The Role of Pre-Treatment Inflammatory Biomarkers in Predicting Tumor Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Variables
- Patients aged 18 years or older;
- Histologically confirmed diagnosis of locally advanced rectal adenocarcinoma;
- Tumor located within 15 cm of the anal verge;
- Undergoing neoadjuvant chemoradiotherapy (CRT) or short-course radiotherapy (RT) followed by surgical resection;
- Availability of complete pre-treatment laboratory data, including inflammatory markers
- Availability of recurrence-free survival and pathological outcome data.
- Presence of synchronous malignancies or history of other primary malignancies;
- Presence of distant metastases at the time of diagnosis (stage IV disease);
- Incomplete treatment data or lack of follow-up information;
- Non-adenocarcinoma histologies (e.g., squamous cell carcinoma, neuroendocrine tumors);
- Patients who did not undergo surgical resection following neoadjuvant therapy.
2.2. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee on Cancer |
| AUC | Area Under the Curve |
| CAPEOX | Capecitabine and Oxaliplatin |
| CRC | Colorectal cancer |
| CRT | Chemoradiotherapy |
| DFS | Disease-free survival |
| NLR | Neutrophil-to-lymphocyte ratio |
| FOLFOX | 5-Fluorouracil, Leucovorin, and Oxaliplatin |
| LMR | Lymphocyte-to-monocyte ratio |
| LVI | Lymphovascular invasion |
| NCCN | National Comprehensive Cancer Network |
| pCR | Pathological complete response |
| ROC | Receiver Operating Characteristic |
| PLR | Platelet-to-lymphocyte ratio |
| PNI | Perineural invasion |
| RC | Rectal cancer |
| RFS | Recurrence-free survival |
| RT | Radiotherapy |
| SNT | Standard neoadjuvant treatment |
| SPSS | Statistical Package for the Social Sciences |
| TNM | Tumor, Node, Metastasis |
| TRG | Tumor regression grade |
| VI | Vascular invasion |
References
- Siegel, R.; Ma, J.; Zou, Z.; Ahmedin, J. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Minsky, B.D.; Mies, C.; Recht, A.; Chaffey, J.T. Resectable adenocarcinoma of the rectosigmoid and rectum. I. Patterns of failure and survival. Cancer. 1988, 61, 1408. [Google Scholar] [CrossRef] [PubMed]
- Adam, I.J.; Martin, I.; Finan, P.; Johnston, D.; Mohamdee, M.; Scott, N.; Dixon, M.; Quirke, P. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 1994, 344, 707. [Google Scholar] [CrossRef] [PubMed]
- Glynne-Jones, R.; Kronfli, M. Locally advanced rectal cancer: A comparison of management strategies. Drugs 2011, 71, 1153–1177. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Stinchcomb, D.G.; Howlader, N.; Horner, M.J.; Mariotto, A.; Miller, B.A.; Feuer, E.J.; Altekruse, S.F.; et al. SEER Cancer Statistics Review, 1975–2011; National Cancer Institute: Bethesda, MD, USA, 2008. [Google Scholar]
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef]
- Zorcolo, L.; Rosman, A.S.; Restivo, A.; Pisano, M.; Nigri, G.R.; Fancellu, A.; Melis, M. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: A meta-analysis. Ann. Surg. Oncol. 2012, 19, 2822–2832. [Google Scholar] [CrossRef]
- de Campos-Lobato, L.F.; Stocchi, L.; da Luz Moreira, A.; Geisler, D.; Dietz, D.W.; Lavery, I.C.; Fazio, V.W.; Kalady, M.F. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann. Surg. Oncol. 2011, 18, 1590–1598. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.-J.; A Calvo, F.; García-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Mohiuddin, M.; Hayne, M.; Regine, W.F.; Hanna, N.; Hagihara, P.F.; McGrath, P.; Marks, G.M. Prognostic significance of post-chemoradiation stage following preoperative chemotherapy and radiation for advanced/recurrent rectal cancers. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1075–1080. [Google Scholar] [CrossRef]
- An, S.; Shim, H.; Kim, K.; Kim, B.; Bang, H.J.; Do, H.; Lee, H.R.; Kim, Y. Pretreatment inflammatory markers predicting treatment outcomes in colorectal cancer. Ann. Coloproctol. 2022, 38, 97–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Theodoropoulos, G.; Wise, W.E.; Padmanabhan, A.; Kerner, B.A.; Taylor, C.W.; Aguilar, P.S.; Khanduja, K.S. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis. Colon. Rectum. 2002, 45, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, C.S.; Salmond, J.M.; Horgan, P.G.; Oien, K.A.; McMillan, D.C. The relationship between the local and systemic inflammatory responses and survival in patients undergoing curative surgery for colon and rectal cancers. J. Gastrointest. Surg. 2009, 13, 2011–2019. [Google Scholar] [CrossRef]
- Ryan, R.; Gibbons, D.; Hyland, J.M.; Treanor, D.; White, A.; E Mulcahy, H.; O’Donoghue, D.P.; Moriarty, M.; Fennelly, D.; Sheahan, K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 2005, 47, 141–146. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls (accessed on 18 May 2022).
