Association of Systemic Inflammatory Response Index and Prognostic Nutritional Index Scores with Sarcopenia in Patients with Metastatic Gastric Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Assessment of Sarcopenia
2.3. Assessment of Inflammatory Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Demographic and Clinical Characteristics of Patients According to the Presence of Sarcopenia
3.3. Sarcopenia Risk Factors
3.4. Factors Determining General Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CAR | CRP Albumin Ratio |
| CT | Computed Tomography |
| DEUFM | Dokuz Eylül University Faculty of Medicine |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| GLOBOCAN | Global Cancer Observatory |
| dNLR | Derivative Neutrophil to Lymphocyte Rate |
| LAR | LDH to Albumin Ratio |
| MLR | Monocyte to Lymphocyte Ratio |
| NLR | Neutrophil to Lymphocyte Ratio |
| PIV | Pan-Immune Inflammatory Value |
| PLR | Platelet to Lymphocyte Ratio |
| PNI | Prognostic Nutritional Index |
| SII | Systemic Inflammatory Index |
| SIRI | Systemic Inflammatory Response Index |
| SMI | Skeletal Muscle Index |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Hacker, U.T.; Hasenclever, D.; Baber, R.; Linder, N.; Busse, H.; Obermannova, R.; Zdrazilova-Dubska, L.; Valik, D.; Lordick, F. Modified Glasgow Prognostic Score (MGPS) Is Correlated with Sarcopenia and Dominates the Prognostic Role of Baseline Body Composition Parameters in Advanced Gastric and Esophagogastric Junction Cancer Patients Undergoing First-Line Treatment from the Phase III EXPAND Trial. Ann. Oncol. 2022, 33, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-J.; Zhao, H.-P.; Yu, Y.; Wang, J.-H.; Guo, L.; Liu, J.-Y.; Pu, J.; Lv, J. Updates on Global Epidemiology, Risk and Prognostic Factors of Gastric Cancer. World J. Gastroenterol. 2023, 29, 2452–2468. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Wong, A.; Zhu, D.; Kraus, D.; Tham, T. Radiologically Defined Sarcopenia Affects Survival in Head and Neck Cancer: A Meta-Analysis. Laryngoscope 2021, 131, 333–341. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A Practical and Precise Approach to Quantification of Body Composition in Cancer Patients Using Computed Tomography Images Acquired during Routine Care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Baracos, V.E.; McCargar, L.J.; Mourtzakis, M.; Mulder, K.E.; Reiman, T.; Butts, C.A.; Scarfe, A.G.; Sawyer, M.B. Body Composition as an Independent Determinant of 5-Fluorouracil–Based Chemotherapy Toxicity. Clin. Cancer Res. 2007, 13, 3264–3268. [Google Scholar] [CrossRef]
