Description of the Human Penile Urethra Epithelium
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
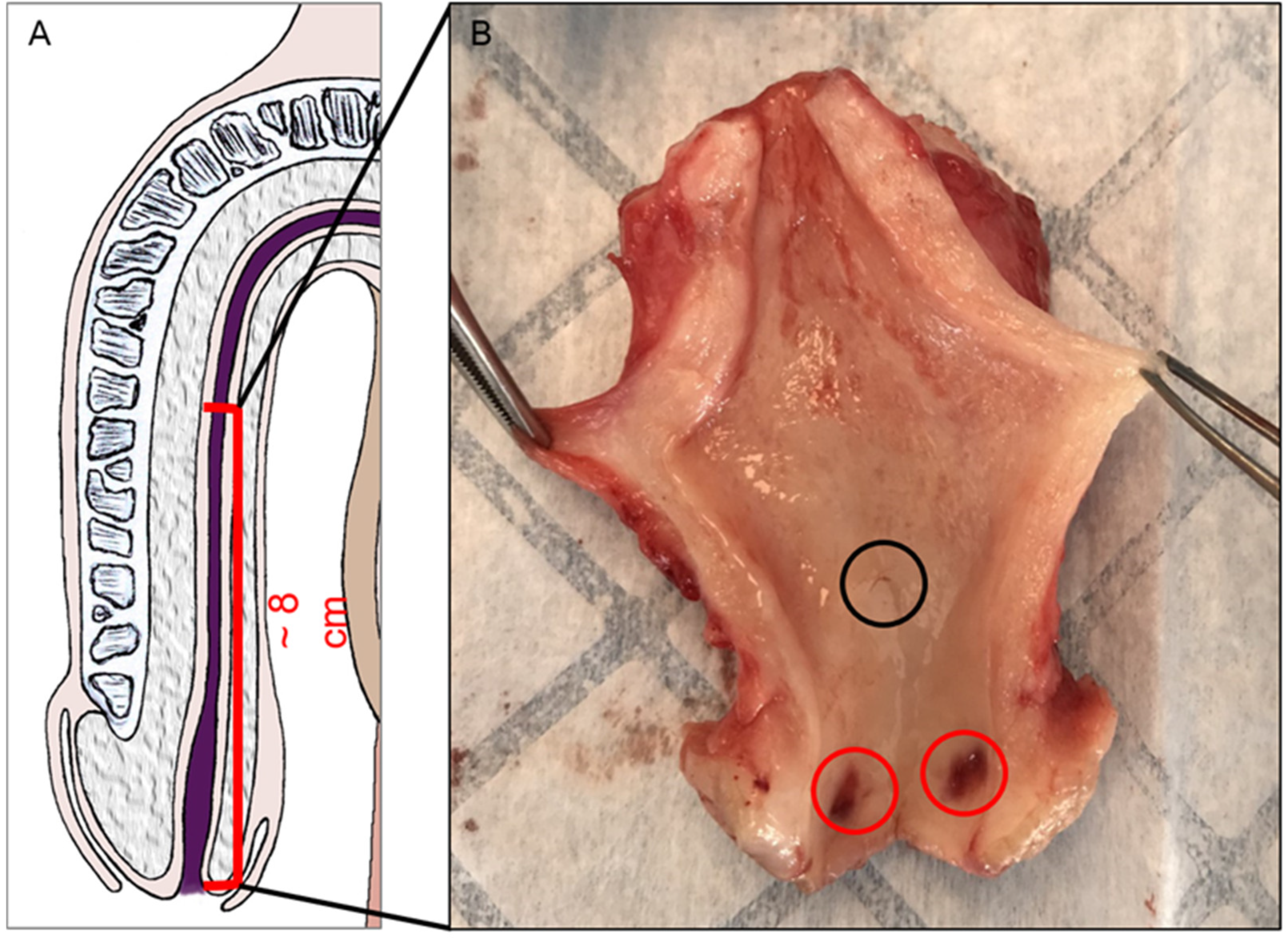
2.2. Organ Collection and Preparation
2.3. Histological Studies
2.4. Thickness Measurement
2.5. Vascular Network Evaluation
2.6. Statistical Analyses
3. Results
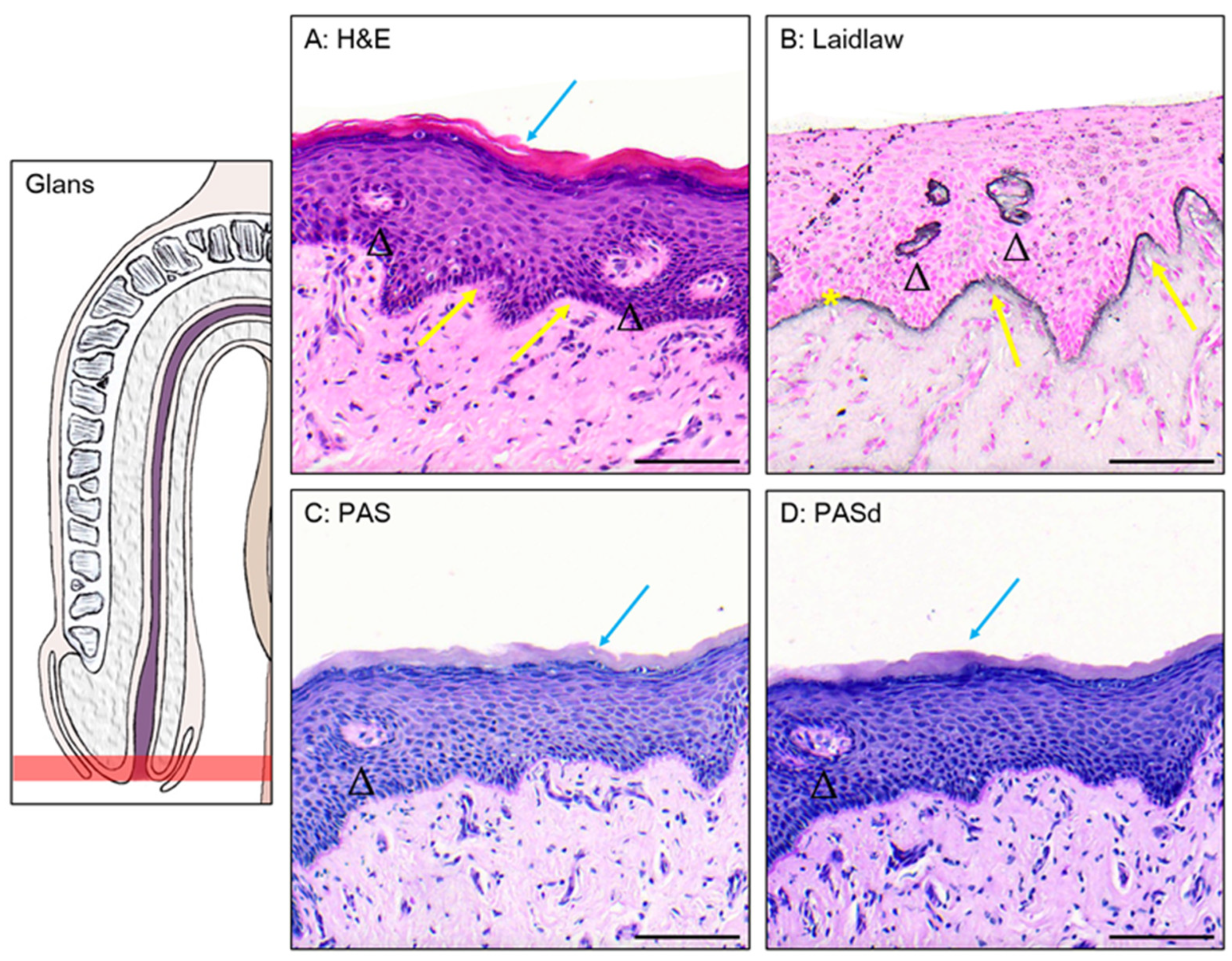
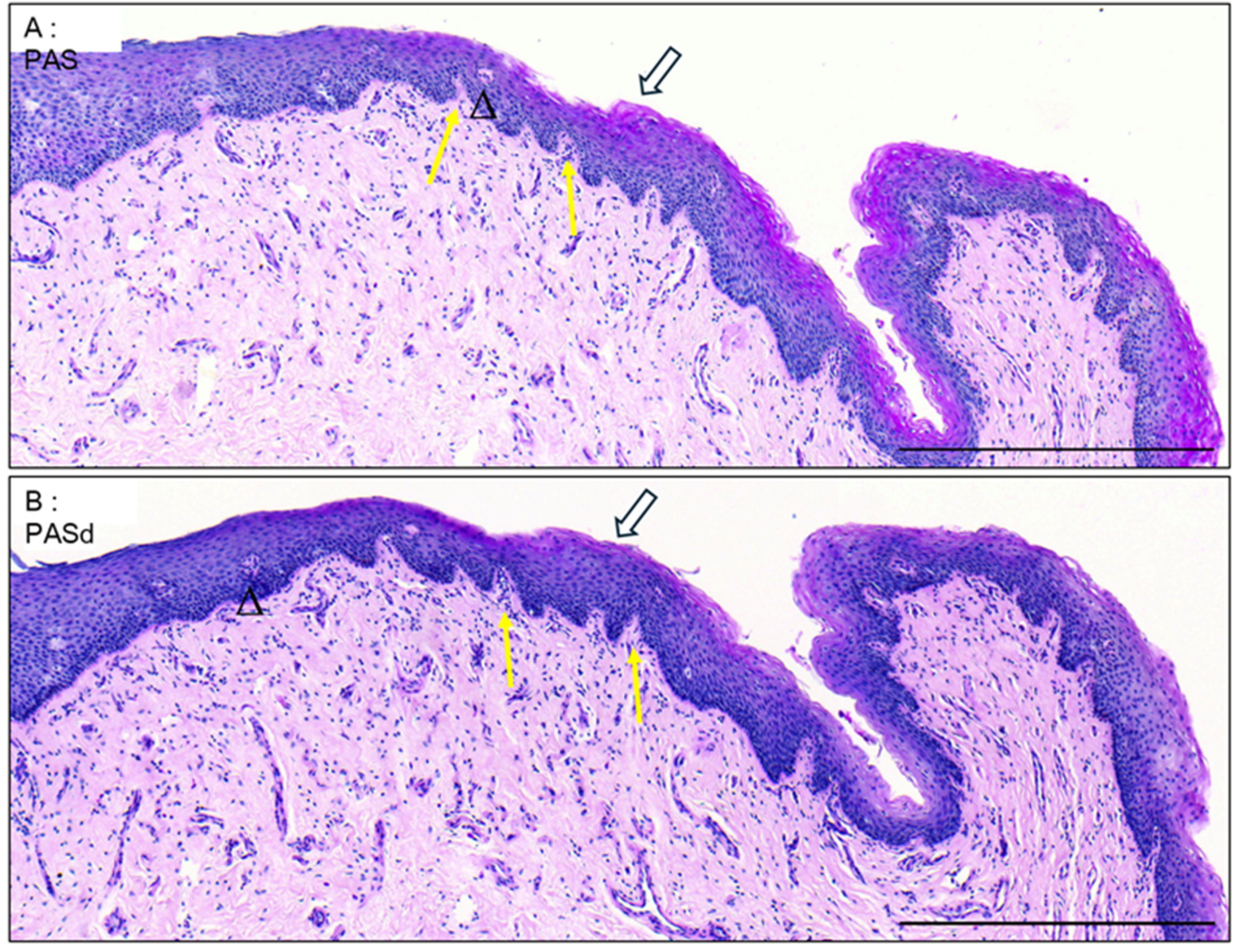
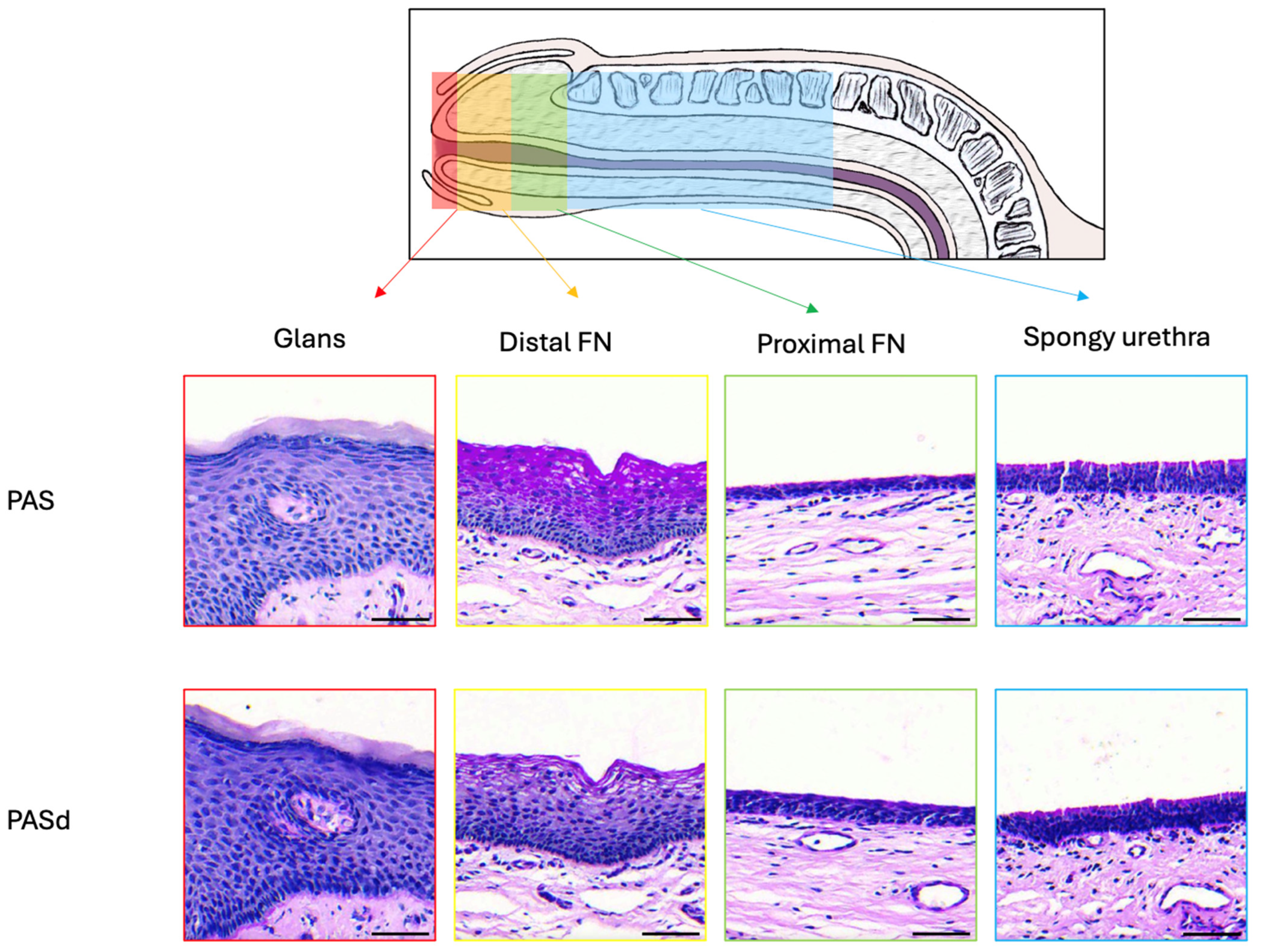
3.1. Epithelium of the Glans
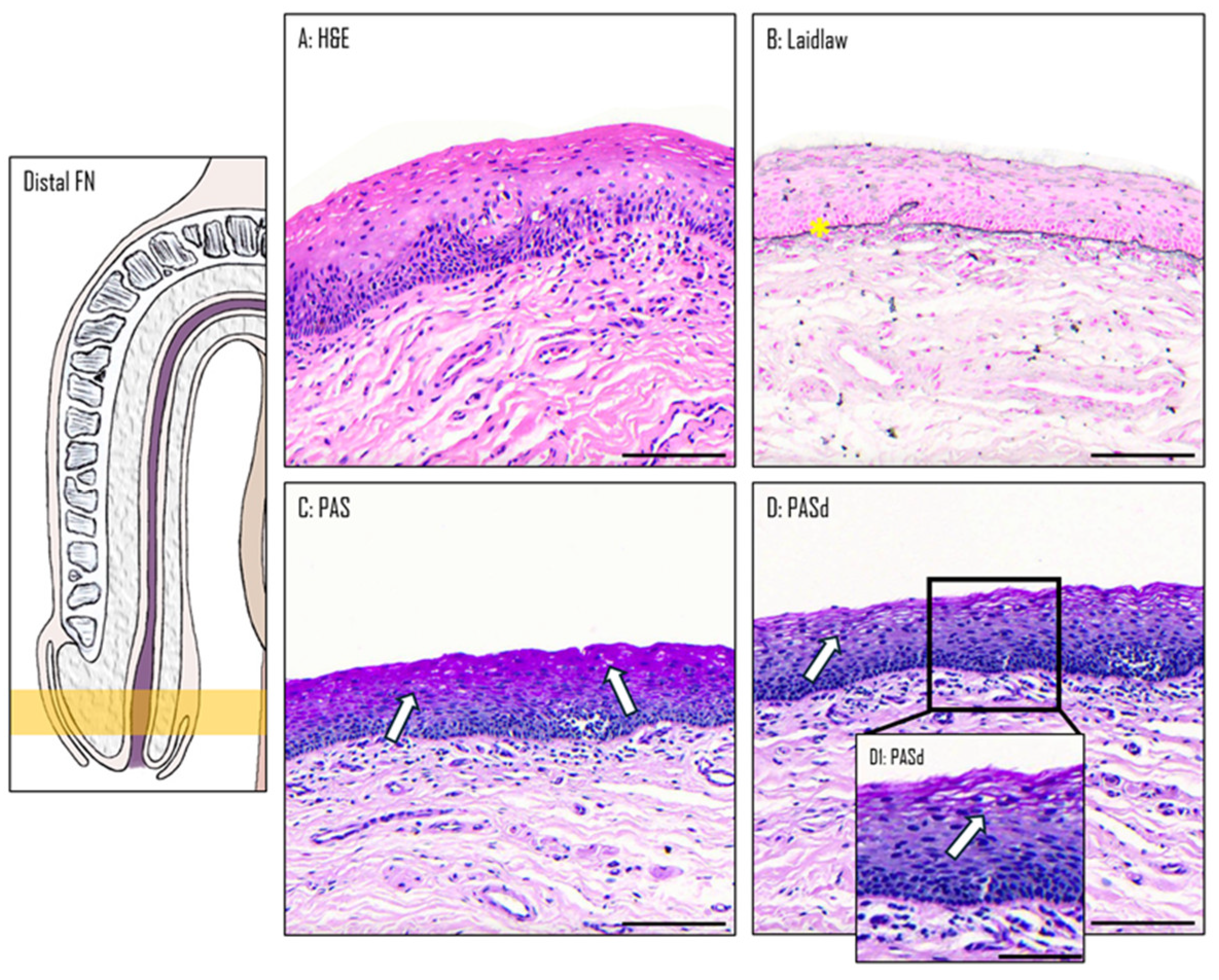
3.2. Epithelium of the Distal Fossa Navicularis (DFN)
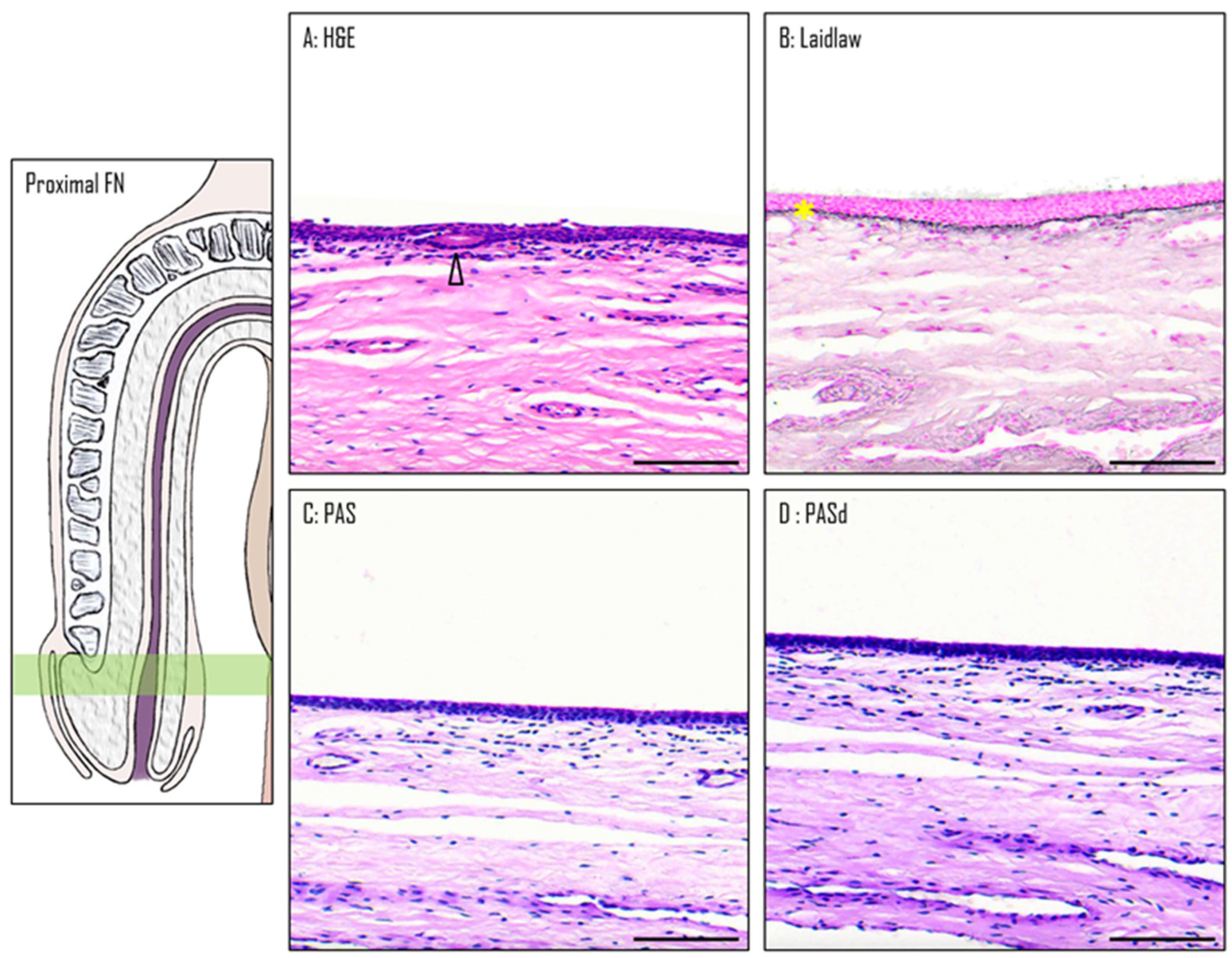
3.3. Epithelium of the Proximal Fossa Navicularis (PFN)
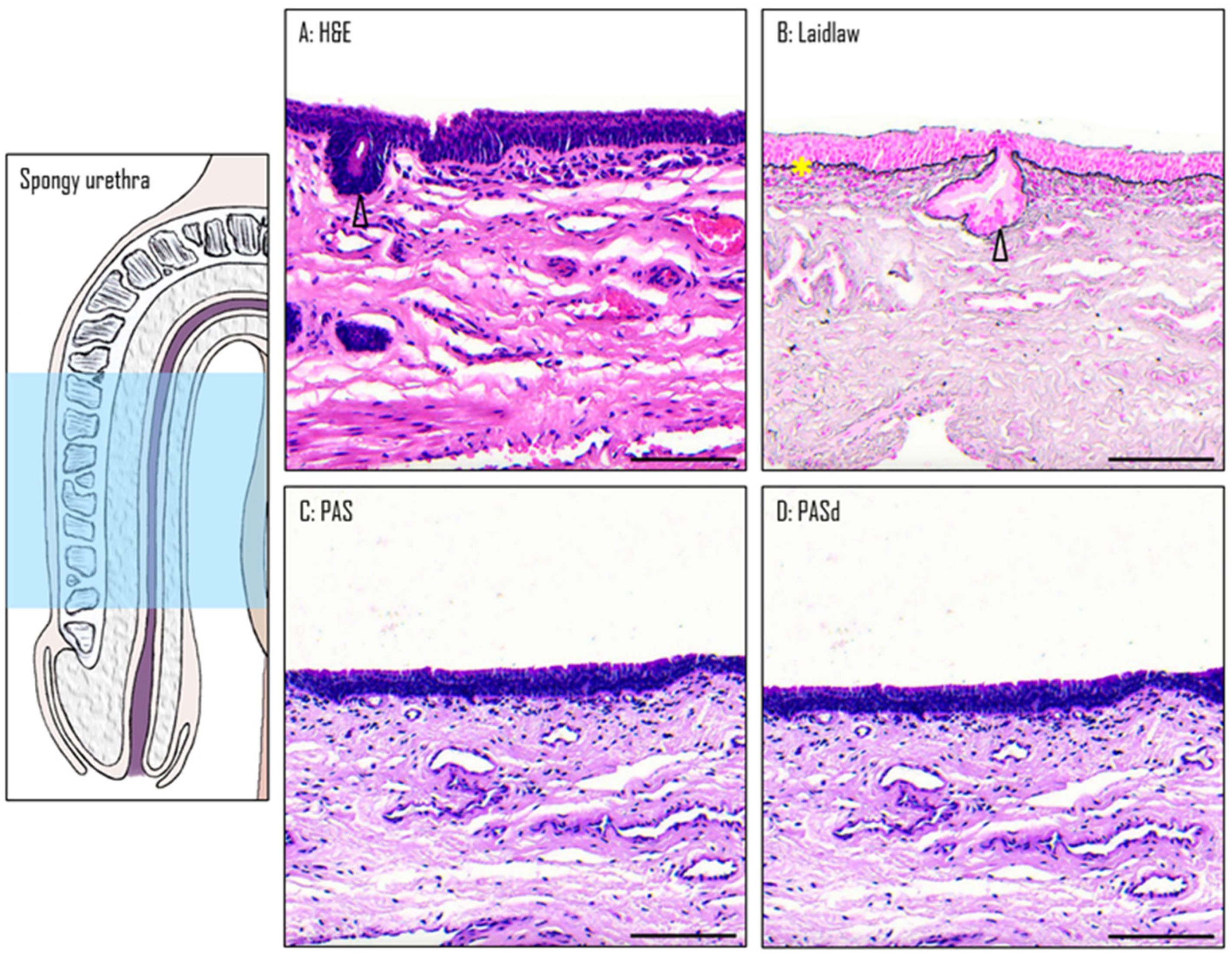
3.4. Epithelium of the Spongy Urethra
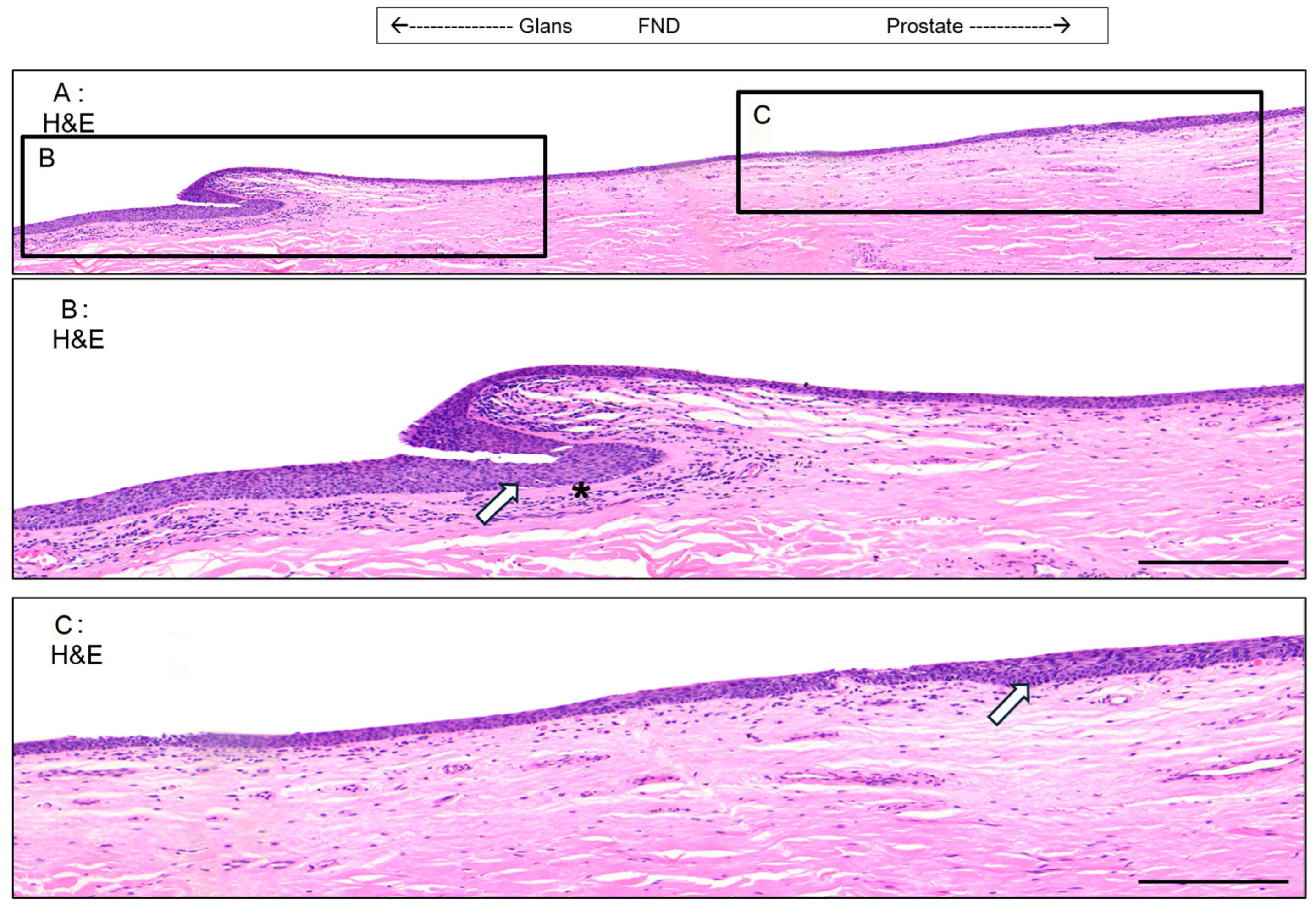
3.5. Valvule-like Structure
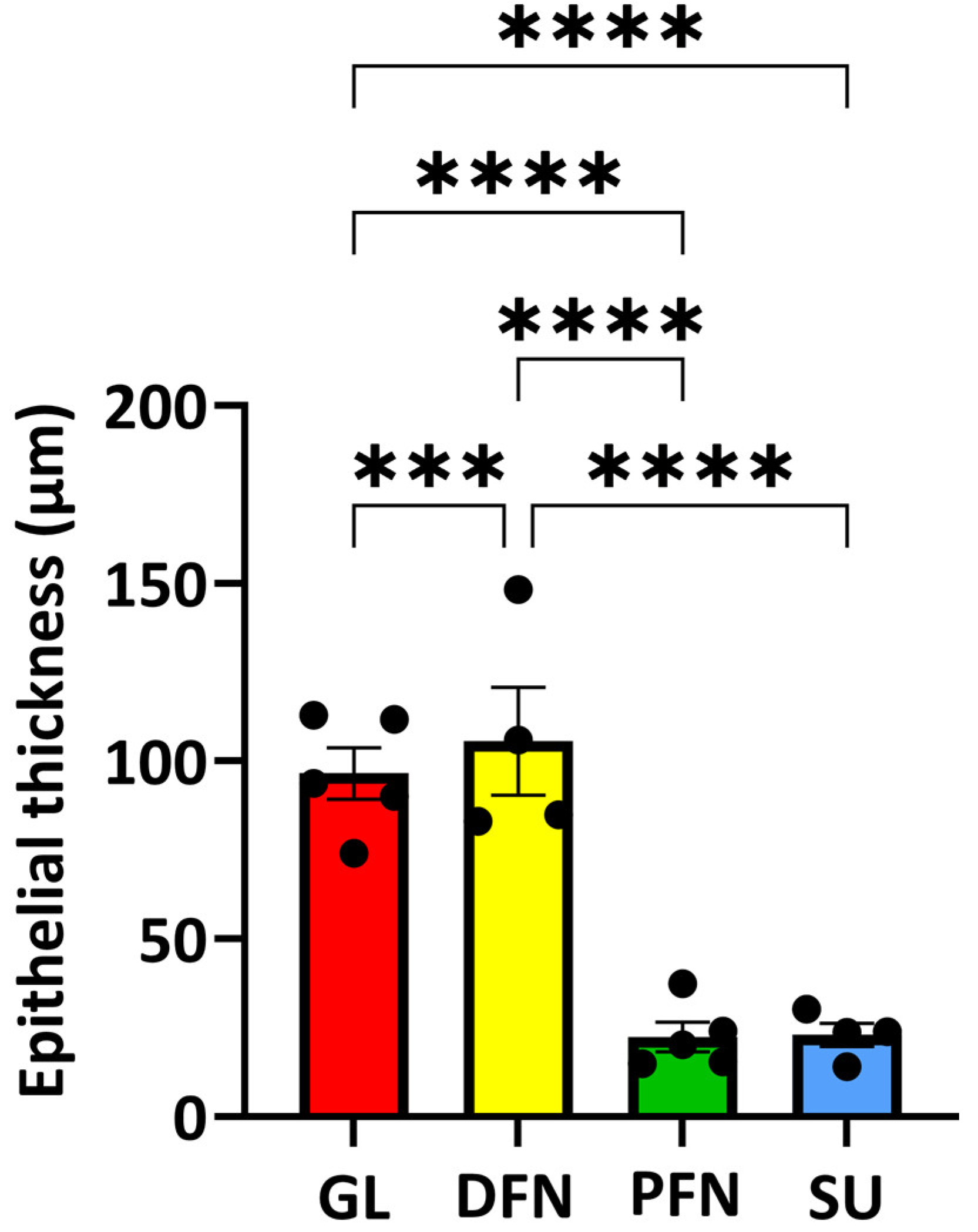
3.6. Thickness of the Diverse Epithelia Along the Urethra
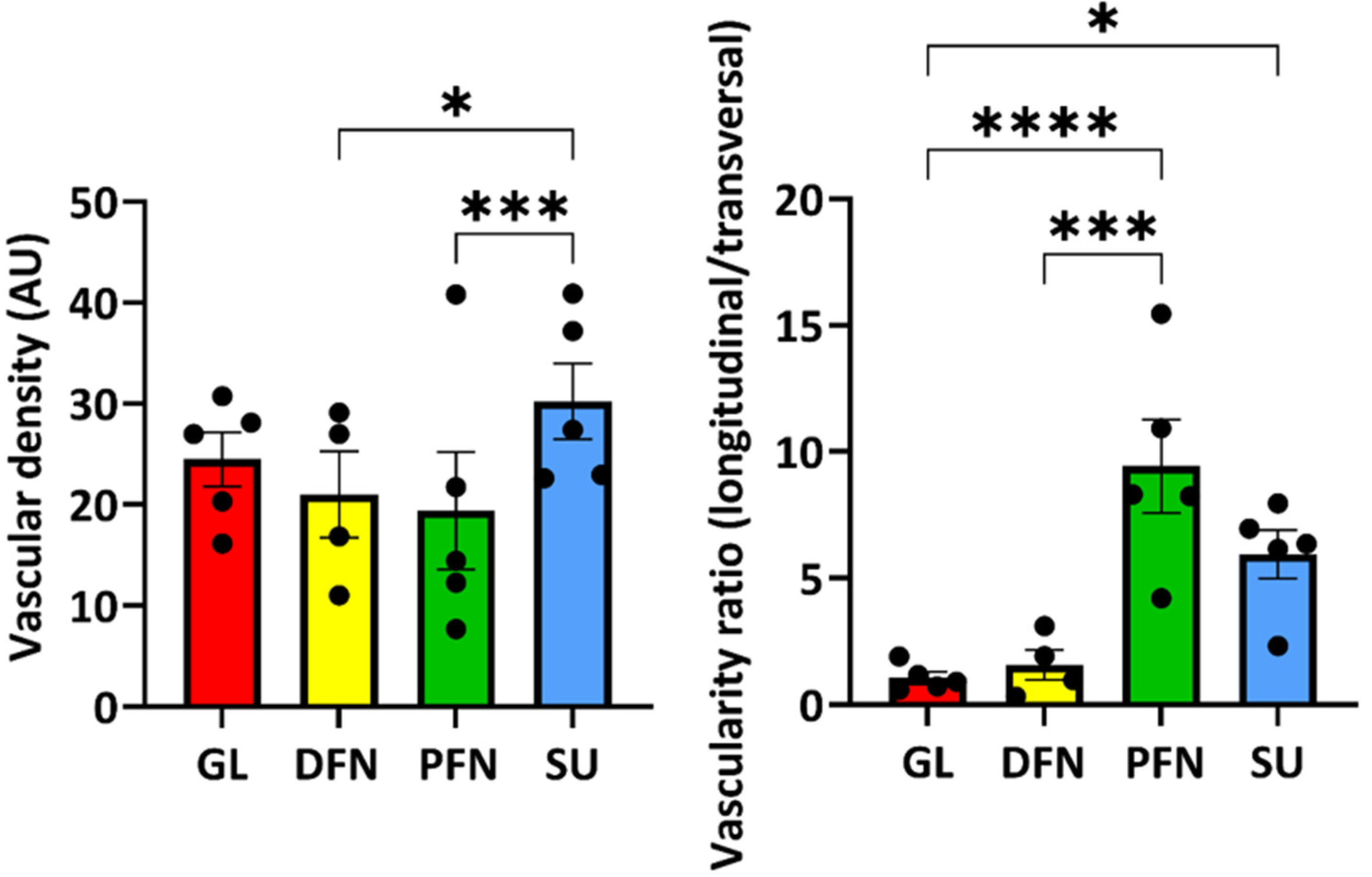
3.7. Organization of the Vascular Network Along the Urethra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elia, E.; Caneparo, C.; McMartin, C.; Chabaud, S.; Bolduc, S. Tissue Engineering for Penile Reconstruction. Bio-Eng. 2024, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Dessanti, A.; Rigamonti, W.; Merulla, V.; Falchetti, D.; Caccia, G. Autologous buccal mucosa graft for hypospadias repair: An initial report. J. Urol. 1992, 147, 1081–1083; discussion 1083–1084. [Google Scholar] [CrossRef]
