Anatomical Variables of the Superior Thyroid Artery on Computed Tomography Angiograms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Imaging Protocol
2.4. Data Collection and Analysis
3. Results
3.1. The Individual Variables of the STA
3.2. The Unilateral Combinations of Variables
3.3. The Bilateral Associations of the Unilateral Combinations of Variables
4. Discussion
4.1. The Limitations of the Study
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECA | external carotid artery |
| GHHB | greater horn of hyoid bone |
| ICA | internal carotid artery |
| CCA | common carotid artery |
| CB | carotid bifurcation |
| STA | superior thyroid artery |
| TLT | thyrolingual trunk |
| TLFT | thyrolinguofacial trunk |
Appendix A
Appendix A.1. Tables
| Parameter | Classification | Description |
|---|---|---|
| STA origin type | A | Origin from the CCA |
| B | Origin from the CB | |
| C | Origin from the ECA | |
| D | Origin from the ICA | |
| E | Origin from the TLT | |
| F | Origin from the TLFT or other rare variants | |
| Topographic origin relative to GHHB | Type 1 | Origin inferior to the GHHB |
| Type 2 | Origin at the level of the GHHB | |
| 2a | Course lateral to the GHHB | |
| 2b | Course medial to the GHHB | |
| 2c | Course posterior to the GHHB | |
| Type 3 | Origin superior to the GHHB | |
| 3a | Course lateral to the GHHB | |
| 3b | Course medial to the GHHB | |
| 3c | Course posterior to the GHHB |
| Unilateral Combinations of Types | Carotid Origin of STA (Types A–C) | Hyoid Level of Origin and Course of STA (Types 1–3) |
|---|---|---|
| A1 | CCA | infrahyoid (type 1) |
| B1 | CB | infrahyoid (type 1) |
| C1 | ECA | infrahyoid (type 1) |
| A2a | CCA | hyoid, lateral to the GHHB |
| A2b | CCA | hyoid, medial to the GHHB |
| A2c | CCA | hyoid, posterior to the GHHB |
| B2a | CB | hyoid, lateral to the GHHB |
| B2b | CB | hyoid, medial to the GHHB |
| B2c | CB | hyoid, posterior to the GHHB |
| C2a | ECA | hyoid, lateral to the GHHB |
| C2b | ECA | hyoid, medial to the GHHB |
| C2c | ECA | hyoid, posterior to the GHHB |
| A3a | CCA | suprahyoid, lateral to the GHHB |
| A3b | CCA | suprahyoid, medial to the GHHB |
| A3c | CCA | suprahyoid, posterior to the GHHB |
| B3a | CB | suprahyoid, lateral to the GHHB |
| B3b | CB | suprahyoid, medial to the GHHB |
| B3c | CB | suprahyoid, posterior to the GHHB |
| C3a | ECA | suprahyoid, lateral to the GHHB |
| C3b | ECA | suprahyoid, medial to the GHHB |
| C3c | ECA | suprahyoid, posterior to the GHHB |
| Type A | Type B | Type C | ABS | |
|---|---|---|---|---|