- Tan, B.H.L.; Brammer, K.; Randhawa, N.; Welch, N.T.; Parsons, S.L.; James, E.J.; Catton, J.A. Sarcopenia Is Associated with Toxicity in Patients Undergoing Neo-Adjuvant Chemotherapy for Oesophago-Gastric Cancer. Eur. J. Surg. Oncol. 2015, 41, 333–338. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Karra, S.; Gurushankari, B.; Rajalekshmy, M.R.; Elamurugan, T.P.; Mahalakshmy, T.; Kate, V.; Nanda, N.; Rajesh, N.G.; Shankar, G. Diagnostic Utility of NLR, PLR and MLR in Early Diagnosis of Gastric Cancer: An Analytical Cross-Sectional Study. J. Gastrointest. Cancer 2023, 54, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Si, H.; Deng, G.; Chen, S.; Zhang, N.; Zhou, Q.; Wang, Z.; Dai, G. A Composite Biomarker of Derived Neutrophil–Lymphocyte Ratio and Platelet–Lymphocyte Ratio Correlates with Outcomes in Advanced Gastric Cancer Patients Treated with Anti-PD-1 Antibodies. Front. Oncol. 2022, 11, 798415. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-C.; Liu, H.; Liu, D.-C.; Tong, C.; Liang, X.-W.; Chen, R.-H. Prognostic Value of Pan-Immune-Inflammation Value in Colorectal Cancer Patients: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 1036890. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, D. The Prognostic Value of Systemic Immune-Inflammation Index (SII) in Patients after Radical Operation for Carcinoma of Stomach in Gastric Cancer. J. Gastrointest. Oncol. 2019, 10, 965–978. [Google Scholar] [CrossRef]
- Ding, P.; Guo, H.; Sun, C.; Yang, P.; Kim, N.H.; Tian, Y.; Liu, Y.; Liu, P.; Li, Y.; Zhao, Q. Combined Systemic Immune-Inflammatory Index (SII) and Prognostic Nutritional Index (PNI) Predicts Chemotherapy Response and Prognosis in Locally Advanced Gastric Cancer Patients Receiving Neoadjuvant Chemotherapy with PD-1 Antibody Sintilimab and XELOX: A Prospective Study. BMC Gastroenterol. 2022, 22, 121. [Google Scholar] [CrossRef]