- Cruz-Diaz, O.; Castellan, M.; Gosalbez, R. Use of buccal mucosa in hypospadias repair. Curr. Urol. Rep. 2013, 14, 366–372. [Google Scholar] [CrossRef]
- Saad, S.; Osman, N.I.; Chapple, C.R. Tissue engineering: Recent advances and review of clinical outcome for urethral strictures. Curr. Opin. Urol. 2021, 31, 498–503. [Google Scholar] [CrossRef]
- Barbagli, G.; Heidenreich, A.; Zugor, V.; Karapanos, L.; Lazzeri, M. Urothelial or oral mucosa cells for tis-sue-engineered urethroplasty: A critical revision of the clinical outcome. Asian J. Urol. 2020, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Pederzoli, F.; Joice, G.; Salonia, A.; Bivalacqua, T.J.; Sopko, N.A. Regenerative. and engineered options for urethroplasty Nat. Rev. Urol. 2019, 16, 453–464. [Google Scholar] [CrossRef]
- Holstein, A.F.; Davidoff, M.S.; Breucker, H.; Countouris, N.; Orlandini, G. Different. epithelia in the distal human male urethra. Cell Tissue Res. 1991, 264, 23–32. [Google Scholar] [CrossRef]
- Hausmann, R.; Schellmann, B. Forensic. value of the Lugol’s staining method: Further studies on glycogenated epithelium in the male urinary tract. Int. J. Legal. Med. 1994, 107, 147–151. [Google Scholar] [CrossRef]