- Buroker, T.; Nigro, N.; Correa, J.; Vaitkevicius, V.K.; Samson, M.; Considine, B. Combination preoperative radiation and chemotherapy in adenocarcinoma of the rectum: Preliminary report. Dis. Colon. Rectum 1976, 19, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, K.C.; Choi, J.H.; Oh, J.H.; Baek, J.-H.; Park, S.H.; Shin, D.B. Chemoradiotherapy followed by surgery in rectal cancer: Improved local control using a moderately high pelvic radiation dose. Jpn. J. Clin. Oncol. 2008, 38, 112–121. [Google Scholar] [CrossRef]
- Wheeler, J.M.; Dodds, E.; Warren, B.F.; Cunningham, C.; George, B.D.; Jones, A.C.; Mortensen, N.J.M. Preoperative chemoradiotherapy and total mesorectal excision surgery for locally advanced rectal cancer: Correlation with rectal cancer regression grade. Dis. Colon. Rectum 2004, 47, 2025–2031. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, L.; Liang, L.; Li, G.; Fan, M.; Wu, Y.; Zhu, J.; Zhang, Z. Baseline neutrophil-lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat. Oncol. 2014, 9, 295. [Google Scholar] [CrossRef]
- Carruthers, R.; Tho, L.M.; Brown, J.; Kakumanu, S.; McCartney, E.; McDonald, A.C. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Assoc. Coloproctol. Great Br. Irel. 2012, 14, 701–707. [Google Scholar] [CrossRef]
- Kitayama, J.; Yasuda, K.; Kawa, K.; Sunami, E.; Nagawa, H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer 2011, 11, 64. [Google Scholar] [CrossRef]
- Mark Krauthamer, M.; Rouvinov, K.; Ariad, S.; Man, S.; Walfish, S.; Pinsk, I.; Sztarker, I.; Charkovsky, T.; Lavrenkov, K. A Study of Inflammation-Based Predictors of Tumor Response to Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Oncology 2013, 85, 27–32. [Google Scholar] [CrossRef]
- Kim, Y.; You, S.H.; Kim, Y.W. Neutrophil-lymphocyte ratio predicts pathologic tumor response and survival after preoperative chemoradiation for rectal cancer. BMC Surg. 2014, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, T.; Akiyoshi, T.; Fujimoto, Y.; Konishi, T.; Nagayama, S.; Fukunaga, Y.; Ueno, M. Prognostic Impact of Neutrophil-to-Lymphocyte Ratio in Patients with Advanced Low Rectal Cancer Treated with Preoperative Chemoradiotherapy. Dig. Surg. 2015, 32, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Chun, M.M.D.; Noh, O.K.; Oh, Y.-T.; Suh, K.W.; Park, J.E.; Cho, O. Sustaining Blood Lymphocyte Count during Preoperative Chemoradiotherapy as a Predictive Marker for Pathologic Complete Response in Locally Advanced Rectal Cancer. Korean Cancer Assoc. 2016, 48, 232–239. [Google Scholar] [CrossRef]