- Hashimoto, I.; Kano, K.; Onuma, S.; Suematsu, H.; Nagasawa, S.; Kanematsu, K.; Furusawa, K.; Hamaguchi, T.; Watanabe, M.; Hayashi, K.; et al. Clinical Effect of the C-Reactive Protein to Serum Albumin Ratio in Patients with Metastatic Gastric or Gastroesophageal Junction Cancer Treated with Trifluridine/Tipiracil. J. Pers. Med. 2023, 13, 923. [Google Scholar] [CrossRef]
- Nakazawa, N.; Sohda, M.; Yamaguchi, A.; Watanabe, T.; Saito, H.; Ubukata, Y.; Kuriyama, K.; Sano, A.; Sakai, M.; Yokobori, T.; et al. An Elevated Serum Lactate Dehydrogenase-to-Albumin Ratio Is a Useful Poor Prognostic Predictor of Nivolumab in Patients with Gastric Cancer. Anticancer Res. 2021, 41, 3925–3931. [Google Scholar] [CrossRef]
- Ruan, G.; Zhang, Q.; Zhang, X.; Tang, M.; Song, M.; Zhang, X.; Li, X.; Zhang, K.; Ge, Y.; Yang, M.; et al. Geriatric Nutrition Risk Index: Prognostic Factor Related to Inflammation in Elderly Patients with Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2021, 12, 1969–1982. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic Value of Sarcopenia in Adults with Solid Tumours: A Meta-Analysis and Systematic Review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Dolan, R.D.; Laird, B.J.A.; Horgan, P.G.; McMillan, D.C. The Prognostic Value of the Systemic Inflammatory Response in Randomised Clinical Trials in Cancer: A Systematic Review. Crit. Rev. Oncol. Hematol. 2018, 132, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Bundred, J.; Tan, B.H.L. Body Composition Assessment and Sarcopenia in Patients with Gastric Cancer: A Systematic Review and Meta-Analysis. Gastric Cancer 2019, 22, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, Y.S.; Kim, E.Y.; Jin, W. Prognostic Significance of CT-Determined Sarcopenia in Patients with Advanced Gastric Cancer. PLoS ONE 2018, 13, e0202700. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Chen, Y.; Kenzik, K.M.; McDonald, A.; Shachar, S.S.; Klepin, H.D.; Kritchevsky, S.; Bhatia, S. Assessment of Sarcopenia Measures, Survival, and Disability in Older Adults Before and After Diagnosis with Cancer. JAMA Netw. Open 2020, 3, e204783. [Google Scholar] [CrossRef]
- Burney, B.O.; Garcia, J.M. Hypogonadism in Male Cancer Patients. J. Cachexia Sarcopenia Muscle 2012, 3, 149–155. [Google Scholar] [CrossRef]