| M, 106 sides | 9 8.49% | 38 35.85% | 56 52.83% | 3 2.83% |
| F, 64 sides | 6 9.38% | 11 17.19% | 46 71.88% | 1 1.56% |
| TOTAL, 170 sides | 15 8.82% | 49 28.82% | 102 60% | 4 2.35% |
| Type A Right | Type A Left | Type B Right | Type B Left | Type C Right | Type C Left | ABS Right | ABS Left | |
|---|---|---|---|---|---|---|---|---|
| M, 106 sides | 3 2.83% | 6 5.66% | 14 13.21% | 24 22.64% | 35 33.02% | 21 19.81% | 1 0.94% | 2 1.89% |
| F, 64 sides | 1 1.56% | 5 7.81% | 4 6.25% | 7 10.94% | 27 42.19% | 19 29.69% | 0 0% | 1 1.56% |
| TOTAL, 170 sides | 4 2.35% | 11 6.47% | 18 10.59% | 31 18.24% | 62 36.47% | 40 23.53% | 1 0.59% | 3 1.76% |
| Type 1 | Type 2a | Type 2b | Type 2c | Type 3a | Type 3b | Type 3c | ABS | |
|---|---|---|---|---|---|---|---|---|
| M (53) | 25 47.17% | 9 16.98% | 0 0% | 1 1.89% | 16 30.19% | 0 0% | 1 1.89% | 1 1.89% |
| F (32) | 13 40.63% | 8 25% | 0 0% | 2 6.25% | 7 21.88% | 0 0% | 2 6.25% | 0 0% |
| TOTAL (85) | 38 44.71% | 17 20% | 0 0% | 3 3.53% | 23 27.06% | 0 0% | 3 3.53% | 1 1.18% |
| Type 1 | Type 2a | Type 2b | Type 2c | Type 3a | Type 3b | Type 3c | ABS | |
|---|---|---|---|---|---|---|---|---|
| M (53) | 25 48.08% | 12 23.08% | 0 0% | 2 3.85% | 9 17.31% | 0 0% | 2 3.85% | 2 3.85% |
| F (32) | 17 53.13% | 6 18.75% | 1 3.13% | 2 6.25% | 4 12.5% | 0 0% | 1 3.13% | 1 3.13% |
| TOTAL (85) | 42 50% | 18 21.43% | 1 1.19% | 4 4.76% | 13 15.48% | 0 0% | 3 3.57% | 3 3.57% |
| Type 1 | Type 2a | Type 2b | Type 2c | Type 3a | Type 3b | Type 3c | ABS | |
|---|---|---|---|---|---|---|---|---|
| M (106) | 50 47.17% | 21 19.81% | 0 0% | 4 3.77% | 25 23.58% | 0 0% | 3 2.83% | 3 2.83% |
| F (64) | 30 46.88% | 14 21.88% | 1 1.56% | 4 6.25% | 11 17.19% | 0 0% | 3 4.69% | 1 1.56% |
| TOTAL (170) | 80 47.06% | 35 20.59% | 1 0.59% | 8 4.71% | 36 21.18% | 0 0% | 6 3.53% | 4 2.35% |
| Unilateral Combinations of Types | Right Side (Count, %) | Left Side (Count, %) |
|---|---|---|
| A1 | 2, 2.35% | 6, 7.06% |
| B1 | 10, 11.76% | 16, 18.82% |
| C1 | 26, 30.59% | 20, 23.53% |
| A2a | 2, 2.35% | 4, 4.71% |
| A2b | ||
| A2c | ||
| B2a | 3, 3.53% | 10, 11.76% |
| B2b | ||
| B2c | 2, 2.35% | 2, 2.35% |
| C2a | 12, 14.12% | 4, 4.71% |
| C2b | 1, 1.18% | |
| C2c | 1, 1.18% | 2, 2.35% |
| A3a | 1, 1.18% | |
| A3b | ||
| A3c | ||
| B3a | 3, 3.53% | 3, 3.53% |
| B3b | ||
| B3c | ||
| C3a | 20, 23.53% | 9, 10.59% |
| C3b | ||
| C3c | 3, 3.53% | 4, 4.71% |
| Unilateral Combinations of Types | Males | Females | ||
|---|---|---|---|---|
| Right Side (Count, %) | Left Side (Count, %) | Right Side (Count, %) | Left Side (Count, %) | |