- Jie Zhang, J.; Zhang, H.Y.; Li, J.; Shao, X.-Y.; Zhang, C.-X. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 68837. [Google Scholar] [CrossRef]
- Kim, N.K.; Baik, H.Y.; Seong, J.S.; Kim, H.; Roh, J.K.; Lee, K.Y.; Sohn, S.K.; Cho, C.H. Impact of Postirradiated Pathologic Downstaging on Local Recurrence and Survival. Oncologic Outcomes After Neoadjuvant Chemoradiation Followed by Curative Resection with Tumor- Specific Mesorectal Excision for Fixed Locally Advanced Rectal Cancer. Ann. Surg. 2006, 244, 1024–1030. [Google Scholar] [CrossRef]
- Kalady, M.F.; Lobato, L.F.C.; Luca Stocchi, L.; Geisler, D.P.; Dietz, D.; Lavery, I.C.; Fazio, V.W. Predictive Factors of Pathologic Complete Response After Neoadjuvant Chemoradiation for Rectal Cancer. Ann. Surg. 2009, 250, 213–220. [Google Scholar] [CrossRef]
- Martin, S.T.; Heneghan, H.M.; Winter, D.C. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br. J. Surg. 2012, 99, 918–928. [Google Scholar] [CrossRef]
- Sato, T.; Ozawa, H.; Hatate, K.; Onosato, W.; Naito, M.; Nakamura, T.; Ihara, A.; Koizumi, W.; Hayakawa, K.; Okayasu, I.; et al. A phase II trial of neoadjuvant preoperative chemoradiotherapy with S-1 plus irinotecan and radiation in patients with locally advanced rectal cancer: Clinical feasibility and response rate. Int. J. Radiat. Oncol. Biol. Phys. 2011, 19, 677–683. [Google Scholar] [CrossRef]


| Stage | Percentage (n = 79) |
|---|---|
| I | 11.4% (9) |
| 2A | 11.4% (9) |
| 2B | 5.1% (4) |
| 2C | 3.8% (3) |
| 3A | 24.1% (19) |
| 3B | 26.6% (21) |
| 3C | 10.1% (8) |
| 4A | 5.1% (4) |
| 4B | 2.5% (2) |
| Parameters | Percentage (n = 79) |
|---|---|
| Anal sphincter distance (cm) | |
| 1–4 | 40.5% (32) |
| 5–10 | 48.1% (38) |
| 11–15 | 11.4% (9) |
| LVI | |
| No | 79.7% (63) |
| Yes | 20.3% (16) |
| PNI | |
| No | 78.5% (62) |
| Yes | 21.5% (17) |
| Circumferential resection margin invasion | |
| No | 100% (79) |
| Yes | 0% (0) |
| Radiotherapy type | |
| RT | 27.8% (22) |
| CRT | 72.2% (57) |
| Parameters | Median (Min–Max) |
|---|---|
| Lymphocyte | 1390 (320–4090) |
| Derived NLR | 2.02 (0.87–7.96) |
