Do Gut Microbiomes Shift After Bariatric Surgery? A Literature Review
Abstract
1. Introduction
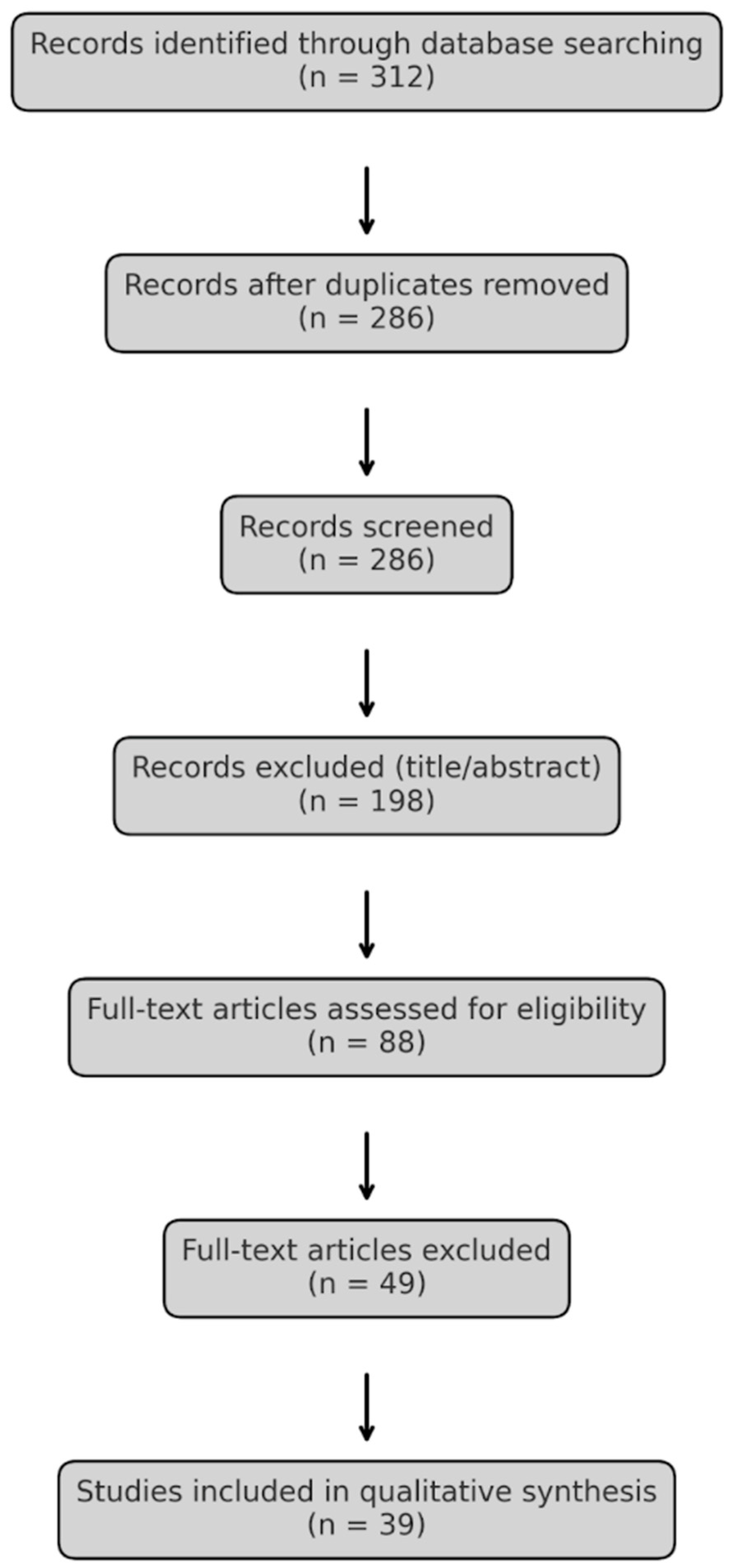
2. Materials and Methods
3. Results
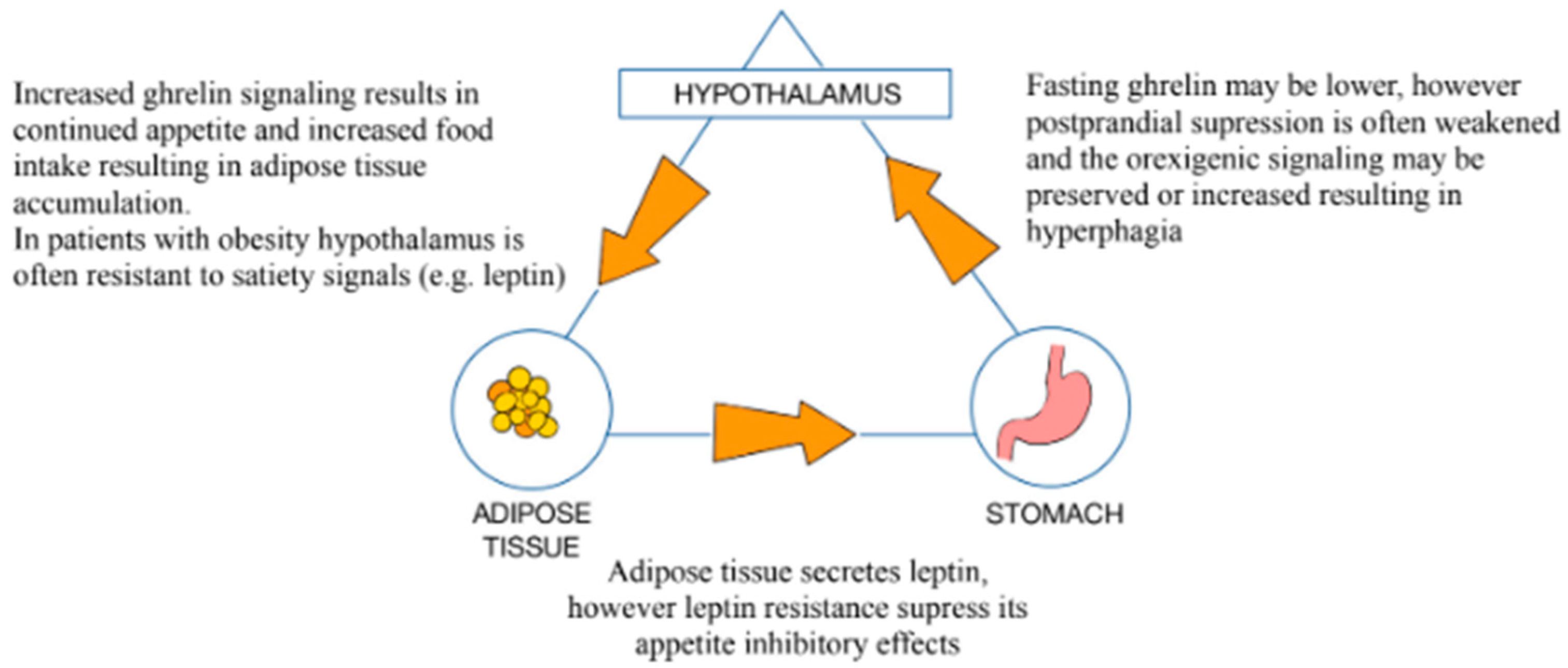
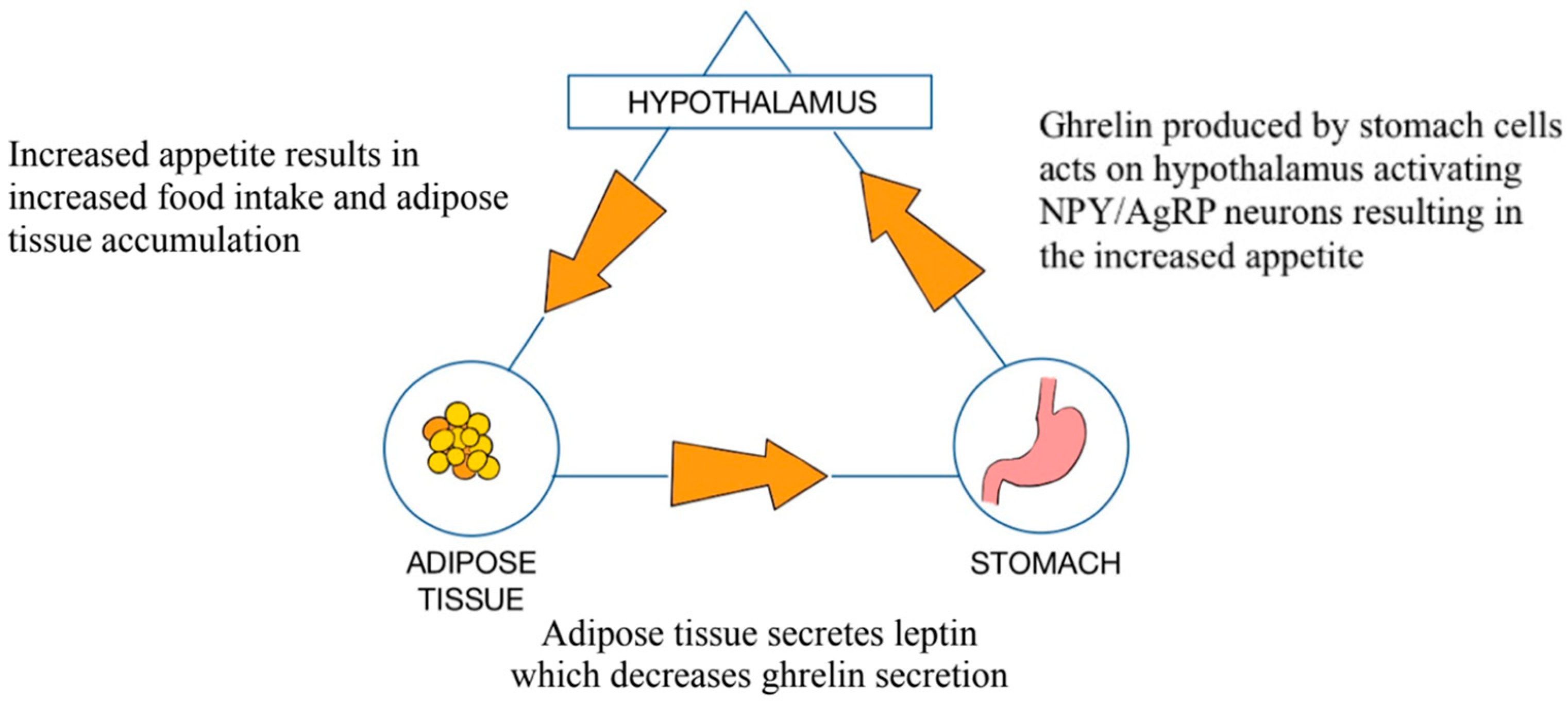
3.1. Role of the GM in the Pathophysiology of Obesity
3.2. Intestinal Hyperpermeability
3.3. The Firmicutes/Bacteroidetes (F/B) Ratio
3.4. Gut Dysbiosis
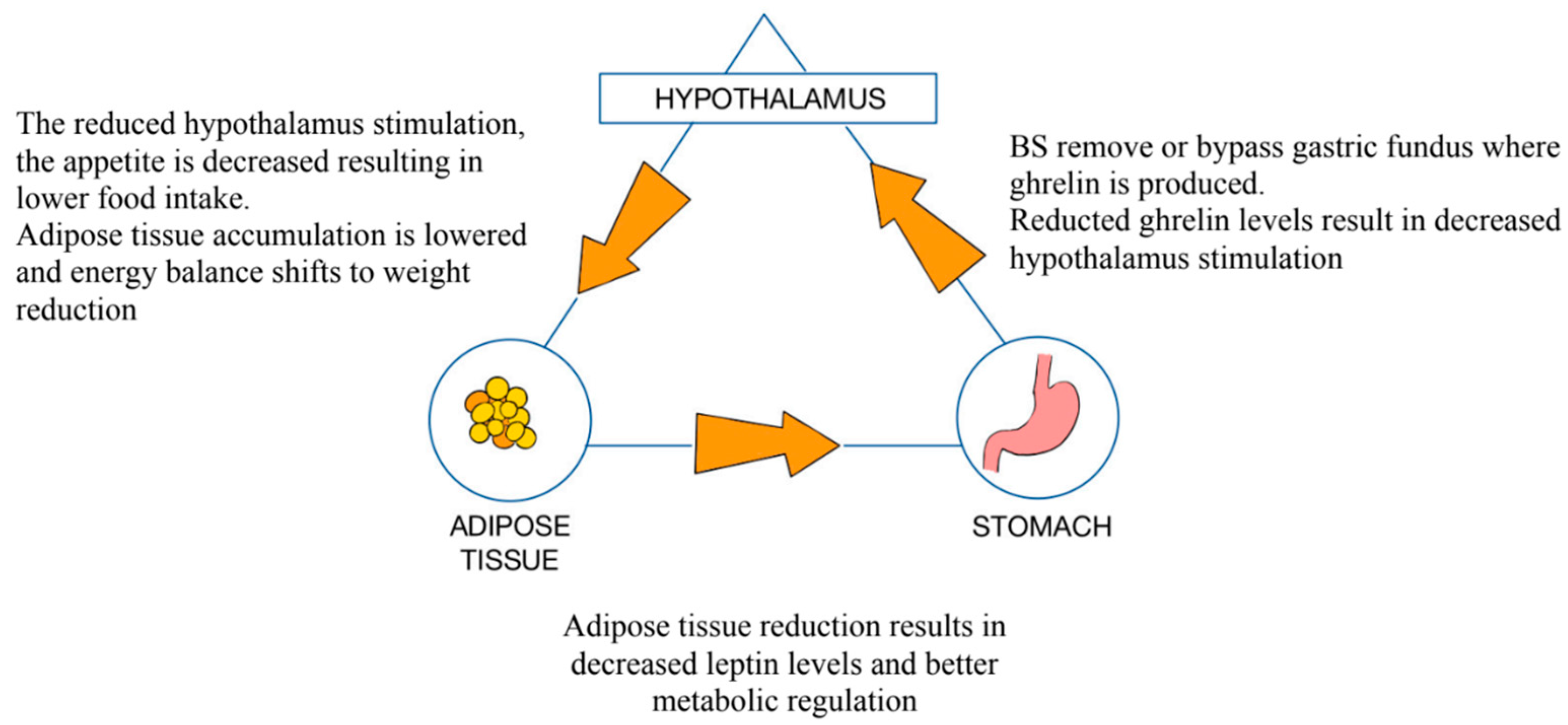
3.5. Roux-en-Y Gastric Bypass (RYGB)
3.6. Sleeve Gastrectomy (SG)
3.7. Mini-Gastric Bypass (MGB)
3.8. Metabolic Improvements and Individual Variability in Microbial Shifts After Bariatric Surgery
3.9. Persistence of Microbiota Changes Post-Bariatric Surgery
3.10. Limitations and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GM | Gut Microbiota |
| SCFAs | Short-Chain Fatty Acids |
| WHO | World Health Organization |
| BMI | Body Mass Index |
| LPSs | Lipopolysaccharides |
| FFAR3 | Free Fatty Acid Receptor 3 |
| PYY | Peptide YY |
| DM2 | Diabetes Mellitus Type 2 |
| TLR4 | Toll-Like Receptor 4 |
| F/B | Firmicutes/Bacteroidetes (ratio) |
| IBS-D | Diarrhea Variant of Irritable Bowel Syndrome |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor Necrosis Factor Alpha |
| INF-1 | Interferon Type I |
| FMT | Fecal Microbiota Transplant |
| CDI | Clostridioides Difficile Infection |
| RYGB | Roux-en-Y Gastric Bypass |
| SG | Sleeve Gastrectomy |
| LGB | Laparoscopic Gastric Banding |
| TJ | Tight Junction |
| TMAO | Trimethylamine-N-Oxide |
| IL-1β | Interleukin 1 Beta |
| CVD | Cardiovascular Disease |
| ASCVD | Atherosclerotic Cardiovascular Disease |
| MGB | Mini-Gastric Bypass |
| SAGB | Single-Anastomosis Gastric Bypass |
| OAGB | One-Anastomosis Gastric Bypass |
| IFSO | International Federation for the Surgery of Obesity and Metabolic Disorders |
