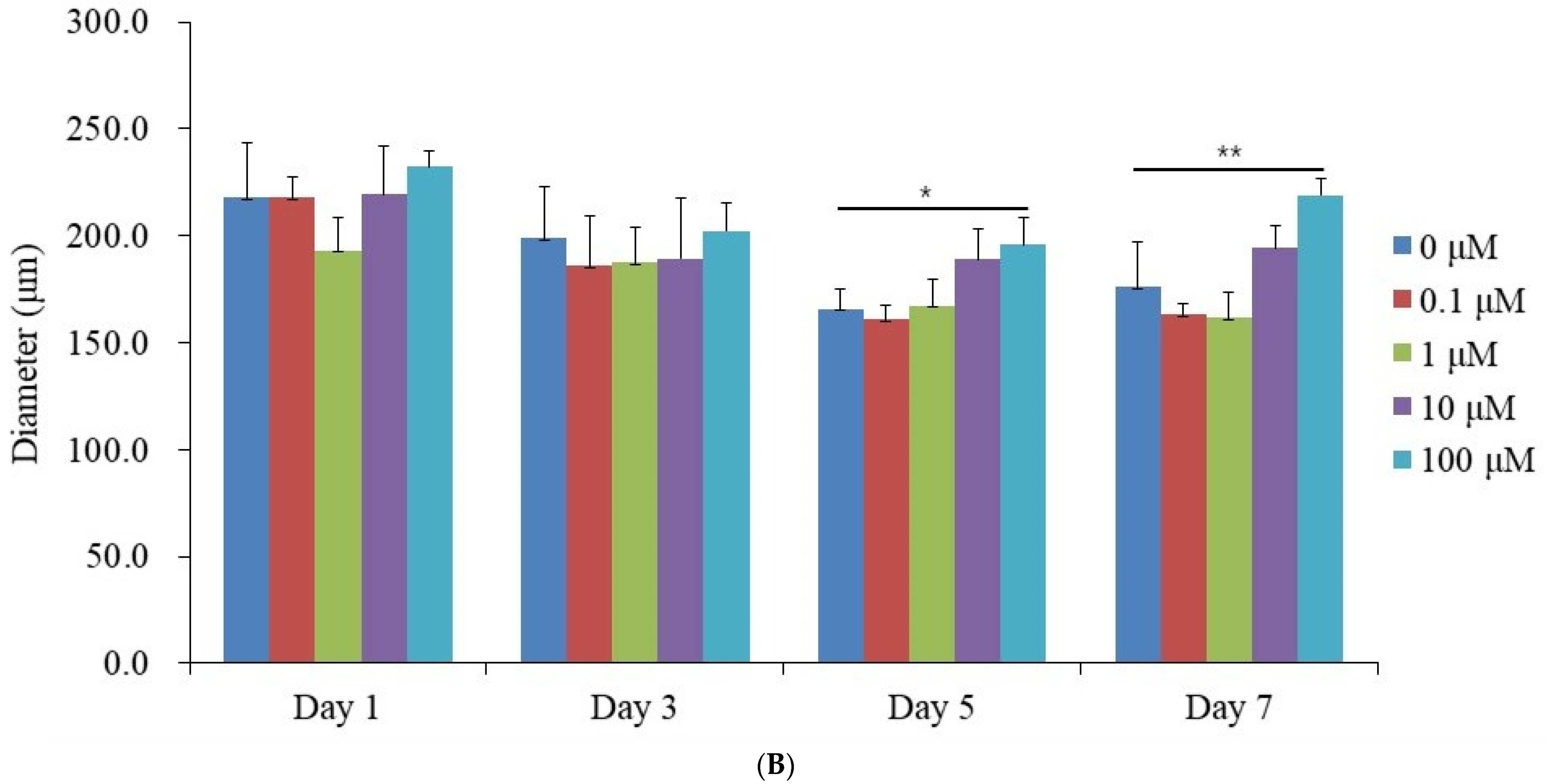
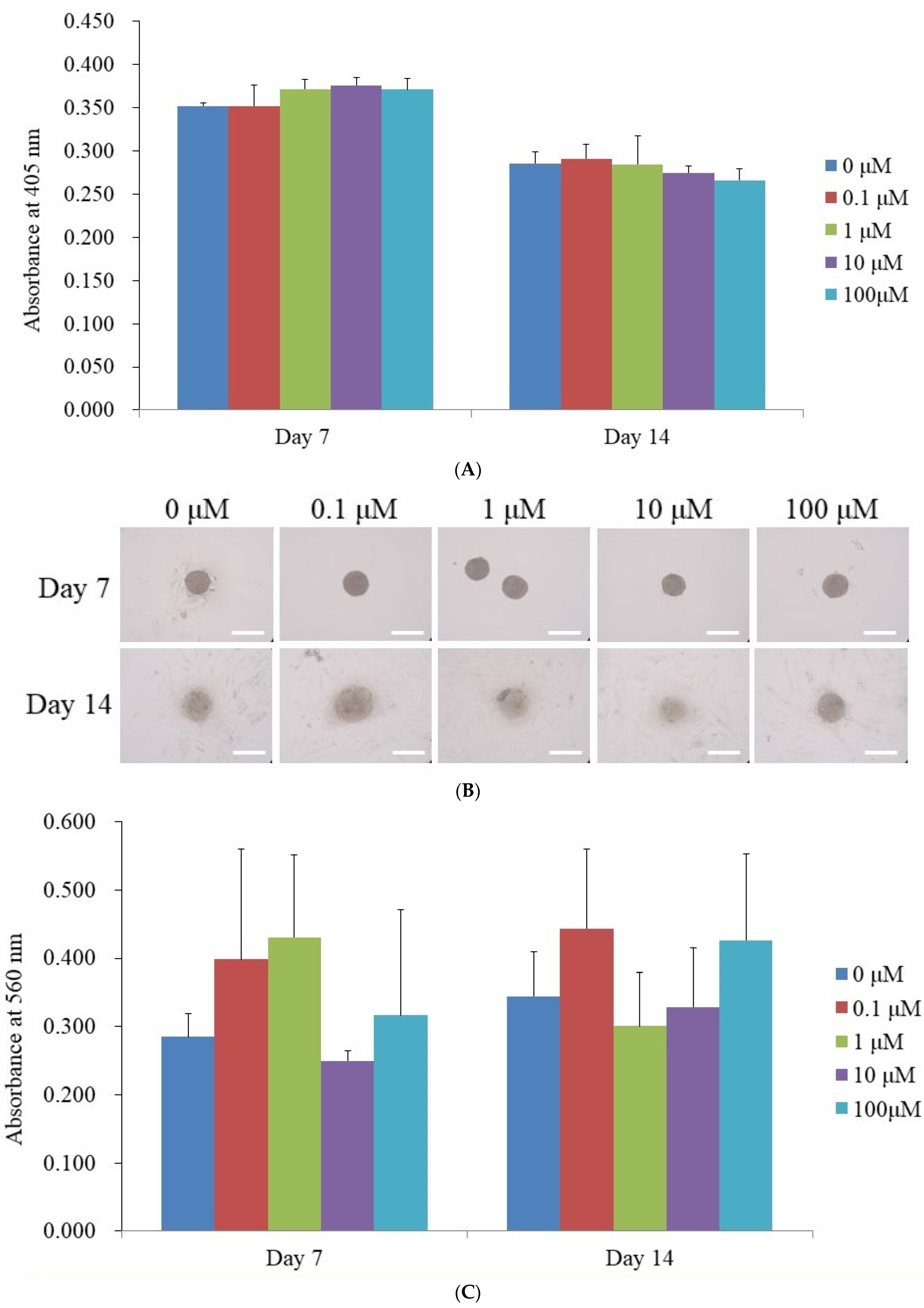
3.1. Generation of Spheroid-Shaped Stem Cell Aggregates
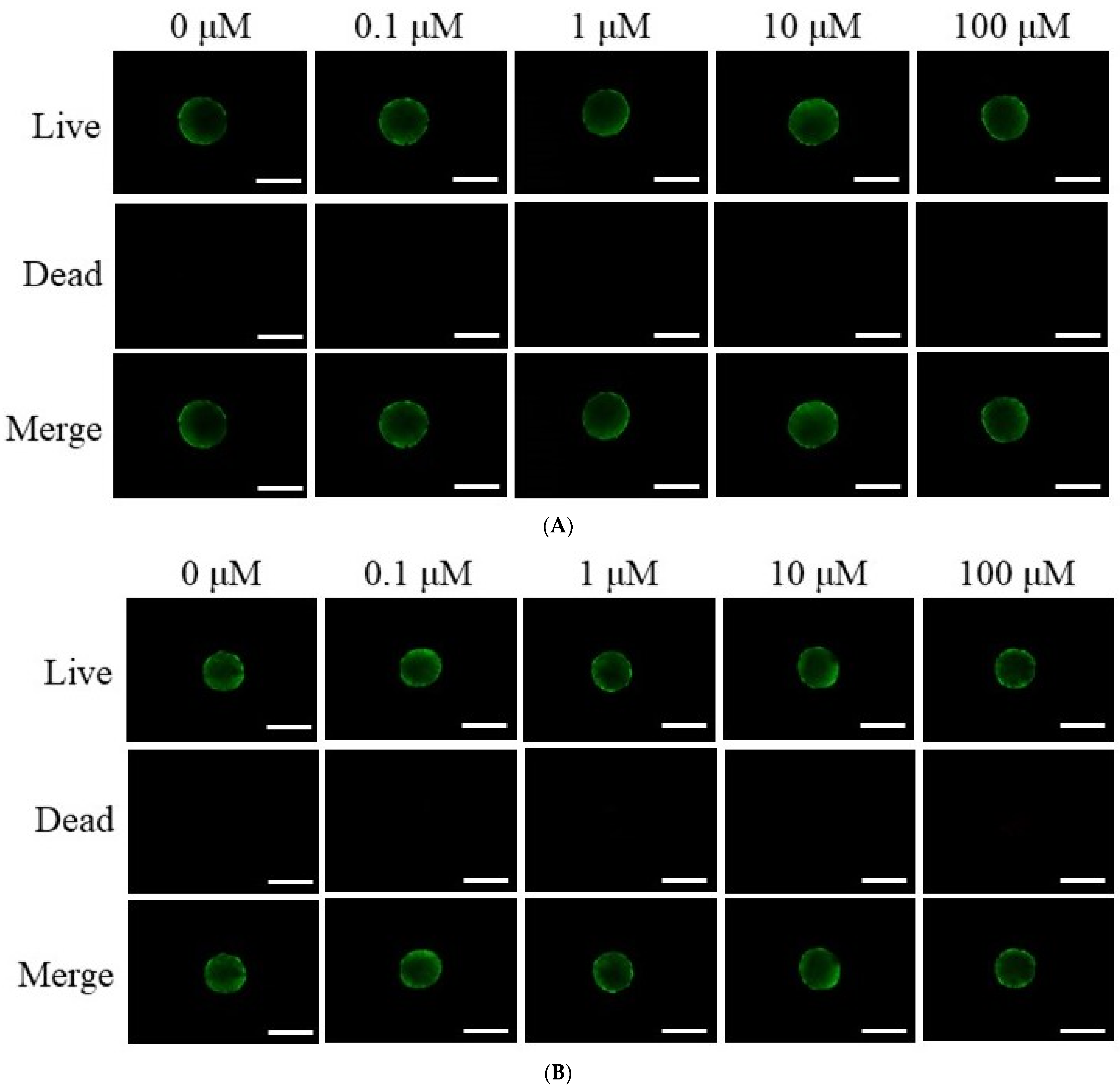
3.2. Assessment of Cell Viability
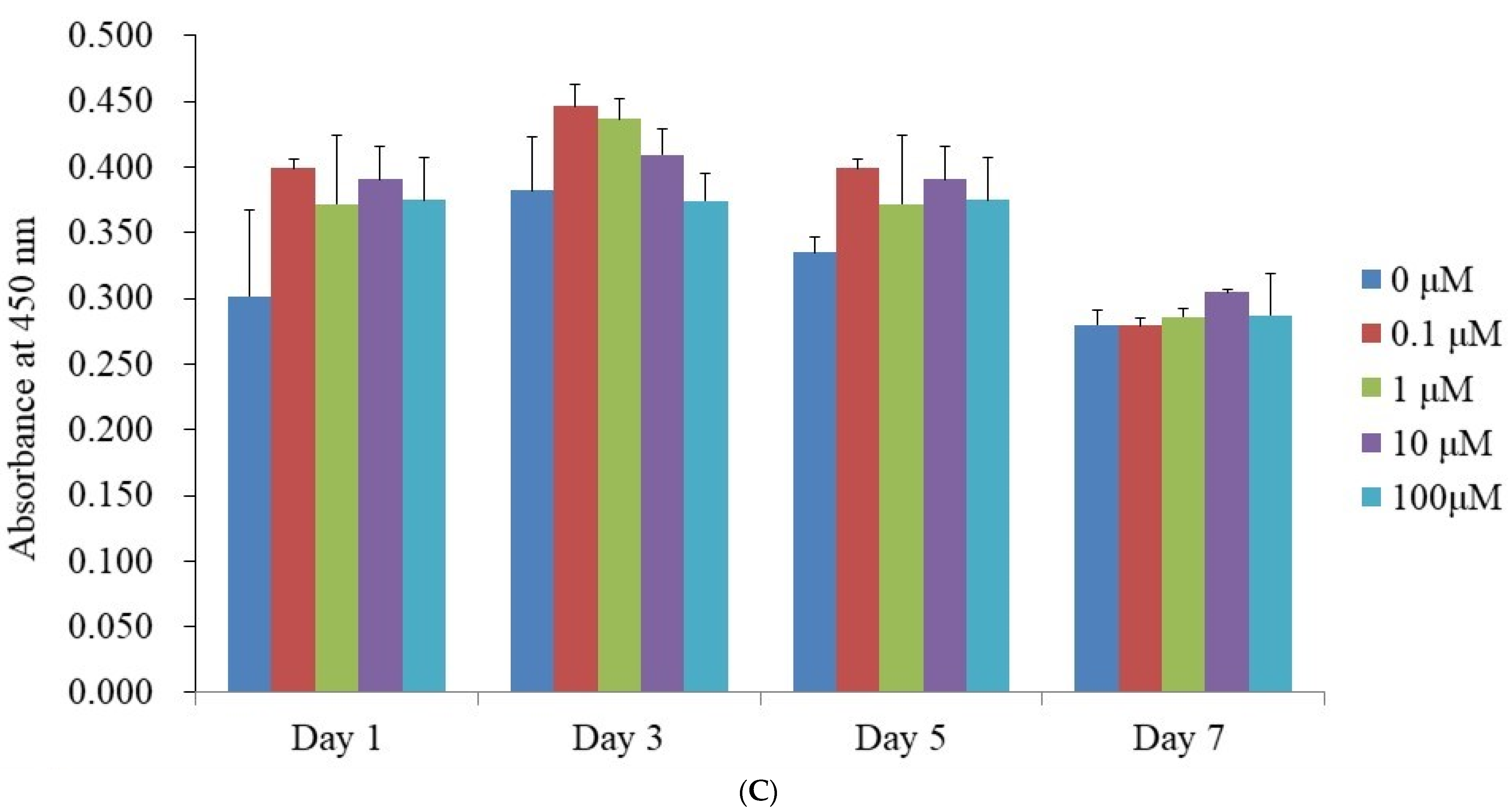
3.3. Alkaline Phosphatase Activity Levels and Calcium Deposition Extent
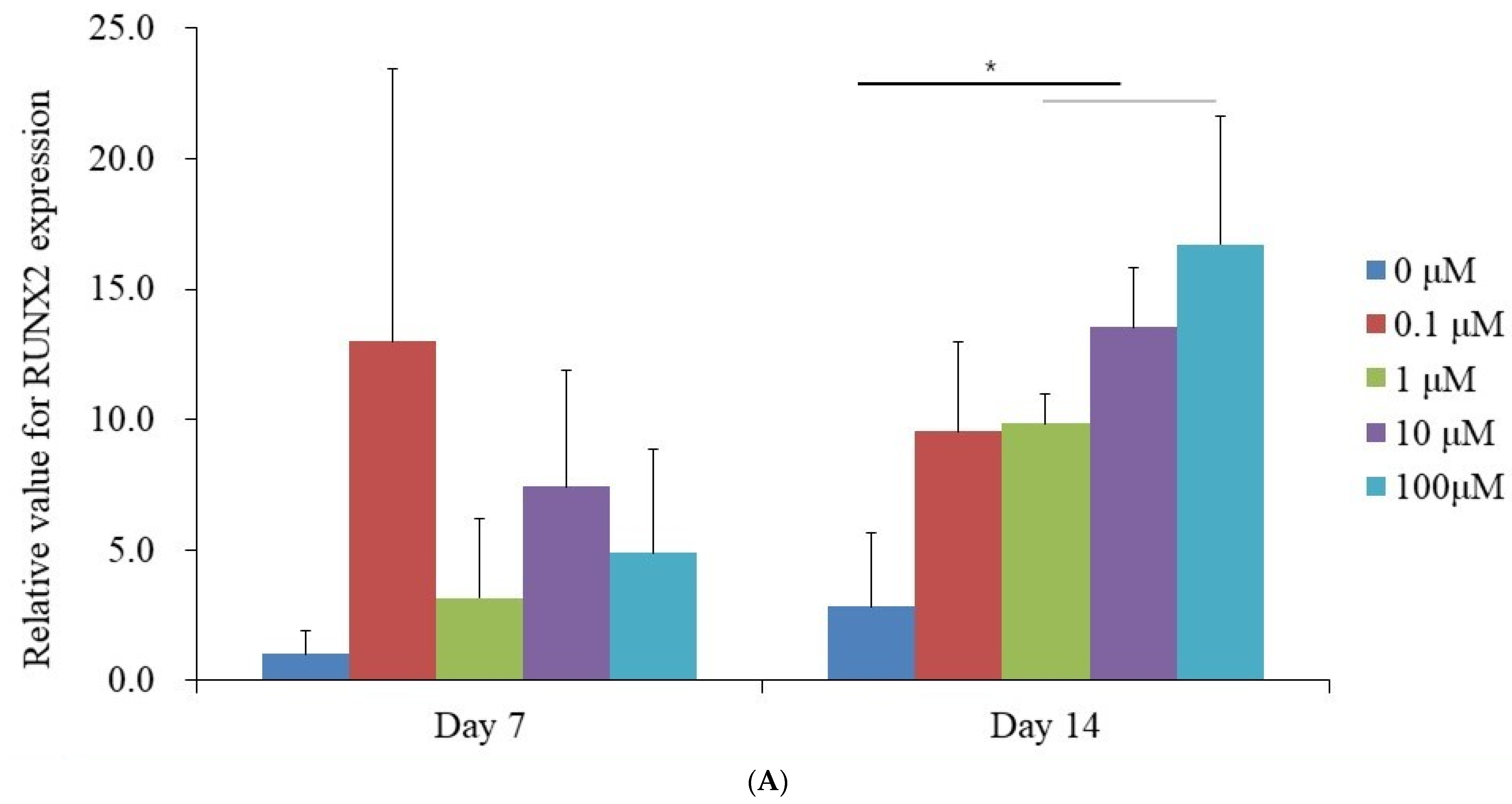
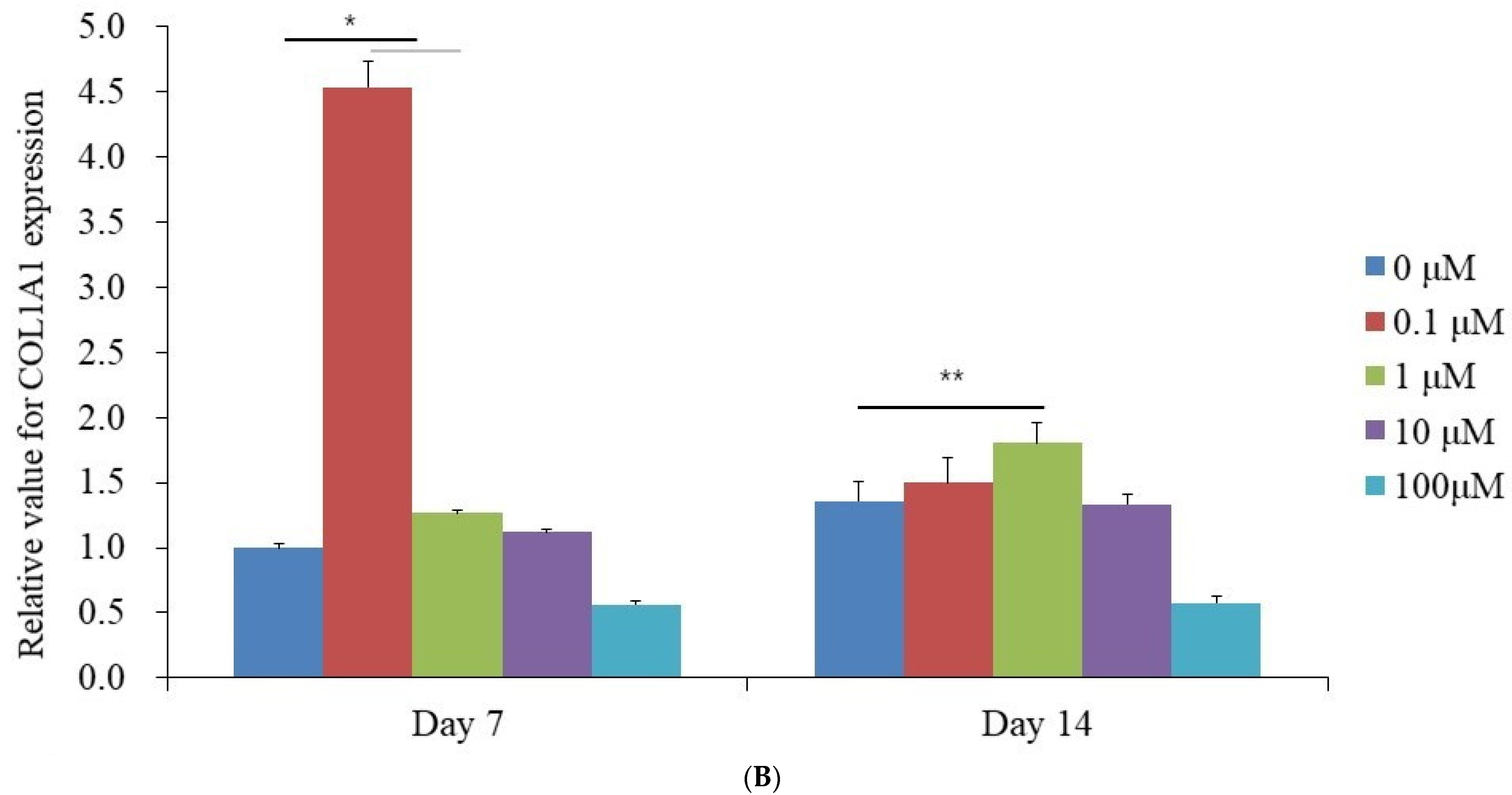
3.4. Evaluation of RUNX2 and COL1A1 Quantitative Real-Time Polymerase Chain Reaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xian, B.; Luo, Z.; Li, K.; Li, K.; Tang, M.; Yang, R.; Lu, S.; Zhang, H.; Ge, J. Dexamethasone Provides Effective Immunosuppression for Improved Survival of Retinal Organoids after Epiretinal Transplantation. Stem Cells Int. 2019, 2019, 7148032. [Google Scholar] [CrossRef]
- Ashour, S.H.; Mudalal, M.; Al-Aroomi, O.A.; Al-Attab, R.; Li, W.; Yin, L. The Effects of Injectable Platelet-Rich Fibrin and Advanced-Platelet Rich Fibrin on Gingival Fibroblast Cell Vitality, Proliferation, Differentiation. Tissue Eng. Regen. Med. 2023, 20, 1161–1172. [Google Scholar] [CrossRef]
- Rawat, S.; Dadhwal, V.; Mohanty, S. Dexamethasone priming enhances stemness and immunomodulatory property of tissue-specific human mesenchymal stem cells. BMC Dev. Biol. 2021, 21, 16. [Google Scholar] [CrossRef]
- Della Bella, E.; Buetti-Dinh, A.; Licandro, G.; Ahmad, P.; Basoli, V.; Alini, M.; Stoddart, M.J. Dexamethasone Induces Changes in Osteogenic Differentiation of Human Mesenchymal Stromal Cells via SOX9 and PPARG, but Not RUNX2. Int. J. Mol. Sci. 2021, 22, 4785. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Kawazoe, N.; Chen, G. Promoted Angiogenesis and Osteogenesis by Dexamethasone-loaded Calcium Phosphate Nanoparticles/Collagen Composite Scaffolds with Microgroove Networks. Sci. Rep. 2018, 8, 14143. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; de Luna-Bertos, E.; Toledano, M.; Manzano-Moreno, F.J.; Costela-Ruiz, V.; Ruiz, C.; Gil, J.; Osorio, R. Dexamethasone and doxycycline functionalized nanoparticles enhance osteogenic properties of titanium surfaces. Dent. Mater. 2023, 39, 616–623. [Google Scholar] [CrossRef]
- Park, J.B.; Kim, I.; Lee, W.; Kim, H. Evaluation of the regenerative capacity of stem cells combined with bone graft material and collagen matrix using a rabbit calvarial defect model. J. Periodontal Implant Sci. 2023, 53, 467–477. [Google Scholar] [CrossRef]
- Khaled, M.M.; Ibrahium, A.M.; Abdelgalil, A.I.; El-Saied, M.A.; El-Bably, S.H. Regenerative Strategies in Treatment of Peripheral Nerve Injuries in Different Animal Models. Tissue Eng. Regen. Med. 2023, 20, 839–877. [Google Scholar] [CrossRef]
- Leypold, T.; Herbsthofer, A.; Craveiro, R.B.; Wolf, M.; Beier, J.P.; Ruhl, T. Effects of cannabinoid receptor activation on Porphyromonas gingivalis lipopolysaccharide stimulation in human periodontal ligament stem cells in vitro. J. Periodontal Implant Sci. 2024, 55, 18. [Google Scholar] [CrossRef]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J. Periodontal Implant Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef]
- Arai, Y.; Lee, S.H. MMP13-Overexpressing Mesenchymal Stem Cells Enhance Bone Tissue Formation in the Presence of Collagen Hydrogel. Tissue Eng. Regen. Med. 2023, 20, 461–471. [Google Scholar] [CrossRef]