| A1 | 2, 3.77% | 3, 5.66% | 3, 9.38% | |
| B1 | 8, 15.09% | 12, 22.64% | 2, 6.25% | 4, 12.5% |
| C1 | 15, 28.3% | 10, 18.87% | 11, 34.38% | 10, 31.25% |
| A2a | 1, 1.89% | 2, 3.77% | 1, 3.13% | 2, 6.25% |
| A2b | ||||
| A2c | ||||
| B2a | 2, 3.77% | 7, 13.21% | 1, 3.13% | 3, 9.38% |
| B2b | ||||
| B2c | 1, 1.89% | 2, 3.77% | 1, 3.13% | |
| C2a | 6, 11.32% | 3, 5.66% | 6, 18.75% | 1, 3.13% |
| C2b | 1, 3.13% | |||
| C2c | 1, 3.13% | 2, 6.25% | ||
| A3a | 1, 1.89% | |||
| A3b | ||||
| A3c | ||||
| B3a | 3, 5.66% | 3, 5.66% | ||
| B3b | ||||
| B3c | ||||
| C3a | 13, 24.53% | 5, 9.43% | 7, 21.88% | 4, 12.5% |
| C3b | ||||
| C3c | 1, 1.89% | 3, 5.66% | 2, 6.25% | 1, 3.13% |
| Type of Bilateral Association | Associated Unilateral Combinations | Count, % | Type of Bilateral Association | Associated Unilateral Combinations | Count, % |
|---|---|---|---|---|---|
| I | B1-A1 | 4, 4.7% | XX | C2a-A2a | 2, 2.35% |
| II | C1-A1 | 3, 3.53% | XXI | C2a-B2a | 1, 1.17% |
| III | A2a-A2a | 1, 1.17% | XXII | C2a-C1 | 4, 4.7% |
| IV | A2a-B2a | 1, 1.17% | XXIII | C2a-C2a | 3, 3.53% |
| V | ABS-ABS | 1, 1.17% | XXIV | C2a-C2c | 1, 1.17% |
| VI | B1-A2a | 1, 1.17% | XXV | C2a-C3c | 1, 1.17% |
| VII | B1-B1 | 3, 3.53% | XXVI | C2c-C2b | 1, 1.17% |
| VIII | B2a-B1 | 2, 2.35% | XXVII | C3a-ABS | 2, 2.35% |
| IX | B1-B2c | 2, 2.35% | XXVIII | C3a-B1 | 2, 2.35% |
| X | B2a-B2a | 2, 2.35% | XXIX | C3a-B2a | 2, 2.35% |
| XI | B2c-A1 | 1, 1.17% | XXX | C3a-B3a | 1, 1.17% |
| XII | B2c-B3a | 1, 1.17% | XXXI | C3a-C1 | 1, 1.17% |
| XIII | B3a-A3a | 1, 1.17% | XXXII | C3a-C2a | 1, 1.17% |
| XIV | B3a-B2a | 2, 2.35% | XXXIII | C3a-C3a | 9, 10.58% |
| XV | C1-B1 | 8, 9.41% | XXXIV | C3a-C3c | 2, 2.35% |
| XVI | C1-B2 | 1, 1.17% | XXXV | C3c-B1 | 1, 1.17% |
| XVII | C1-B3a | 1, 1.17% | XXXVI | C3c-C1 | 1, 1.17% |
| XVIII | C1-C1 | 13, 15.29% | XXXVII | C3c-C3c | 1, 1.17% |
| XIX | C1-C2c | 1, 1.17% |
| Gender/Type | Type III | Type V | Type VII | Type X | Type XVIII | Type XXIII | Type XXXIII | Type XXXVII |
|---|---|---|---|---|---|---|---|---|
| M = 53 | 1 | 1 | 3 | 1 | 6 | 2 | 5 | 0 |
| F = 32 | 0 | 0 | 0 | 1 | 7 | 1 | 4 | 1 |
| TOTAL | 1 (1.17%) | 1 (1.17%) | 3 (3.52%) | 2 (2.35%) | 13 (15.29%) | 3 (3.52%) | 9 (10.58%) | 1 (1.17%) |
| Bilateral Associations | Types | M | F | Bilateral Associations | Types | M | F |
|---|---|---|---|---|---|---|---|
| B1-A1 | I | 3 | 1 | C2a-B2a | XXI | 1 | 0 |
| C1-A1 | II | 2 | 1 | C2a-C1 | XXII | 1 | 3 |
| A2a-B2a | IV | 0 | 1 | C2a-C2c | XXIV | 0 | 1 |
| B1-A2a | VI | 0 | 1 | C2a-C3c | XXV | 1 | 0 |