References
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.A.; Schoonjans, K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, Y.; Zhang, Y.; Luo, F.; Song, K.; Wang, G.; Ling, F. Vitamin B12 produced by Cetobacterium somerae improves host resistance against pathogen infection through strengthening the interactions within gut microbiota. Microbiome 2023, 11, 135. [Google Scholar] [CrossRef]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2018, 102, 415–425. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Satoh-Takayama, N. Stomach microbiota, Helicobacter pylori, and group 2 innate lymphoid cells. Exp. Mol. Med. 2020, 52, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Wong, G.L.-H.; To, K.-F.; Wong, V.W.-S.; Lai, L.H.; Chow, D.K.-L.; Lau, J.Y.-W.; Sung, J.J.-Y.; Ding, C. Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal anti-inflammatory drug use. PLoS ONE 2009, 4, e7985. [Google Scholar] [CrossRef]
- Kim, J.-H.; Wolfe, B. Bariatric/metabolic surgery: Short- and long-term safety. Curr. Atheroscler. Rep. 2012, 14, 597–605. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Debédat, J.; Clément, K.; Aron-Wisnewsky, J. Gut Microbiota Dysbiosis in Human Obesity: Impact of Bariatric Surgery. Curr. Obes. Rep. 2019, 8, 229–242. [Google Scholar] [CrossRef]
- Munukka, E.; Wiklund, P.; Pekkala, S.; Völgyi, E.; Xu, L.; Cheng, S.; Lyytikäinen, A.; Marjomäki, V.; Alen, M.; Vaahtovuo, J.; et al. Women with and without metabolic disorder differ in their gut microbiota composition. Obesity 2012, 20, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N.; Verma, P.; Kumar, R.; Kumar, S. Probiotic microbes: Biodiversity, mechanisms of action and potential role in human health. In Proceedings of the National Conference on Advances in Food Science and Technology, Paris, France, 23–25 October 2017; Volume 33. [Google Scholar]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed]