- Hossler, F.E. Ultrastructure Atlas of Human Tissues; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Krstić, R.V. Urogenital Apparatus Human Microscopic Anatomy; Springer: Berlin/Heidelberg, Germany, 1991; pp. 295–437. [Google Scholar]
- Barbieri, J.S.; Wanat, K.; Seykora, J. Skin: Basic Structure and Function Pathobiology of Human Disease. In Pathobiology of Human Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1134–1144. [Google Scholar]
- Saba, I.; Barat, C.; Chabaud, S.; Reyjon, N.; Leclerc, M.; Jakubowska, W.; Orabi, H.; Lachhab, A.; Pelletier, M.; Tremblay, M.J.; et al. Immunocompetent Human 3D Organ-Specific Hormone-Responding Vaginal Mucosa Model of HIV-1 Infection Tissue. Eng. Part C Methods 2021, 27, 152–166. [Google Scholar] [CrossRef]
- Mohtashami, M.; Mohamadi, M.; Azimi-Nezhad, M.; Saeidi, J.; Nia, F.F.; Ghasemi, A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol. Appl. Biochem. 2021, 68, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Komine-Aizawa, S.; Kuramochi, T.; Ito, S.; Trinh, Q.D.; Pham, N.T.K.; Sasano, M.; Hayakawa, S. Lactobacillus crispatus accelerates re-epithelialization in vaginal epithelial cell line MS74. Am. J. Reprod. Immunol. 2018, 80, e13027. [Google Scholar] [CrossRef]
- Bowie, W.R.; Pollock, H.M.; Forsyth, P.S.; Floyd, J.F.; Alexander, E.R.; Wang, S.P.; Holmes, K.K. Bacteriology of the urethra in normal men and men with nongonococcal urethritis. J. Clin. Microbiol. 1977, 6, 482–488. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal pH and microbicidal lactic acid when Lactobacilli dominate the microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Aldunate, M.; Tyssen, D.; Johnson, A.; Zakir, T.; Sonza, S.; Moench, T.; Cone, R.; Tachedjian, G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013, 68, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Atassi, F.; Servin, A.L. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol. Lett. 2010, 304, 29–38. [Google Scholar] [CrossRef]
- Spurbeck, R.R.; Arvidson, C.G. Lactobacilli at the Front Line of Defense Against Vaginally Acquired Infections. Futur. Microbiol. 2011, 6, 567–582. [Google Scholar] [CrossRef]
- Unemo, M.; Bradshaw, C.S.; Hocking, J.S.; de Vries, H.J.C.; Francis, S.C.; Mabey, D.; Marrazzo, J.M.; Sonder, G.J.B.; Schwebke, J.R.; Hoornenborg, E.; et al. Sexually transmitted infections: Challenges ahead. Lancet. Infect. Dis. 2017, 17, e235–e279. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; van den Broek, M.; Balzarini, J.; Vanderleyden, J.; Lebeer, S. Vaginal microbiota and its role in HIV trans-mission and infection. FEMS Microbiol. Rev. 2013, 37, 762–792. [Google Scholar] [CrossRef]
- Martin, D.H. The Microbiota of the Vagina and Its Influence on Women’s Health and Disease. Am. J. Med. Sci. 2012, 343, 2–9. [Google Scholar] [CrossRef]