| B2a-B1 | VIII | 2 | 0 | C2c-C2b | XXVI | 0 | 1 |
| B1-B2c | IX | 2 | 0 | C3a-ABS | XXVII | 1 | 1 |
| B2c-A1 | XI | 0 | 1 | C3a-B1 | XXVIII | 1 | 1 |
| B2c-B3a | XII | 1 | 0 | C3a-B2a | XXIX | 1 | 1 |
| B3a-A3a | XIII | 1 | 0 | C3a-B3a | XXX | 1 | 0 |
| B3a-B2a | XIV | 2 | 0 | C3a-C1 | XXXI | 1 | 0 |
| C1-B1 | XV | 6 | 2 | C3a-C2a | XXXII | 1 | 0 |
| C1-B2 | XVI | 1 | 0 | C3a-C3c | XXXIV | 2 | 0 |
| C1-B3a | XVII | 1 | 0 | C3c-B1 | XXXV | 0 | 1 |
| C1-C2c | XIX | 1 | 1 | C3c-C1 | XXXVI | 1 | 0 |
| C2a-A2a | XX | 1 | 1 |
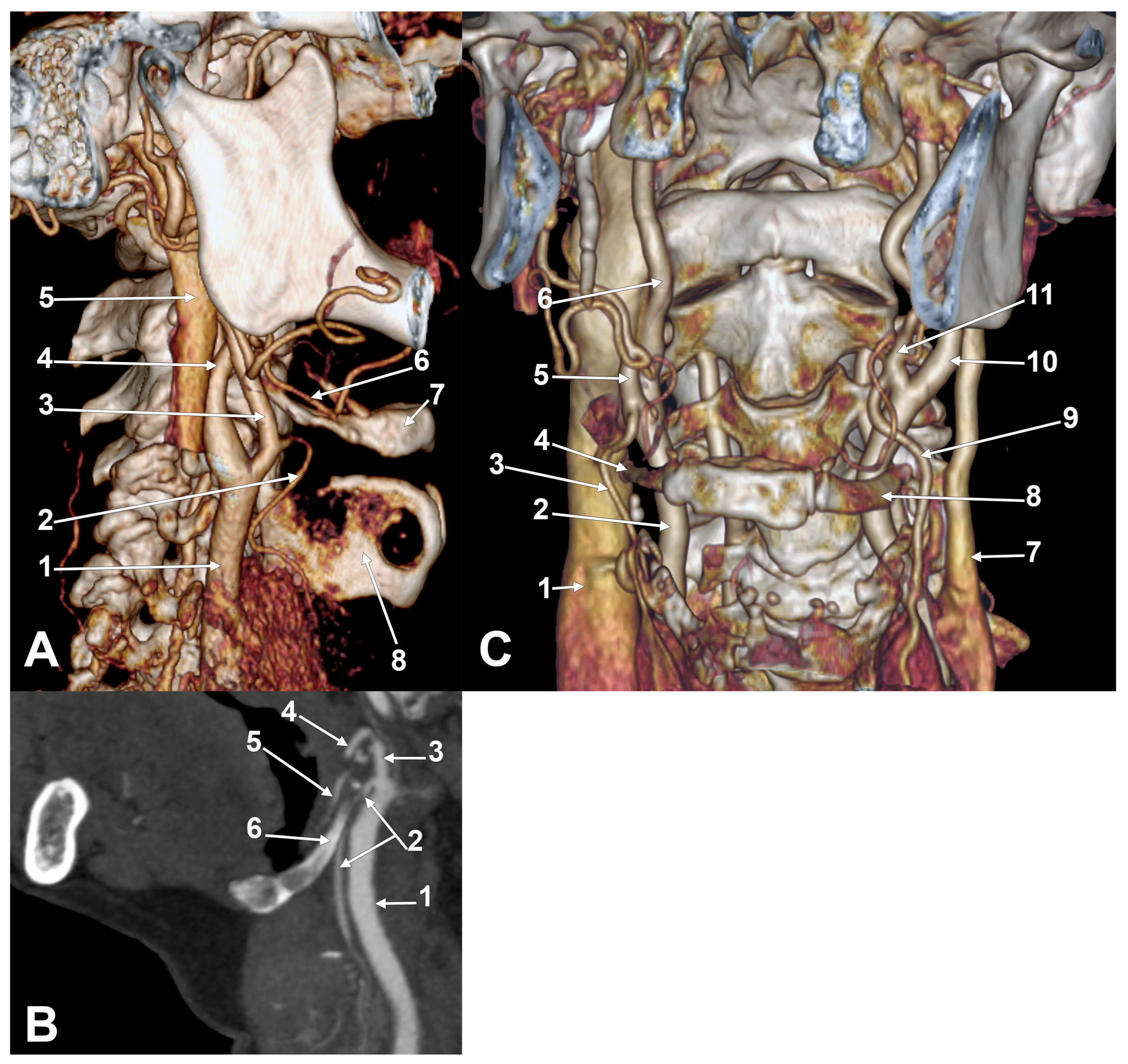
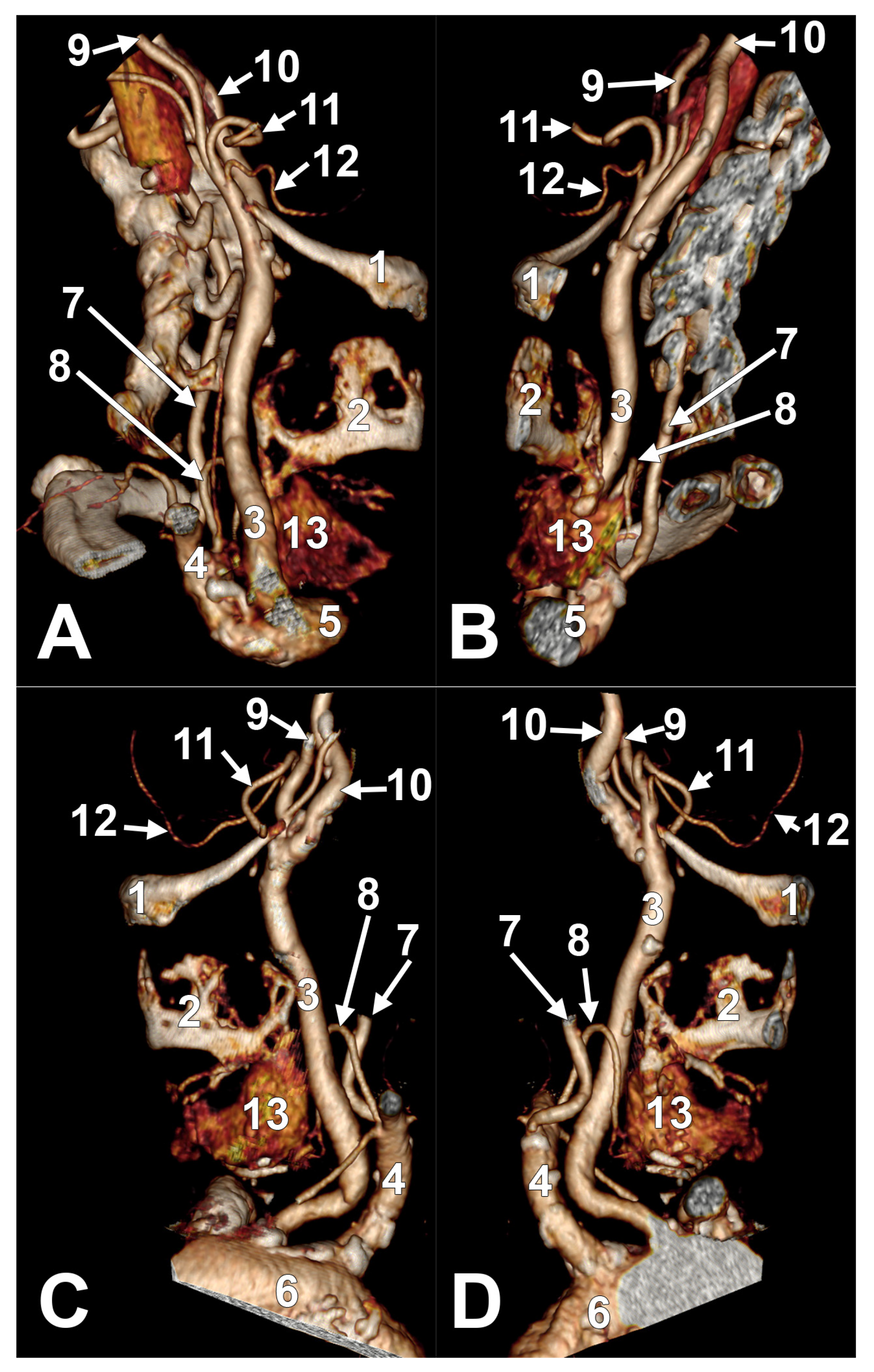
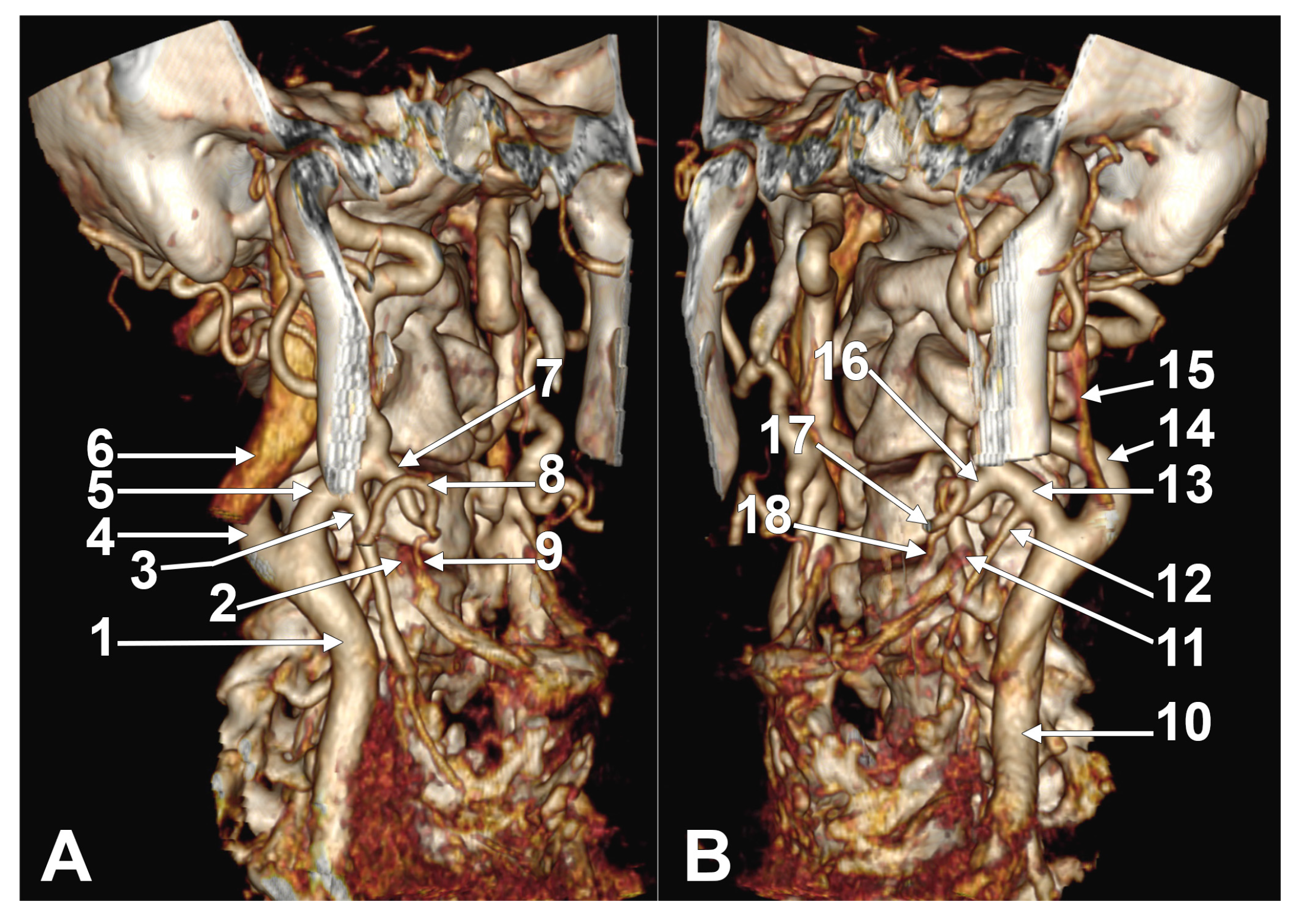
Appendix A.2. Supplemental Figures

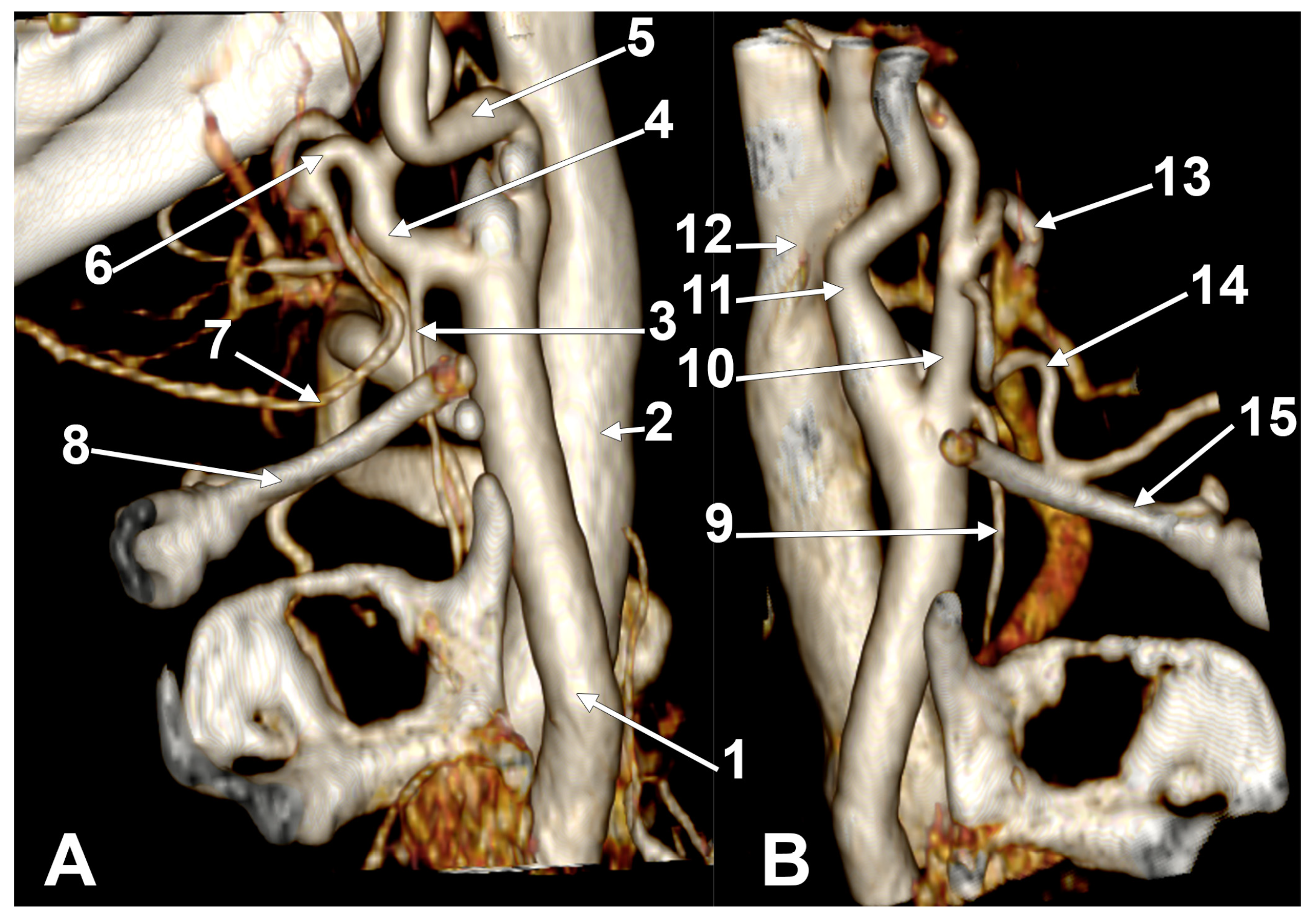
References
- Lo, A.; Oehley, M.; Bartlett, A.; Adams, D.; Blyth, P.; Al-Ali, S. Anatomical variations of the common carotid artery bifurcation. ANZ J. Surg. 2006, 76, 970–972. [Google Scholar] [CrossRef]
- Klosek, S.K.; Rungruang, T. Topography of carotid bifurcation: Considerations for neck examination. Surg. Radiol. Anat. 2008, 30, 383–387. [Google Scholar] [CrossRef]
- Lucev, N.; Bobinac, D.; Maric, I.; Drescik, I. Variations of the great arteries in the carotid triangle. Otolaryngol. Head Neck Surg. 2000, 122, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Kurkcuoglu, A.; Aytekin, C.; Oktem, H.; Pelin, C. Morphological variation of carotid artery bifurcation level in digital angiography. Folia Morphol 2015, 74, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Rouviere, H.; Delmas, A. Anatomie Humaine. Tête et Cou; Masson: Paris, France, 1985; Volume 1. [Google Scholar]
- Al-Rafiah, A.; AA, E.L.-H.; Aal, I.H.; Zaki, A.I. Anatomical study of the carotid bifurcation and origin variations of the ascending pharyngeal and superior thyroid arteries. Folia Morphol. 2011, 70, 47–55. [Google Scholar]
- Denli Yalvac, E.S.; Balak, N.; Atalay, B.; Bademci, M.S.; Kocaaslan, C.; Oztekin, A.; Ankarali, H.; Aydin, E. A New Method for Determining the Level of the Carotid Artery Bifurcation. J. Craniofacial Surg. 2019, 30, e523–e527. [Google Scholar] [CrossRef]
- Sasikumar, N.; Vijayalakshmi, S.; Raghunath, G.; Karunakaran, B.; Nithya, S.; Ks, P.D.; Kumaresan, M.; Gurusamy, K.; Francis, Y.M.; Dharshini, P. Morphometric Study and Branching Patterns of External Carotid Artery Using Computed Tomography Angiography Among the South Indian Population: A Retrospective Study. Cureus 2023, 15, e35624. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.; Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Lippert, H.; Pabst, R. Arterial Variations in Man: Classification and Frequency; J.P. Bergmann Verlag: München, Germany, 1985. [Google Scholar]