- Theodorakis, N.; Nikolaou, M. The Human Energy Balance: Uncovering the Hidden Variables of Obesity. Diseases 2025, 13, 55. [Google Scholar] [CrossRef]
- Saeed, S.; Bonnefond, A.; Froguel, P. Obesity: Exploring its connection to brain function through genetic and genomic perspectives. Mol. Psychiatry 2025, 30, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Kahan, L.G.; Mehrzad, R. Environmental factors related to the obesity epidemic. In Obesity; Mehrzad, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 117–139. [Google Scholar]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Smith, B.R.; Schauer, P.; Nguyen, N.T. Surgical Approaches to the Treatment of Obesity: Bariatric Surgery. Endocrinol. Metab. Clin. N. Am. 2008, 37, 943–964. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Kurniawan, A. Appetite problem in cancer patients: Pathophysiology, diagnosis, and treatment. Cancer Treat. Res. Commun. 2021, 27, 100336. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Harakeh, S.M.; Khan, I.; Kumosani, T.; Barbour, E.; Almasaudi, S.B.; Bahijri, S.M.; Alfadul, S.M.; Ajabnoor, G.M.A.; Azhar, E.I. Gut Microbiota: A Contributing Factor to Obesity. Front. Cell. Infect. Microbiol. 2016, 6, 95. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Muñoz-Garach, A.; Clemente-Postigo, M.; Tinahones, F.J. Importance of gut microbiota in obesity. Eur. J. Clin. Nutr. 2019, 72, 26–37. [Google Scholar] [CrossRef]
- Enache, R.-M.; Profir, M.; Roşu, O.A.; Creţoiu, S.M.; Gaspar, B.S. The Role of Gut Microbiota in the Onset and Progression of Obesity and Associated Comorbidities. Int. J. Mol. Sci. 2024, 25, 12321. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef]