- Yu, R.; Wong, M.; Leung, J.; Lee, J.; Auyeung, T.W.; Woo, J. Incidence, Reversibility, Risk Factors and the Protective Effect of High Body Mass Index against Sarcopenia in Community-Dwelling Older Chinese Adults. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 15–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Duan, X.; Han, X.; Wang, J.; Han, G.; Mi, L.; Shi, J.; Li, N.; Yin, X.; Hou, J.; et al. Sarcopenia and Systemic Inflammation Response Index Predict Response to Systemic Therapy for Hepatocellular Carcinoma and Are Associated with Immune Cells. Front. Oncol. 2022, 12, 854096. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, W.; Huang, Y.; Chen, W.; Wu, R.; Chen, X.; Lou, N.; Wang, P. Sarcopenia Is Associated with the Neutrophil/Lymphocyte and Platelet/Lymphocyte Ratios in Operable Gastric Cancer Patients: A Prospective Study. Cancer Manag. Res. 2018, 10, 4935–4944. [Google Scholar] [CrossRef]
- Lin, J.-X.; Lin, J.-P.; Xie, J.-W.; Wang, J.; Lu, J.; Chen, Q.-Y.; Cao, L.; Lin, M.; Tu, R.; Zheng, C.-H.; et al. Prognostic Value and Association of Sarcopenia and Systemic Inflammation for Patients with Gastric Cancer Following Radical Gastrectomy. Oncologist 2019, 24, e1091–e1101. [Google Scholar] [CrossRef]
- Ding, P.; Lv, J.; Sun, C.; Chen, S.; Yang, P.; Tian, Y.; Zhou, Q.; Guo, H.; Liu, Y.; Zhao, Q. Combined Systemic Inflammatory Immunity Index and Prognostic Nutritional Index Scores as a Screening Marker for Sarcopenia in Patients with Locally Advanced Gastric Cancer. Front. Nutr. 2022, 9, 981533. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, H.S.; Beom, S.H.; Rha, S.Y.; Chung, H.C.; Kim, J.H.; Chun, Y.J.; Lee, S.W.; Choe, E.-A.; Heo, S.J.; et al. Marked Loss of Muscle, Visceral Fat, or Subcutaneous Fat After Gastrectomy Predicts Poor Survival in Advanced Gastric Cancer: Single-Center Study from the CLASSIC Trial. Ann. Surg. Oncol. 2018, 25, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Lilong, Z.; Kuang, T.; Li, M.; Li, X.; Hu, P.; Deng, W.; Wang, W. Sarcopenia Affects the Clinical Efficacy of Immune Checkpoint Inhibitors in Patients with Gastrointestinal Cancers. Clin. Nutr. 2024, 43, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ge, H.; Miao, Z.; Shao, S.; Shi, H.; Dong, C. Dynamic Changes in the Systemic Inflammation Response Index Predict the Outcome of Resectable Gastric Cancer Patients. Front. Oncol. 2021, 11, 577043. [Google Scholar] [CrossRef] [PubMed]