- Schwebke, J.R. Abnormal vaginal flora as a biological risk factor for acquisition of HIV infection and sexually transmitted diseases. J. Infect. Dis. 2005, 192, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Desmond, R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am. J. Obstet. Gynecol. 2007, 196, 517.e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Caneparo, C.; Chabaud, S.; Bolduc, S. Reconstruction of Vascular and Urologic Tubular Grafts by Tissue Engineering. Processes 2021, 9, 513. [Google Scholar] [CrossRef]
- Booth, D.; Afshari, R.; Ghovvati, M.; Shariati, K.; Sturm, R.; Annabi, N. Advances in 3D bioprinting for urethral tissue reconstruction. Trends Biotechnol. 2024, 42, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Pastorek, D.; Culenova, M.; Csobonyeiova, M.; Skuciova, V.; Danisovic, L.; Ziaran, S. Tissue Engineering of the Urethra: From Bench to Bedside. Biomedicines 2021, 9, 1917. [Google Scholar] [CrossRef]
- Casarin, M.; Morlacco, A.; Moro, F.D. Tissue Engineering and Regenerative Medicine in Pediatric Urology: Urethral and Urinary Bladder Reconstruction. Int. J. Mol. Sci. 2022, 23, 6360. [Google Scholar] [CrossRef]
- Duan, L.; Wang, Z.; Fan, S.; Wang, C.; Zhang, Y. Research progress of biomaterials and innovative technologies in urinary tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1258666. [Google Scholar] [CrossRef]