- Singh, A.V.; Bhardwaj, P.; Laux, P.; Pradeep, P.; Busse, M.; Luch, A.; Hirose, A.; Osgood, C.J.; Stacey, M.W. AI and ML-based risk assessment of chemicals: Predicting carcinogenic risk from chemical-induced genomic instability. Front. Toxicol. 2024, 6, 1461587. [Google Scholar] [CrossRef] [PubMed]
- Pokrzywnicka, P.; Gumprecht, J. Intestinal microbiota and its relationship with diabetes and obesity. Clin. Diabetol. 2017, 5, 164–172. [Google Scholar] [CrossRef]
- Dreyer, J.L.; Liebl, A.L. Early colonization of the gut microbiome and its relationship with obesity. Hum. Microbiome J. 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Cano, R.; Bermúdez, V.; Galban, N.; Garrido, B.; Santeliz, R.; Gotera, M.P.; Duran, P.; Boscan, A.; Carbonell-Zabaleta, A.-K.; Durán-Agüero, S.; et al. Dietary polyphenols and gut microbiota cross-talk: Molecular and therapeutic perspectives for cardiometabolic disease: A narrative review. Int. J. Mol. Sci. 2024, 25, 9118. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Yáñez, A.; Price, J.G.; Chow, A.; Merad, M.; Goodridge, H.S.; Mazmanian, S.K. Gut Microbiota Promote Hematopoiesis to Control Bacterial Infection. Cell Host Microbe 2014, 15, 374–381. [Google Scholar] [CrossRef]
- Ng, S.C.; Bernstein, C.N.; Vatn, M.H.; Lakatos, P.L.; Loftus, E.V., Jr.; Tysk, C.; O’Morain, C.; Moum, B.; Colombel, J.-F.; on behalf of the Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD). Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 2013, 62, 630–649. [Google Scholar] [CrossRef]