- Alkurt, E.G.; Durak, D.; Turhan, V.B.; Sahiner, I.T. Effect of C-Reactive Protein-to-Albumin Ratio on Prognosis in Gastric Cancer Patients. Cureus 2022, 14, e23972. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; Del Signore, S.; Anker, S.D.; Bejuit, R.; Bordes, P.; Cherubini, A.; Cruz-Jentoft, A.J.; et al. Multicomponent Intervention to Prevent Mobility Disability in Frail Older Adults: Randomised Controlled Trial (SPRINTT Project). BMJ 2022, 377, e068788. [Google Scholar] [CrossRef]
- De Felice, F.; Malerba, S.; Nardone, V.; Salvestrini, V.; Calomino, N.; Testini, M.; Boccardi, V.; Desideri, I.; Gentili, C.; De Luca, R.; et al. Progress and Challenges in Integrating Nutritional Care into Oncology Practice: Results from a National Survey on Behalf of the NutriOnc Research Group. Nutrients 2025, 17, 188. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef]
- Erul, E.; Guven, D.C.; Ozbay, Y.; Altunbulak, A.Y.; Kahvecioglu, A.; Ercan, F.; Yesil, M.F.; Ucdal, M.T.; Cengiz, M.; Yazici, G.; et al. Evaluation of Sarcopenia as a Prognostic Biomarker in Locally Advanced Head and Neck Squamous Cell Carcinoma. Biomark. Med. 2023, 17, 87–99. [Google Scholar] [CrossRef]
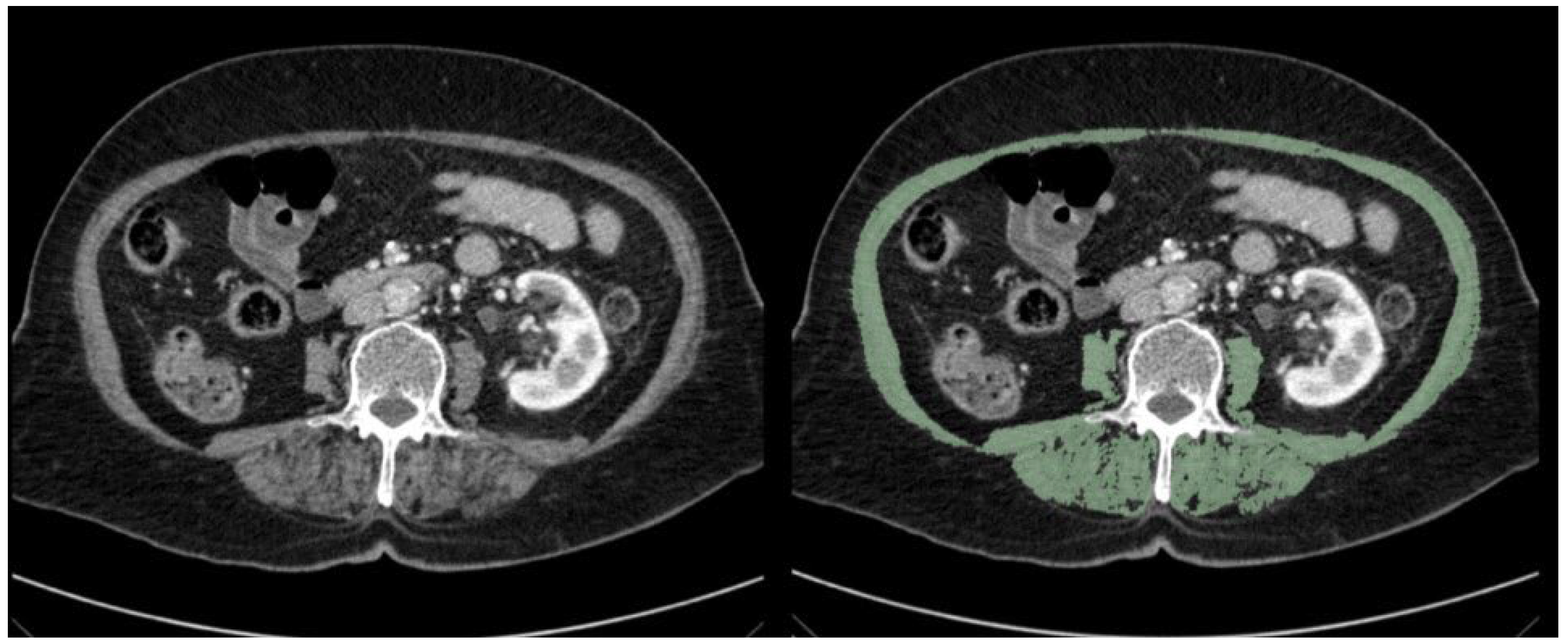

| Parameters | n (%) | |
|---|---|---|
| Median age, years (min–max) | 58 (26–84) | |
| Age group | <60 years | 72 (40.7) |
| >60 years | 105 (59.3) | |
| Sex | Woman | 53 (29.9) |
| Man | 124 (70.1) | |
| ECOG | 0–1 | 148 (83.6) |
| 2–3 | 29 (16.4) | |
| Histopathological type | Adenocarcinoma | 118 (66.7) |
| Signet ring | 58 (32.8) | |
| Adenosquamous | 1 (0.6) | |
| BMI groups | Underweight (<18.5 kg/m2) | 16 (9) |
| Normal (≥18.5 and <25 kg/m2) | 106 (59.9) | |
| Overweight (≥25 and <30 kg/m2) | 43 (24.3) | |
| Obese (≥30 kg/m2) | 12 (6.8) | |
| Sarcopenia | Yes | 127 (71.8) |
| No | 50 (28.2) | |
| Metastasis at diagnosis | Yes | 125 (70.6) |
| No | 52 (29.4) | |
| Liver metastasis | 75 (42.4) | |
| Lung metastasis | 43 (24.3) | |
| Bone metastasis | 38 (21.5) | |
| Peritoneal metastasis | 126 (71.2) | |
| Distant lymph node metastasis | 82 (46.3) | |
| Other metastasis | 70 (39.5) | |
| Sarcopenia (n = 127) | No Sarcopenia (n = 50) | p | ||
|---|---|---|---|---|
| Median age, years (min–max) | 63 (31–84) | 59 (26–84) | 0.074 | |