| NLR | 3.04 (0.46–11.24) |
| PLR | 189.7 (52.72–779.62) |
| LMR | 2.41 (0.80–8.13) |
| Hemoglobin | 12.6 (7.5–16.10) |
| Neutrophil | 4520 (1940–12,050) |
| Age | 56 (32–83) |
| Parameters | Time to Relapse (Months) Median (Min–Max) | p |
|---|---|---|
| Differentiation n = 65 | 0.197 | |
| G1 = 53 | 16 (6–50) | |
| G2 = 4 | 11 (5–14) | |
| G3 = 8 | 27.5 (6–43) | |
| Tumor Regression n = 79 | 0.776 | |
| G0 = 10 | 12.5 (6–50) | |
| G1 = 24 | 15.5 (5–47) | |
| G2 = 29 | 15 (6–44) | |
| G3 = 16 | 18 (7–50) | |
| Response n = 79 | 0.685 | |
| Complete Response = 10 | 12.5 (6–50) | |
| Non-Complete = 69 | 15 (5–50) |
| Parameters n = 79 Tumor Regression Grade | G0 Median (Min–Max) | G1 Median (Min–Max) | G2 Median (Min–Max) | G3 Median (Min–Max) | p |
|---|---|---|---|---|---|
| Hemoglobin | 13.05 (11.1–15.7) | 12.55 (7.5–16.1) | 12.6 (9–16.5) | 12.65 (9.6–16) | p > 0.05 |
| Neutrophil | 4760 (2520–6330) | 5270 (2600–7860) | 3990 (1940–8370) | 4465 (2550–12,050) | 0.210 |
| Lymphocyte | 1315 (540–3200) | 1505 (600–4090) | 1170 (320–3970) | 1770 (610–2830) | 0.533 |
| Derived-NLR | 2.04 (1.32–4.66) | 2.02 (0.87–4.16) | 2.00 (0.87–4.59) | 1.815 (1.07–7.96) | 0.941 |
| NLR | 2.815 (1.91–11.24) | 3.42 (1.1–7.21) | 2.86 (0.46–10.09) | 2.74 (1.39–10.68) | 0.921 |
| PLR | 192.27 (12.18–779.62) | 183.25 (62.97–347) | 222.00 (57.72–603) | 142.66 (66.00–491.80) | 0.957 |
| Parameters n = 65 Differentiation | G1 Median (Min–Max) | G2 Median (Min–Max) | G3 Median (Min–Max) | p |
|---|---|---|---|---|
| Hemoglobin | 12.55 (7.5–16.1) | 12.6 (9–16.5) | 12.65 (9.6–16) | p > 0.05 |
| Neutrophil | 4520 (1940–12,050) | 4895 (3260–7150) | 5055 (2560–8440) | 0.891 |
| Lymphocyte | 1390 (320–4090) | 1805 (900–2100) | 830 (600–3350) | 0.400 |
| Derived-NLR | 1.96 (0.87–4.66) | 1.85 (1.18–3.25) | 2.81 (1.33–7.96) | 0.197 |
| NLR | 2.99 (0.46–11.24) | 2.63 (1.55–5.02) | 4.85 (1.74–10.68) | 0.278 |
| PLR | 189.51 (52.72–779.62) | 176.36 (95.85–326.66) | 256.00 (91.64–468.30) | 0.608 |
| Recurrence Time | Hemoglobin | Neutrophil | Lymphocyte | D-NLR | NLR | PLR |
|---|---|---|---|---|---|---|
| All Groups n = 79 Tumor Regression | Rho: 0.132 p: 0.246 | Rho: −0.130 p: 0.253 | Rho: 0.045 p: 0.694 | Rho: −0.129 p: 0.257 | Rho: −0.089 p: 0.438 | Rho: −0.175 p: 0.112 |