- Murk, W.; Risnes, K.R.; Bracken, M.B. Prenatal or Early-Life Exposure to Antibiotics and Risk of Childhood Asthma: A Systematic Review. Pediatrics 2011, 127, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Li, J.V.; Ashrafian, H.; Sarafian, M.; Homola, D.; Rushton, L.; Barker, G.; Cabrera, P.M.; Lewis, M.R.; Darzi, A.; Lin, E.; et al. Roux-en-Y gastric bypass-induced bacterial perturbation contributes to altered host-bacterial co-metabolic phenotype. Microbiome 2021, 9, 139. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Chen, Y.; Lian, G.; Wang, J.; Zhang, J.; Shan, K.; Shang, L.; Tian, F.; Jing, C. Role of Gut Microbiome and Microbial Metabolites in Alleviating Insulin Resistance After Bariatric Surgery. Obes. Surg. 2021, 31, 327–336. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Gutiérrez-Repiso, C.; Gómez-Pérez, A.M.; García-Fuentes, E.; Tinahones, F.J.; Moreno-Indias, I. Gut microbiota adaptation after weight loss by Roux-en-Y gastric bypass or sleeve gastrectomy bariatric surgeries. Surg. Obes. Relat. Dis. 2019, 15, 1888–1895. [Google Scholar] [CrossRef]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Bariatric Surgery in Obesity: Effects on Gut Microbiota and Micronutrient Status. Nutrients 2020, 12, 235. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, Q.; Zhong, H.; Li, D.; Yu, S.; Yang, H.; Wang, C.; Yin, Z. Roux-en-Y Gastric Bypass Improved Insulin Resistance via Alteration of the Human Gut Microbiome and Alleviation of Endotoxemia. BioMed Res. Int. 2021, 2021, 5554991. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef] [PubMed]
- Casselbrant, A.; Elias, E.; Fändriks, L.; Wallenius, V. Expression of tight-junction proteins in human proximal small intestinal mucosa before and after Roux-en-Y gastric bypass surgery. Surg. Obes. Relat. Dis. 2015, 11, 45–53. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Roca-Rodriguez, M.d.M.; Camargo, A.; Ocaña-Wilhelmi, L.; Cardona, F.; Tinahones, F.J. Lipopolysaccharide and lipopolysaccharide-binding protein levels and their relationship to early metabolic improvement after bariatric surgery. Surg. Obes. Relat. Dis. 2015, 11, 933–939. [Google Scholar] [CrossRef]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut Microbiota-Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef]