- Natsis, K.; Raikos, A.; Foundos, I.; Noussios, G.; Lazaridis, N.; Njau, S.N. Superior thyroid artery origin in Caucasian Greeks: A new classification proposal and review of the literature. Clin. Anat. 2011, 24, 699–705. [Google Scholar] [CrossRef]
- Vazquez, T.; Cobiella, R.; Maranillo, E.; Valderrama, F.J.; McHanwell, S.; Parkin, I.; Sanudo, J.R. Anatomical variations of the superior thyroid and superior laryngeal arteries. Head Neck 2009, 31, 1078–1085. [Google Scholar] [CrossRef]
- Motwani, R.; Jhajhria, S.K. Variant Branching Pattern of Superior Thyroid Artery and Its Clinical Relevance: A Case Report. J. Clin. Diagn. Res. 2015, 9, AD05–AD06. [Google Scholar] [CrossRef]
- Aggarwal, N.R.; Krishnamoorthy, T.; Devasia, B.; Menon, G.; Chandrasekhar, K. Variant origin of superior thyroid artery, occipital artery and ascending pharyngeal artery from a common trunk from the cervical segment of internal carotid artery. Surg. Radiol. Anat. 2006, 28, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Han, S.H.; Oh, C.S.; Chung, I.H. Superior and middle thyroid arteries arising from the common carotid artery. Surg. Radiol. Anat. 2011, 33, 645–647. [Google Scholar] [CrossRef]
- Triantafyllou, G.; Paschopoulos, I.; Duparc, F.; Tsakotos, G.; Tsiouris, C.; Olewnik, L.; Georgiev, G.; Zielinska, N.; Piagkou, M. The superior thyroid artery origin pattern: A systematic review with meta-analysis. Surg. Radiol. Anat. 2024, 46, 1549–1560. [Google Scholar] [CrossRef]
- Dessie, M.A. Variations of the origin of superior thyroid artery and its relationship with the external branch of superior laryngeal nerve. PLoS ONE 2018, 13, e0197075. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.C.; Vrapciu, A.D.; Jianu, A.M.; Hostiuc, S.; Rusu, M.C. The retromandibular loop of the external carotid artery. Ann. Anat. Anat. Anz. 2024, 253, 152226. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulou, S.; Mavridis, I. Emerging patterns of the human superior thyroid artery and review of its clinical anatomy. Surg. Radiol. Anat. 2014, 36, 33–38. [Google Scholar] [CrossRef]
- Gupta, P.; Bhalla, A.S.; Thulkar, S.; Kumar, A.; Mohanti, B.K.; Thakar, A.; Sharma, A. Variations in superior thyroid artery: A selective angiographic study. Indian J. Radiol. Imaging 2014, 24, 66–71. [Google Scholar] [CrossRef]
- Ongeti, K.W.; Ogeng’o, J.A. Variant origin of the superior thyroid artery in a Kenyan population. Clin. Anat. 2012, 25, 198–202. [Google Scholar] [CrossRef]