- Zhou, A.K.; Jou, E.; Lu, V.; Zhang, J.; Chabra, S.; Abishek, J.; Wong, E.; Zeng, X.; Guo, B. Using Pre-Clinical Studies to Explore the Potential Clinical Uses of Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem cells. Tissue Eng. Regen. Med. 2023, 20, 793–809. [Google Scholar] [CrossRef]
- Suhandi, C.; Mohammed, A.F.A.; Wilar, G.; El-Rayyes, A.; Wathoni, N. Effectiveness of Mesenchymal Stem Cell Secretome on Wound Healing: A Systematic Review and Meta-analysis. Tissue Eng. Regen. Med. 2023, 20, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Na, J.; Karima, G.; Amirthalingam, S.; Hwang, N.S.; Kim, H.D. Mesenchymal Stem Cell Spheroids: A Promising Tool for Vascularized Tissue Regeneration. Tissue Eng. Regen. Med. 2024, 21, 673–693. [Google Scholar] [CrossRef]
- Lee, K.; Ko, E.; Park, Y. Adipose Tissue-Derived Mesenchymal Stem Cell Inhibits Osteoclast Differentiation through Tumor Necrosis Factor Stimulated Gene-6. Tissue Eng. Regen. Med. 2024, 21, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Frank, M.; Staehlke, S.; Springer, A.; Hahn, O.; Meyer, J.; Peters, K. 3D Spheroid Cultivation Alters the Extent and Progression of Osteogenic Differentiation of Mesenchymal Stem/Stromal Cells Compared to 2D Cultivation. Biomedicines 2023, 11, 1049. [Google Scholar] [CrossRef]
- Cho, C.S.; Jo, I. Bone Morphogenic Protein-2-Conjugated Three-Dimensional-Printed Poly (L-Lactic Acid) (PLLA) Scaffold is likely Promising as an Effective Bone Substitute. Tissue Eng. Regen. Med. 2023, 20, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, G.; Hong, H.S.; Kim, S.; Son, Y. Platelet-Derived Growth Factor-BB Priming Enhances Vasculogenic Capacity of Bone Marrow-Derived Endothelial Precursor Like Cells. Tissue Eng. Regen. Med. 2023, 20, 695–704. [Google Scholar] [CrossRef]