| Age group | ≥60.0 years | 46 (36.2) | 26 (52) | 0.054 |
| <60.0 years | 81 (63.8) | 24 (48) | ||
| Sex | Man | 98 (77.2) | 26 (52.0) | 0.001 |
| Woman | 29 (22.8) | 24 (48.0) | ||
| ECOG | 2–3 | 106 (83.5) 106 (83.5) | 42 (84) 42 (84) | 0.931 |
| 0–1 | 21 (16.5) | 8 (16) | ||
| BMI | 22.3 (12.5–53.2) | 24.4 (13.7–35.1) | <0.001 | |
| BMI group | Normal | 80 (63) | 26 (52) | 0.023 |
| Underweight | 15 (11.8) | 1 (2) | ||
| Overweight | 25 (19.7) | 18 (36) | ||
| Obese | 7 (5.5) | 5 (10) | ||
| Metastasis at diagnosis | Yes | 90 (70.9) | 35 (70) | 0.909 |
| No | 37 (29.1) | 15 (30) | ||
| Liver metastasis | 57 (44.9) | 18 (36) | 0.282 | |
| Lung metastasis | 36 (28.3) | 7 (14) | 0.045 | |
| Bone metastasis | 25 (19.7) | 13 (26) | 0.347 | |
| Peritoneal metastasis | 91 (71.7) | 35 (70) | 0.827 | |
| Distant lymph node metastasis | 59 (46.5) | 23 (46) | 0.956 | |
| Other metastasis | 56 (44.1) | 14 (28) | 0.06 | |
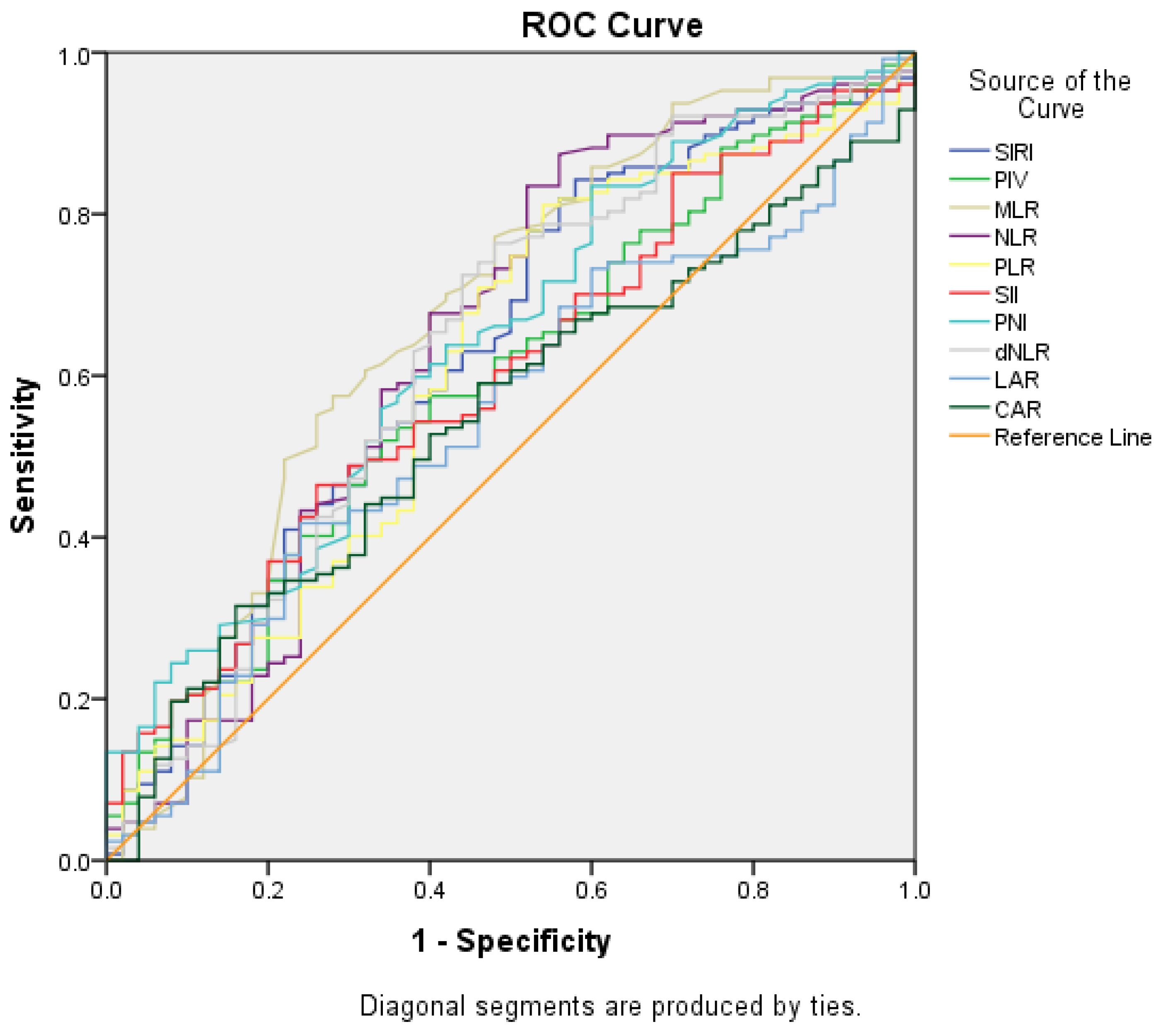
| SIRI | 2.4 (0.3–21.9) | 1.7 (0.5–20.3) | 0.008 | |
| PIV | 637 (30.1–8580.9) | 495.6 (62.4–3587.7) | 0.051 | |
| NLR | 3.6 (0.8–39.7) | 2.7 (1.0–12) | 0.004 | |
| MLR | 0.5 (0.1–1.9) | 0.3 (0.1–2) | <0.001 | |
| PLR | 206.0 (34.1–912.9) | 160.7 (65.8–675) | 0.025 | |
| SII | 1150 (100.4–8580.9) | 874.9 (208–3800) | 0.046 | |
| PNI | 44.4 (21.7–61.7) | 47.1 (34.9–65.2) | 0.004 | |
| dNLR | 2.4 (0.6–23.8) | 1.7 (0.8–7.8) | 0.007 | |
| LAR | 52.8 (27.8–2074.3) | 49.2 (29.1–468.2) | 0.343 | |
| CAR | 2.9 (0.1–80.6) | 2.2 (0.2–107.5) | 0.327 | |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p | OR | CI 95% | p | |
| Age | ||||||
| <60 years vs. ≥60 years | 1.9 | 1.0–3.7 | 0.056 | |||
| Sex | ||||||
| Woman vs. man | 3.1 1.63.1 | 1.6–6.2 1.6–6.2 | 0.001 0.001 | |||
| BMI | 0.040 | |||||
| Obese vs. normal | 2.2 | 0.6–7.5 | 0.210 | |||
| Obese vs. underweight | 10.7 | 1.0–109.8 | 0.046 | |||
| Obese vs. overweight | 1.0 | 0.3–3.6 | 0.990 | |||
| Lung metastasis | ||||||
| None vs. present | 2.4 | 1.0–5.9 | 0.050 | |||
| SIRI | ||||||
| ≤1.14 vs. >1.14 | 3.9 | 1.9–8.1 | <0.001 | 2.4 | 1.0–5.9 | 0.044 |
| PIV | ||||||
| ≤630.41 vs. >630.41 | 2.1 | 1.1–4.1 | 0.033 | |||
| NLR | ||||||
| ≤2.18 vs. >2.18 | 5.5 | 2.5–11.7 | <0.001 | |||
| MLR | ||||||
| ≤0.43 vs. >0.43 | 3.5 | 1.7–7.1 | 0.001 | |||