- Ozgur, Z.; Govsa, F.; Celik, S.; Ozgur, T. Clinically relevant variations of the superior thyroid artery: An anatomic guide for surgical neck dissection. Surg. Radiol. Anat. 2009, 31, 151–159. [Google Scholar] [CrossRef]
- Alagoz, M.S.; Cagri Uysal, A.; Tuccar, E.; Sensoz, O. How cranial could the sternocleidomastoid muscle be split? J. Craniofac. Surg. 2005, 16, 201–204. [Google Scholar] [CrossRef]
- Won, S.Y. Anatomical considerations of the superior thyroid artery: Its origins, variations, and position relative to the hyoid bone and thyroid cartilage. Anat. Cell Biol. 2016, 49, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Metgudmath, R.B.; Belaldavar, B.P. Topographic Evaluation of Superior Thyroid Artery-A Terrain to be Well Versed for Surgeon’s Knife. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 5994–6000. [Google Scholar] [CrossRef]
- Joshi, A.; Gupta, S.; Vaniya, V. Anatomical variation in the origin of superior thyroid artery and it’s relation with external laryngeal nerve. Natl. J. Med. Res. 2014, 4, 138–141. [Google Scholar]
- Rimi, K.R.; Ara, S.; Hossain, M.; Shefyetullah, K.; Naushaba, H.; Bose, B. Postmortem study of thyroid arteries in Bangladeshi people. Bangladesh J. Anat. 2009, 7, 26–33. [Google Scholar] [CrossRef]
- Shankar, V.V.; Komala, N.; Shetty, S. A cross-sectional study of superior thyroid artery in human cadavers. Int. J. Anat. Res. 2017, 5, 4751–4755. [Google Scholar] [CrossRef]
- Sharma, A. Variation in the Origin of Superior Thyroid Artery and it’s relation with external laryngeal nerve: A cadaveric study. Acad. Anat. Intl 2019, 5, 6–9. [Google Scholar] [CrossRef][Green Version]
- Shivaleela, C.; Anupama, D.; Lakshmi Prabha Subhash, R. Study of anatomical variations in the origin of superior thyroid artery. Int. J. Anat. Res. 2016, 4, 1765–1768. [Google Scholar] [CrossRef]
- Tsegay, A.T.; Berhe, T.; Amdeslase, F.; Hayelom, H. Variations on arterial supply of thyroid gland and its clinical significance in selected universities of North Ethiopia. Int. J. Anat. Res. 2019, 7, 6830–6834. [Google Scholar] [CrossRef]
- Poutoglidis, A.; Savvakis, S.; Karamitsou, P.; Forozidou, E.; Paraskevas, G.; Lazaridis, N.; Fyrmpas, G.; Karamitsou, A.; Skalias, A. Is the origin of the superior thyroid artery consistent? A systematic review of 5488 specimens. Am. J. Otolaryngol. 2023, 44, 103823. [Google Scholar] [CrossRef]