- Dobson, L.K.; Zeitouni, S.; McNeill, E.P.; Bearden, R.N.; Gregory, C.A.; Saunders, W.B. Canine Mesenchymal Stromal Cell-Mediated Bone Regeneration is Enhanced in the Presence of Sub-Therapeutic Concentrations of BMP-2 in a Murine Calvarial Defect Model. Front. Bioeng. Biotechnol. 2021, 9, 764703. [Google Scholar] [CrossRef]
- Nakayama, Y.; Ogihara-Takeda, M.; Saito, Y.; Yamaguchi, A.; Ogata, Y. Early wound healing at 1 week postoperatively in periodontal tissue regeneration therapy: Enamel matrix derivative versus recombinant human fibroblast growth factor. J. Periodontal Implant Sci. 2024, 54, 405–418. [Google Scholar] [CrossRef]
- Michelo, C.M.; Fasse, E.; van Cranenbroek, B.; Linda, K.; van der Meer, A.; Abdelrazik, H.; Joosten, I. Added effects of dexamethasone and mesenchymal stem cells on early Natural Killer cell activation. Transpl. Immunol. 2016, 37, 1–9. [Google Scholar] [CrossRef]
- Rivera-Cruz, C.M.; Shearer, J.J.; Figueiredo Neto, M.; Figueiredo, M.L. The Immunomodulatory Effects of Mesenchymal Stem Cell Polarization within the Tumor Microenvironment Niche. Stem Cells Int. 2017, 2017, 4015039. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pang, B.; Li, Y.; Zhu, D.; Pang, T.; Liu, Y. Dexamethasone has variable effects on mesenchymal stromal cells. Cytotherapy 2012, 14, 423–430. [Google Scholar] [CrossRef]