- Shanmugham, M.; Bellanger, S.; Leo, C.H. Gut-Derived Metabolite, Trimethylamine-N-oxide (TMAO) in Cardio-Metabolic Diseases: Detection, Mechanism, and Potential Therapeutics. Pharmaceuticals 2023, 16, 504. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Hov, J.R.; Nestvold, T.K.; Thoresen, H.; Berge, R.K.; Svardal, A.; Lappegård, K.T. Major Increase in Microbiota-Dependent Proatherogenic Metabolite TMAO One Year After Bariatric Surgery. Metab. Syndr. Relat. Disord. 2016, 14, 197–201. [Google Scholar] [CrossRef]
- Seeras, K.; Sankararaman, S.; Lopez, P.P. Sleeve Gastrectomy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Murphy, R.; Tsai, P.; Jüllig, M.; Liu, A.; Plank, L.; Booth, M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes. Surg. 2017, 27, 917–925. [Google Scholar] [CrossRef]
- Hamamah, S.; Hajnal, A.; Covasa, M. Influence of Bariatric Surgery on Gut Microbiota Composition and Its Implication on Brain and Peripheral Targets. Nutrients 2024, 16, 1071. [Google Scholar] [CrossRef]
- Sanmiguel, C.P.; Jacobs, J.; Gupta, A.; Ju, T.B.; Stains, J.; Coveleskie, K.; Lagishetty, V.; Balioukova, A.; Chen, Y.; Dutson, E.; et al. Surgically Induced Changes in Gut Microbiome and Hedonic Eating as Related to Weight Loss: Preliminary Findings in Obese Women Undergoing Bariatric Surgery. Psychosom. Med. 2017, 79, 880–887. [Google Scholar] [CrossRef]