- Jackson, A.R.; Narla, S.T.; Bates, C.M.; Becknell, B. Urothelial progenitors in development and repair. Pediatr. Nephrol. 2022, 37, 1721–1731. [Google Scholar] [CrossRef]
- Xuan, Z.; Zachar, V.; Pennisi, C.P. Sources Selection Microenvironmental Preconditioning of Cells for Urethral Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 14074. [Google Scholar] [CrossRef]
- Caneparo, C.; Chabaud, S.; Fradette, J.; Bolduc, S. Engineered human organ-specific urethra as a functional substitute. Sci. Rep. 2022, 12, 21346. [Google Scholar] [CrossRef]
- Imbeault, A.; Bernard, G.; Rousseau, A.; Morissette, A.; Chabaud, S.; Bouhout, S.; Bolduc, S. An endothelialized urothelial cell-seeded tubular graft for urethral replacement. Can. Urol. Assoc. J. 2013, 7, E4–E9. [Google Scholar] [CrossRef][Green Version]
- Dictionnaire médical de l’Académie de Médecine. Available online: https://www.academie-medecine.fr/le-dictionnaire/index.php?q=glandes+intra-%C3%A9pith%C3%A9liales+de+l%5C%27ur%C3%A8thre (accessed on 1 May 2024).
- Isola, M.; Cossu, M.; DeLisa, A.; Isola, R.; Massa, D.; Casti, A.; Solinas, P.; Lantini, M.S. Oxytocin Immunoreactivity in the Hu-man Urethral (Littre’s) Glands. J. Reprod. Dev. 2010, 56, 94–97. [Google Scholar] [CrossRef]