- Tzortzis, A.S.; Antonopoulos, I.; Pechlivanidou, E.; Chrysikos, D.; Pappas, N.; Troupis, T. Anatomical variations of the superior thyroid artery: A systematic review. Morphol. Bull. De. L’association Des. Anat. 2023, 107, 100597. [Google Scholar] [CrossRef]
- Calota, R.N.; Rusu, M.C.; Dumitru, C.C.; Moraru, L.; Tudose, R.C. Retropharyngeal course of the superior thyroid artery—A novel finding. Surg. Radiol. Anat. 2025, 47, 115. [Google Scholar] [CrossRef] [PubMed]
- Toni, R.; Della Casa, C.; Castorina, S.; Malaguti, A.; Mosca, S.; Roti, E.; Valenti, G. A meta-analysis of superior thyroid artery variations in different human groups and their clinical implications. Ann. Anat. Anat. Anz. 2004, 186, 255–262. [Google Scholar] [CrossRef]
- Toni, R.; Della Casa, C.; Mosca, S.; Malaguti, A.; Castorina, S.; Roti, E. Anthropological variations in the anatomy of the human thyroid arteries. Thyroid 2003, 13, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Nunez, M.; Menchaca-Gutierrez, J.L.; Pinales-Razo, R.; Elizondo-Riojas, G.; Quiroga-Garza, A.; Fernandez-Rodarte, B.A.; Elizondo-Omana, R.E.; Guzman-Lopez, S. Origin variations of the superior thyroid, lingual, and facial arteries: A computed tomography angiography study. Surg. Radiol. Anat. 2020, 42, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Moriggl, B.; Sturm, W. Absence of three regular thyroid arteries replaced by an unusual lowest thyroid artery (A. thyroidea ima): A case report. Surg. Radiol. Anat. 1996, 18, 147–150. [Google Scholar] [CrossRef]
- Esen, K.; Ozgur, A.; Balci, Y.; Tok, S.; Kara, E. Variations in the origins of the thyroid arteries on CT angiography. Jpn. J. Radiol. 2018, 36, 96–102. [Google Scholar] [CrossRef]
- Mehta, V.; Suri, R.K.; Arora, J.; Rath, G.; Das, S. Anomalous superior thyroid artery. Kathmandu Univ. Med. J. 2010, 8, 429–431. [Google Scholar] [CrossRef]
- Rusu, M.C.; Tudose, R.C.; Vrapciu, A.D.; Popescu, S.A. Lowered hyoid bone overlapping the thyroid cartilage in CT angiograms. Surg. Radiol. Anat. 2024, 46, 333–339. [Google Scholar] [CrossRef]
- Xia, C.; Li, Z. Recurrent Ischemic Strokes Due to Anatomic Variation of the Hyoid Bone and Thyroid Cartilage. Radiology 2024, 313, e241186. [Google Scholar] [CrossRef]
- Corbett, C.R.; Young, A.E.; Gaunt, J.; Unal, H.; Unal, G. The effect of ligation of the inferior thyroid artery upon thyroid remnant function. Surg. Gynecol. Obstet. 1988, 166, 418–420. [Google Scholar]
- Bednarz, M.; Gromaszek, M.; Daniluk, A.; Iwaniuk, K.; Samczuk, M.; Białkowska, Z.; Buczek, J.; Stachowicz, H.; Gawłowicz, Ł.; Ostański, J. Superior thyroid artery–variations of origin and clinical significance. J. Pre-Clin. Clin. Res. 2024, 18, 168–174. [Google Scholar] [CrossRef]