- Yang, J.; Chen, L.; Shang, X.-Y.; Chen, Y.-L.; Zhao, S.-S.; Jin, S.; Liu, H.-X.; Du, J. Roux-en-Y gastric bypass-induced perturbative changes in microbial communities and metabolic pathways in rats. Front. Microbiol. 2022, 13, 1034839. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Tan, X.; Yan, H.; Shen, Q.; Hua, R.; Shao, Y.; Yao, Q. Sleeve gastrectomy decreases high-fat diet induced colonic pro-inflammatory status through the gut microbiota alterations. Front. Endocrinol. 2023, 14, 1091040. [Google Scholar] [CrossRef] [PubMed]
- Chaim, E.A.; Ramos, A.C.; Cazzo, E. Mini-gastric bypass: Description of the technique and preliminary results. Abcd-Arquivos Bras. Cir. Dig. Arch. Dig. Surg. 2017, 30, 264–266. [Google Scholar] [CrossRef]
- De Luca, M.; Tie, T.; Ooi, G.; Higa, K.; Himpens, J.; Carbajo, M.-A.; Mahawar, K.; Shikora, S.; Brown, W.A. Mini Gastric Bypass-One Anastomosis Gastric Bypass (MGB-OAGB)-IFSO Position Statement. Obes. Surg. 2018, 28, 1188–1206. [Google Scholar] [CrossRef]
- Rutledge, R.; Kular, K.; Manchanda, N. The Mini-Gastric Bypass original technique. Int. J. Surg. 2019, 61, 38–41. [Google Scholar] [CrossRef]
- Amin, U.; Huang, D.; Dhir, A.; Shindler, A.E.; Franks, A.E.; Thomas, C.J. Effects of gastric bypass bariatric surgery on gut microbiota in patients with morbid obesity. Gut Microbes 2024, 16, 2427312. [Google Scholar] [CrossRef]
- Tseng, H.H.; Wu, C.Y. The gut microbiome in obesity. J. Formos. Med. Assoc. 2019, 118 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef]
- Koutoukidis, D.A.; Jebb, S.A.; Zimmerman, M.; Otunla, A.; Henry, J.A.; Ferrey, A.; Schofield, E.; Kinton, J.; Aveyard, P.; Marchesi, J.R. The association of weight loss with changes in the gut microbiota diversity, composition, and intestinal permeability: A systematic review and meta-analysis. Gut Microbes 2022, 14, 2020068. [Google Scholar] [CrossRef]
| Bariatric Procedure | Increased Bacterial Populations | Decreased Bacterial Populations | Additional Notes |