| G0 n = 10 | Rho: 0.088 p: 0.808 | Rho: 0.085 p: 0.815 | Rho: 0.444 p: 0.199 | Rho: −0.122 p: 0.738 | Rho: −0.366 p: 0.298 | Rho: −0.292 p: 0.413 |
| G1 n = 24 | Rho: 0.337 p: 0.107 | Rho: −0.213 p: 0.317 | Rho: −0.214 p: 0.315 | Rho: 0.019 p: 0.929 | Rho: 0.148 p: 0.489 | Rho: −0.009 p: 0.965 |
| G2 n = 29 | Rho: −0.012 p: 0.950 | Rho: −0.057 p: 0.769 | Rho: 0.021 p: 0.912 | Rho: −0.043 p: 0.825 | Rho: 0.082 p: 0.672 | Rho: −0.043 p: 0.824 |
| G3 n = 16 | Rho: 0.029 p: 0.914 | Rho: −0.355 p: 0.177 | Rho: 0.243 p: 0.364 | Rho: −0.514 p: 0.042 * | Rho: −0.514 p: 0.042 * | Rho: −0.522 p: 0.038 * |
| Recurrence Time | Hemoglobin | Neutrophil | Lymphocyte | D-NLR | NLR | PLR |
|---|---|---|---|---|---|---|
| All Groups n = 65 Differentiation | Rho: 0.148 p: 0.239 | Rho: −0.178 p: 0.157 | Rho: 0.060 p: 0.635 | Rho: −0.227 p: 0.068 | Rho: −0.126 p: 0.318 | Rho: −0.205 p: 0.102 |
| G1 n = 53 | Rho: 0.337 p: 0.107 | Rho: −0.145 p: 0.300 | Rho: 0.139 p: 0.320 | Rho: −0.343 p: 0.012 * | Rho: −0.208 p: 0.135 | Rho: −0.279 p: 0.043 * |
| G2 n = 4 | Rho: −0.012 p: 0.950 | Rho: −0.316 p: 0.684 | Rho: −0.738 p: 0.262 | Rho: 0.738 p: 0.262 | Rho: 0.738 p: 0.262 | Rho: 0.949 p: 0.051 |
| G3 n = 8 | Rho: 0.029 p: 0.914 | Rho: −0.180 p: 0.670 | Rho: −0.263 p: 0.528 | Rho: −0.024 p = 0.955 | Rho: 0.060 p = 0.888 | Rho: −0.096 p = 0.821 |
| Parameters PCR n = 79 | Median (Min–Max) Non-CR n = 69 | Median (Min–Max) Complete Response n = 10 | p |
|---|---|---|---|
| Neutrophil | 4520 (1940–12,050) | 4760 (2520–6330) | 0.889 |
| Lymphocyte | 1390 (320–4090) | 1315 (540–3200) | 0.912 |
| D-NLR | 2.01 (0.87–7.96) | 2.04 (1.32–4.66) | 0.819 |
| NLR | 3.07 (0.46–10.68) | 2.81 (1.91–11.24) | 0.831 |
| PLR | 189.51 (52.72–603.00) | 192.27 (112.18–779.62) | 0.930 |
| LMR | 2.29 (0.80–6.37) | 2.65 (0.83–8.13) | 0.327 |
| Parameters Group 1 (G1) Group 2 (G2 and G3) Differentiation n = 65 | Cutoff Value | Sensitivity | Specificity | Area Under the Curve (AUC) | p |
|---|---|---|---|---|---|
| Hemoglobin | 11.30 | 66.7% | 35.8% | 0.484 + −0.107 | 0.866 |
| Neutrophil | 4420 | 75.0% | 47.2% | 0.549 + −0.088 | 0.600 |
| Lymphocyte | 1220 | 50.0% | 45.3% | 0.447 + −0.087 | 0.565 |
| D-NLR | 2.06 | 75.0% | 62.3% | 0.605 + −0.095 | 0.257 |
| NLR | 2.76 | 75.0% | 45.3% | 0.573 + −0.091 | 0.432 |
| PLR | 170.6 | 66.7% | 47.2% | 0.554 + −0.093 | 0.560 |
| LMR | 2.13 | 83.3% | 49.1% | 0.612 + −0.080 | 0.227 |