- Zechhi-Orlandini, S.; Gulisano, M.; Orlandini, G.E.; Holstein, A.F. Scanning Electron Microscopic Observations on the Epithelium of the Human Spongy Urethra. Andrologia 2009, 20, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Dalghi, M.G.; Montalbetti, N.; Carattino, M.D.; Apodaca, G. The Urothelium: Life in a Liquid Environment. Physiol. Rev. 2020, 100, 1621–1705. [Google Scholar] [CrossRef]
- Serbanoiu, A.; Ion, R.T.; Filipoiu, F.; Tulin, A.; Enyedi, M. Dissection of the Male Urethra Demonstrating Its Topographical Specificity. Cureus 2024, 16, e65946. [Google Scholar] [CrossRef] [PubMed]
- Kajbafzadeh, A. Congenital. Urethral Anomalies in Boys. Part II. Urol. J. 2005, 2, 125–131. [Google Scholar]
- Shenoy, S.P.; Marla, P.K.; Venugopal, P.; Adappa, K.K.; Tantry, T.P.; Shankar, M.; Rai, G.D. An Endoscopic Study of the La-cuna Magna and Reappraisal of Its Clinical Significance in Contemporary Urological Practice. Urology 2011, 78, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Pariser, J.J.; Kim, N. Transgender. vaginoplasty: Techniques and outcomes. Transl. Androl. Urol. 2019, 8, 241–247. [Google Scholar] [CrossRef]
- Dessy, L.A.; Mazzocchi, M.; Corrias, F.; Ceccarelli, S.; Marchese, C.; Scuderi, N. The. use of cultured autologous oral epithelial cells for vaginoplasty in male-to-female transsexuals: A feasibility, safety, and advantageousness clinical pilot study. Plast. Reconstr. Surg. 2014, 133, 158–161. [Google Scholar] [CrossRef]