|---|---|---|---|
| Roux-en-Y Gastric Bypass (RYGB) | Veillonella spp., Acidaminococcus spp., Slackia spp., Granulicatella spp., Akkermansia spp., Escherichia spp., Klebsiella spp., Gammaproteobacteria | Gemella spp., Clostridioides difficile, Clostridium hiranonis, Lachnospiraceae, Ruminococcaceae | Increased microbial metabolites (TMAO); potential gut permeability changes; reduced LPS levels post-surgery. |
| Sleeve Gastrectomy (SG) | Bacteroidetes, Actinobacteria, Blautia spp., Akkermansia spp., Eubacterium spp., Lactobacillus spp., Cyanobacteria, Haemophilus spp. | Firmicutes, Desulfovibrio spp. | Lowered F/B 1 ratio; increased anti-inflammatory species; enhanced ketone body metabolism. |
| Mini-Gastric Bypass (MGB) | Limited research available | Limited research available | Similar weight reduction outcomes to RYGB; improved glycemic control and lipid profile. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorysz, Z.; Kowalewski, P.; Walędziak, M.; Różańska-Walędziak, A. Do Gut Microbiomes Shift After Bariatric Surgery? A Literature Review. Medicina 2025, 61, 849. https://doi.org/10.3390/medicina61050849
Sorysz Z, Kowalewski P, Walędziak M, Różańska-Walędziak A. Do Gut Microbiomes Shift After Bariatric Surgery? A Literature Review. Medicina. 2025; 61(5):849. https://doi.org/10.3390/medicina61050849
Chicago/Turabian StyleSorysz, Zofia, Piotr Kowalewski, Maciej Walędziak, and Anna Różańska-Walędziak. 2025. "Do Gut Microbiomes Shift After Bariatric Surgery? A Literature Review" Medicina 61, no. 5: 849. https://doi.org/10.3390/medicina61050849
APA StyleSorysz, Z., Kowalewski, P., Walędziak, M., & Różańska-Walędziak, A. (2025). Do Gut Microbiomes Shift After Bariatric Surgery? A Literature Review. Medicina, 61(5), 849. https://doi.org/10.3390/medicina61050849