| Parameters Group 1 (G0, G1) Group 2 (G2, G3) Tumor Regression | Cutoff Value | Sensitivity | Specificity | Area Under the Curve (AUC) | p |
|---|---|---|---|---|---|
| Hemoglobin | 12.35 | 50% | 42.2% | 0.489 + −0.066 | 0.866 |
| Neutrophil | 4270 | 73.5% | 53.3% | 0.622 + −0.064 | 0.065 |
| Lymphocyte | 1005 | 73.5% | 44.4% | 0.572 + −0.065 | 0.276 |
| D-NLR | 1.65 | 70.6% | 40% | 0.495 + −0.066 | 0.941 |
| NLR | 2.35 | 79.4% | 40% | 0.527 + −0.065 | 0.681 |
| PLR | 162.6 | 61.8% | 46.7% | 0.493 + −0.065 | 0.913 |
| LMR | 12.05 | 59.4% | 38.1% | 0.523 + −0.068 | 0.731 |
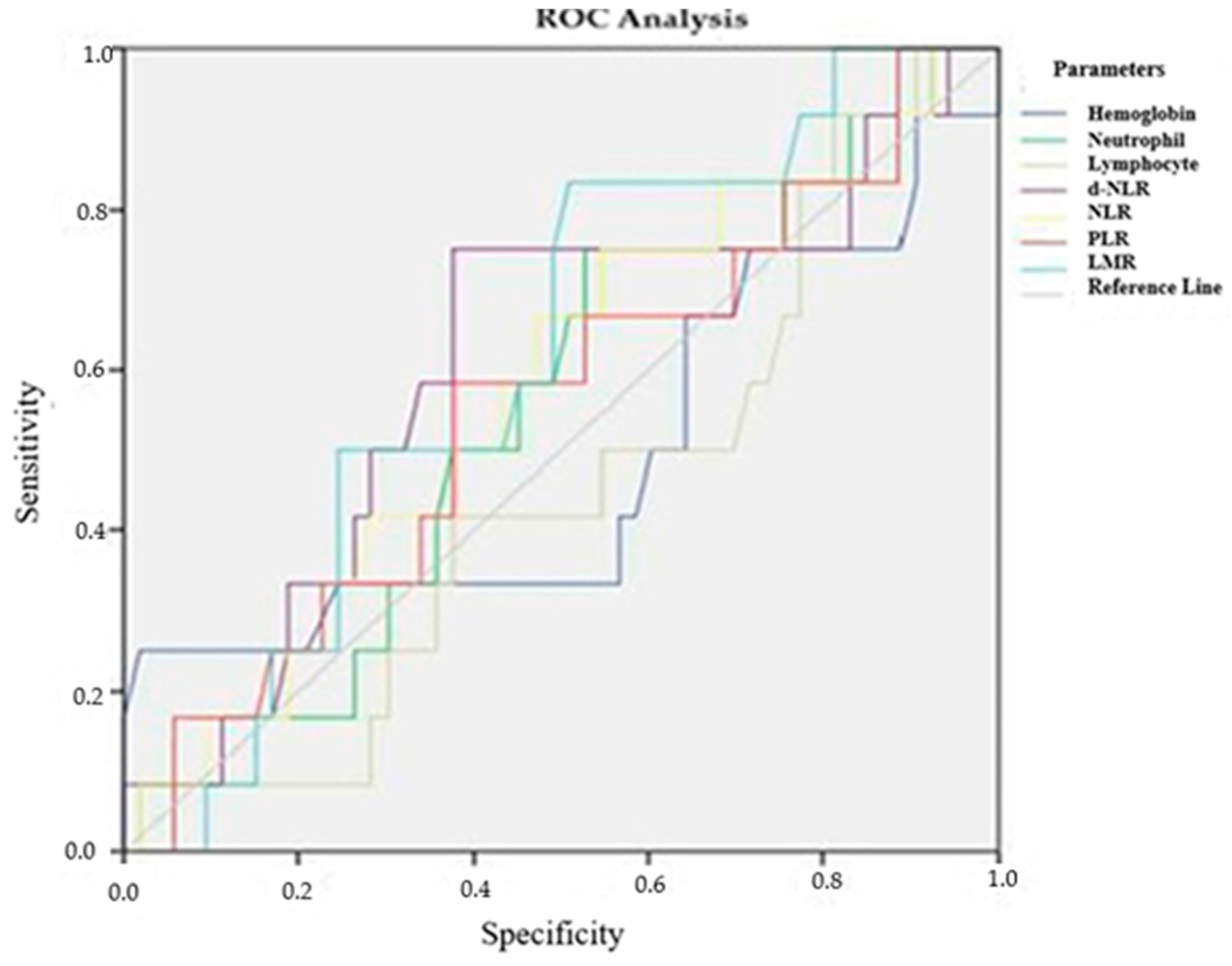
| Parameters Recurrence Time | Cutoff Value | Sensitivity | Specificity | Area Under the Curve (AUC) | p |
|---|---|---|---|---|---|
| Hemoglobin | 13.55 | 52.6% | 26.7% | 0.643 + −0.080 | 0.062 |
| Neutrophil | 5175 | 52.6% | 41.7% | 0.525 + −0.082 | 0.748 |
| Lymphocyte | 1665 | 52.6% | 36.7% | 0.597 + −0.081 | 0.205 |
| D-NLR | 1.98 | 63.2% | 60% | 0.572 + −0.082 | 0.347 |
| NLR | 2.73 | 63.2% | 65% | 0.618 + −0.085 | 0.121 |
| PLR | 162.6 | 63.2% | 65% | 0.621 + −0.088 | 0.113 |
| LMR | 3.55 | 63.2% | 23.3% | 0.593 + −0.082 | 0.224 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altıntaş, Y.E.; Bilici, A.; Yıldız, Ö.; Kınıkoğlu, O.; Ölmez, Ö.F. The Role of Pre-Treatment Inflammatory Biomarkers in Predicting Tumor Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Medicina 2025, 61, 865. https://doi.org/10.3390/medicina61050865
Altıntaş YE, Bilici A, Yıldız Ö, Kınıkoğlu O, Ölmez ÖF. The Role of Pre-Treatment Inflammatory Biomarkers in Predicting Tumor Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Medicina. 2025; 61(5):865. https://doi.org/10.3390/medicina61050865
Chicago/Turabian StyleAltıntaş, Yunus Emre, Ahmet Bilici, Özcan Yıldız, Oğuzcan Kınıkoğlu, and Ömer Fatih Ölmez. 2025. "The Role of Pre-Treatment Inflammatory Biomarkers in Predicting Tumor Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer" Medicina 61, no. 5: 865. https://doi.org/10.3390/medicina61050865
APA StyleAltıntaş, Y. E., Bilici, A., Yıldız, Ö., Kınıkoğlu, O., & Ölmez, Ö. F. (2025). The Role of Pre-Treatment Inflammatory Biomarkers in Predicting Tumor Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Medicina, 61(5), 865. https://doi.org/10.3390/medicina61050865