- Thomas, J.B.; Antiga, L.; Che, S.L.; Milner, J.S.; Steinman, D.A.; Spence, J.D.; Rutt, B.K.; Steinman, D.A. Variation in the carotid bifurcation geometry of young versus older adults: Implications for geometric risk of atherosclerosis. Stroke 2005, 36, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Kanetaka, H.; Kim, Y.H.; Okayama, K.; Kano, M.; Kikuchi, M. Age-related morphological changes in the human hyoid bone. Cells Tissues Organs 2005, 180, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Gurlek Celik, N.; Oktay, M. Evaluation of hyoid bone position, shape, area, volume, and tongue volume. Surg. Radiol. Anat. 2024, 47, 30. [Google Scholar] [CrossRef]
- Bann, D.V.; Kim, Y.; Zacharia, T.; Goldenberg, D. The effect of aging on the anatomic position of the thyroid gland. Clin. Anat. 2017, 30, 205–212. [Google Scholar] [CrossRef]
- Aristokleous, N.; Seimenis, I.; Papaharilaou, Y.; Georgiou, G.C.; Brott, B.C.; Eracleous, E.; Anayiotos, A.S. Effect of posture change on the geometric features of the healthy carotid bifurcation. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 148–154. [Google Scholar] [CrossRef]


| Origin of STA | Pooled Prevalence |
|---|---|
| CCA | 13.09% |
| CB | 25.36% |
| ECA | 56.94% |
| ICA | 0.00% (2 cases) |
| TLT from the CCA | 0.01% |
| TLT from the CB | 0.00% (11 cases) |
| TLT from the ECA | 0.61% |
| TLFT from the CB | 0.00% (2 cases) |
| TLFT from the ECA | 0.09% |
| Absent STA | 0.09% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calotă, R.N.; Rusu, M.C.; Rusu, M.I.; Dumitru, C.C.; Vrapciu, A.D. Anatomical Variables of the Superior Thyroid Artery on Computed Tomography Angiograms. Medicina 2025, 61, 775. https://doi.org/10.3390/medicina61050775
Calotă RN, Rusu MC, Rusu MI, Dumitru CC, Vrapciu AD. Anatomical Variables of the Superior Thyroid Artery on Computed Tomography Angiograms. Medicina. 2025; 61(5):775. https://doi.org/10.3390/medicina61050775
Chicago/Turabian StyleCalotă, Rodica Narcisa, Mugurel Constantin Rusu, Marius Ioan Rusu, Cătălin Constantin Dumitru, and Alexandra Diana Vrapciu. 2025. "Anatomical Variables of the Superior Thyroid Artery on Computed Tomography Angiograms" Medicina 61, no. 5: 775. https://doi.org/10.3390/medicina61050775
APA StyleCalotă, R. N., Rusu, M. C., Rusu, M. I., Dumitru, C. C., & Vrapciu, A. D. (2025). Anatomical Variables of the Superior Thyroid Artery on Computed Tomography Angiograms. Medicina, 61(5), 775. https://doi.org/10.3390/medicina61050775