- Lee, H.; Hwa, S.; Cho, S.; Kim, J.H.; Song, H.J.; Ko, Y.; Park, J.B. Impact of Polydeoxyribonucleotides on the Morphology, Viability, and Osteogenic Differentiation of Gingiva-Derived Stem Cell Spheroids. Medicina 2024, 60, 1610. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, M.; Liu, Y.; Yang, Y.; Xiao, F.; Fan, J.; Yu, H.; Qiu, Y.; Liu, B.; Wang, W.; Yuhui, Q. Anti-gastric cancer activity and mechanism of natural compound “Heilaohulignan C” isolated from Kadsura coccinea. Phytother Res. 2021, 35, 3977–3987. [Google Scholar] [CrossRef]
- Byeon, S.M.; Bae, T.S.; Lee, M.H.; Ahn, S.G. Guided bone regeneration of calcium phosphate-coated and strontium ranelate-doped titanium mesh in a rat calvarial defect model. J. Periodontal Implant Sci. 2024, 54, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Zhou, W.J.; Liu, Z.H. Metformin enhances the osteogenic activity of rat bone marrow mesenchymal stem cells by inhibiting oxidative stress induced by diabetes mellitus: An in vitro and in vivo study. J. Periodontal Implant Sci. 2023, 53, 54–68. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.B. Dimethyl Sulfoxide Leads to Decreased Osteogenic Differentiation of Stem Cells Derived from Gingiva via Runx2 and Collagen I Expression. Eur. J. Dent. 2019, 13, 131–136. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, J.H.; Kim, Y.J.; Hwang, I.; Kim, Y.Y.; Kim, H.S.; Park, D.Y. Highly accurate measurement of the relative abundance of oral pathogenic bacteria using colony-forming unit-based qPCR. J. Periodontal Implant Sci. 2024, 54, 444–457. [Google Scholar] [CrossRef]
- Yuasa, M.; Yamada, T.; Taniyama, T.; Masaoka, T.; Xuetao, W.; Yoshii, T.; Horie, M.; Yasuda, H.; Uemura, T.; Okawa, A.; et al. Dexamethasone enhances osteogenic differentiation of bone marrow- and muscle-derived stromal cells and augments ectopic bone formation induced by bone morphogenetic protein-2. PLoS ONE 2015, 10, e0116462. [Google Scholar] [CrossRef]