- Sueters, J.; Groenman, F.A.; Bouman, M.-B.; Roovers, J.P.W.; de Vries, R.; Smit, T.H.; Huirne, J.A.F. Tissue. Engineering Ne-ovagina for Vaginoplasty in Mayer–Rokitansky–Küster–Hauser Syndrome and Gender Dysphoria Patients: A Systematic Review. Tissue Eng. Part B Rev. 2023, 29, 28–46. [Google Scholar] [CrossRef]
- Bustos, S.S.; Bustos, V.P.; Mascaro, A.; Ciudad, P.; Forte, A.J.; Del Corral, G.; Manrique, O.J. Complications. and Patient-reported Outcomes in Transfemale Vaginoplasty: An Updated Systematic Review and Meta-analysis. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3510. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duval, M.; Brownell, D.; Chabaud, S.; Laungani, A.; Philippe, E.; Bolduc, S. Description of the Human Penile Urethra Epithelium. Medicina 2025, 61, 788. https://doi.org/10.3390/medicina61050788
Duval M, Brownell D, Chabaud S, Laungani A, Philippe E, Bolduc S. Description of the Human Penile Urethra Epithelium. Medicina. 2025; 61(5):788. https://doi.org/10.3390/medicina61050788
Chicago/Turabian StyleDuval, Matisse, David Brownell, Stéphane Chabaud, Alexis Laungani, Eric Philippe, and Stéphane Bolduc. 2025. "Description of the Human Penile Urethra Epithelium" Medicina 61, no. 5: 788. https://doi.org/10.3390/medicina61050788
APA StyleDuval, M., Brownell, D., Chabaud, S., Laungani, A., Philippe, E., & Bolduc, S. (2025). Description of the Human Penile Urethra Epithelium. Medicina, 61(5), 788. https://doi.org/10.3390/medicina61050788