| PLR | ||||||
| ≤155.65 vs. >155.65 | 3.1 | 1.6–6 | 0.001 | |||
| SII | ||||||
| ≤1227.50 vs. >1227.50 | 2.6 | 1.3–5.3 | 0.010 | 1.0 | 0.4–2.5 | 0.979 |
| PNI | ||||||
| ≥50.35 vs. <50.35 | 3.4 | 1.6–7.0 | 0.001 | 2.4 | 1.1–5.4 | 0.034 |
| dNLR | ||||||
| ≤1.70 vs. >1.70 | 3.5 | 1.8–7.0 | <0.001 | 1.8 | 0.7–4.6 | 0.194 |
| mOS | 95% CI | p Value | |
|---|---|---|---|
| Sarcopenia | 0.010 | ||
| Yes | 11.5 ± 0.8 | 9.8–13.3 | |
| No | 17.5 ± 4.6 | 8.5–26.4 | |
| SIRI | <0.001 | ||
| ≤1.77 | 18.5 ± 2.1 | 14.3–22.6 | |
| >1.77 | 9.7 ± 1.2 | 7.3–12.1 | |
| NLR | <0.001 | ||
| ≤2.18 | 23.0 ± 2.3 | 18.5–27.5 | |
| >2.18 | 10.8 ± 1.2 | 8.5–13.0 | |
| SII | 0.016 | ||
| ≤621.73 | 18.0 ± 4.1 | 10.0–26.1 | |
| >621.73 | 11.5 ± 1.0 | 9.6–13.4 | |
| PNI | <0.001 | ||
| ≥46.95 | 16.2 ± 2.5 | 11.3–21.1 | |
| <46.95 | 10.4 ± 1.3 | 7.9–13.0 | |
| LAR | <0.001 | ||
| ≤45.98 | 19.5 ± 1.2 | 17.3–21.8 | |
| >45.98 | 9.4 ± 1.1 | 7.2–11.6 | |
| CAR | <0.001 | ||
| ≤1.89 | 22.4 ± 2.2 | 18.1–26.6 | |
| >1.89 | 9.7 ± 1.2 | 7.5–12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanbur, B.; Unek, I.T.; Uzun, M.; Ozturk, C.; Yarol, R.C.; Balci, A. Association of Systemic Inflammatory Response Index and Prognostic Nutritional Index Scores with Sarcopenia in Patients with Metastatic Gastric Cancer. Medicina 2025, 61, 785. https://doi.org/10.3390/medicina61050785
Kanbur B, Unek IT, Uzun M, Ozturk C, Yarol RC, Balci A. Association of Systemic Inflammatory Response Index and Prognostic Nutritional Index Scores with Sarcopenia in Patients with Metastatic Gastric Cancer. Medicina. 2025; 61(5):785. https://doi.org/10.3390/medicina61050785
Chicago/Turabian StyleKanbur, Busra, Ilkay Tugba Unek, Mehmet Uzun, Caner Ozturk, Raif Can Yarol, and Ali Balci. 2025. "Association of Systemic Inflammatory Response Index and Prognostic Nutritional Index Scores with Sarcopenia in Patients with Metastatic Gastric Cancer" Medicina 61, no. 5: 785. https://doi.org/10.3390/medicina61050785
APA StyleKanbur, B., Unek, I. T., Uzun, M., Ozturk, C., Yarol, R. C., & Balci, A. (2025). Association of Systemic Inflammatory Response Index and Prognostic Nutritional Index Scores with Sarcopenia in Patients with Metastatic Gastric Cancer. Medicina, 61(5), 785. https://doi.org/10.3390/medicina61050785