- Tangtrongsup, S.; Kisiday, J.D. Effects of Dexamethasone Concentration and Timing of Exposure on Chondrogenesis of Equine Bone Marrow-Derived Mesenchymal Stem Cells. Cartilage 2016, 7, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Feng, X.; Wang, K.; Song, Y.; Luo, R.; Yang, C. Dexamethasone promotes mesenchymal stem cell apoptosis and inhibits osteogenesis by disrupting mitochondrial dynamics. FEBS Open Bio. 2020, 10, 211–220. [Google Scholar] [CrossRef]
- Abu-Absi, S.F.; Hu, W.S.; Hansen, L.K. Dexamethasone effects on rat hepatocyte spheroid formation and function. Tissue Eng. 2005, 11, 415–426. [Google Scholar] [CrossRef]
- Meesuk, L.; Suwanprateeb, J.; Thammarakcharoen, F.; Tantrawatpan, C.; Kheolamai, P.; Palang, I.; Tantikanlayaporn, D.; Manochantr, S. Osteogenic differentiation and proliferation potentials of human bone marrow and umbilical cord-derived mesenchymal stem cells on the 3D-printed hydroxyapatite scaffolds. Sci. Rep. 2022, 12, 19509. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Chen, R.; Wang, M.; Qi, Q.; Tang, Y.; Guo, Z.; Wu, S.; Li, Q. Sequential anti-inflammatory and osteogenic effects of a dual drug delivery scaffold loaded with parthenolide and naringin in periodontitis. J. Periodontal Implant Sci. 2023, 53, 20–37. [Google Scholar] [CrossRef]
- Feng, J.; Meng, Z. Insulin growth factor-1 promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells through the Wnt/β-catenin pathway. Exp. Ther. Med. 2021, 22, 891. [Google Scholar] [CrossRef]
- Devos, H.; Zoidakis, J.; Roubelakis, M.G.; Latosinska, A.; Vlahou, A. Reviewing the Regulators of COL1A1. Int. J. Mol. Sci. 2023, 24, 10004. [Google Scholar] [CrossRef]
- Wu, H.; Liao, X.; Wu, T.; Xie, B.; Ding, S.; Chen, Y.; Song, L.; Wei, B. Mechanism of MiR-145a-3p/Runx2 pathway in dexamethasone impairment of MC3T3-E1 osteogenic capacity in mice. PLoS ONE 2024, 19, e0309951. [Google Scholar] [CrossRef] [PubMed]
- De Pace, R.; Iaquinta, M.R.; Benkhalqui, A.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Mazziotta, C.; Rotondo, J.C.; Bononi, I.; Tognon, M.; et al. Revolutionizing bone healing: The role of 3D models. Cell Regen. 2025, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.C.; Hoch, A.I.; Harvestine, J.N.; Zhou, D.; Leach, J.K. Mesenchymal Stem Cell Spheroids Retain Osteogenic Phenotype Through α2β1 Signaling. Stem Cells Transl. Med. 2016, 5, 1229–1237. [Google Scholar] [CrossRef]
- Liu, X.; Astudillo Potes, M.D.; Dashtdar, B.; Schreiber, A.C.; Tilton, M.; Li, L.; Elder, B.D.; Lu, L. 3D Stem Cell Spheroids with 2D Hetero-Nanostructures for In Vivo Osteogenic and Immunologic Modulated Bone Repair. Adv. Healthc. Mater. 2024, 13, e2303772. [Google Scholar] [CrossRef] [PubMed]
- Melnik, D.; Sahana, J.; Corydon, T.J.; Kopp, S.; Nassef, M.Z.; Wehland, M.; Infanger, M.; Grimm, D.; Krüger, M. Dexamethasone Inhibits Spheroid Formation of Thyroid Cancer Cells Exposed to Simulated Microgravity. Cells 2020, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Yoshida, K.; Yang, D.; Haneji, T. Protein phosphatase 2A Cα regulates osteoblast differentiation and the expressions of bone sialoprotein and osteocalcin via osterix transcription factor. J. Cell. Physiol. 2013, 228, 1031–1037. [Google Scholar] [CrossRef]
- Getova, V.E.; Orozco-García, E.; Palmers, S.; Krenning, G.; Narvaez-Sanchez, R.; Harmsen, M.C. Extracellular Vesicles from Adipose Tissue-Derived Stromal Cells Stimulate Angiogenesis in a Scaffold-Dependent Fashion. Tissue Eng. Regen. Med. 2024, 21, 881–895. [Google Scholar] [CrossRef]
| Gene | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| RUNX2 | AAGTGCGGTGCAAACTTTCT | TCTCGGTGGCTGCTAGTGA |
| COL1A1 | CCAGAAGAACTGGTACATCAGCAA | TGGTTTCTTCTCCTCTGCGC |
| β-actin | TGGCACCCAG CACAATGAA | CTAAGTCATAGTCCGCCTAGAAGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, J.-H.; Lee, H.-J.; Park, J.-B. Impact of Dexamethasone on Three-Dimensional Stem Cell Spheroids: Morphology, Viability, Osteogenic Differentiation. Medicina 2025, 61, 871. https://doi.org/10.3390/medicina61050871
Lee H, Kim J-H, Lee H-J, Park J-B. Impact of Dexamethasone on Three-Dimensional Stem Cell Spheroids: Morphology, Viability, Osteogenic Differentiation. Medicina. 2025; 61(5):871. https://doi.org/10.3390/medicina61050871
Chicago/Turabian StyleLee, Heera, Ju-Hwan Kim, Hyun-Jin Lee, and Jun-Beom Park. 2025. "Impact of Dexamethasone on Three-Dimensional Stem Cell Spheroids: Morphology, Viability, Osteogenic Differentiation" Medicina 61, no. 5: 871. https://doi.org/10.3390/medicina61050871
APA StyleLee, H., Kim, J.-H., Lee, H.-J., & Park, J.-B. (2025). Impact of Dexamethasone on Three-Dimensional Stem Cell Spheroids: Morphology, Viability, Osteogenic Differentiation. Medicina, 61(5), 871. https://doi.org/10.3390/medicina61050871








