Tubeless Percutaneous Nephrolithotomy in the Barts ‘Flank-Free’ Modified Supine Position with 24-Hour Discharge: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
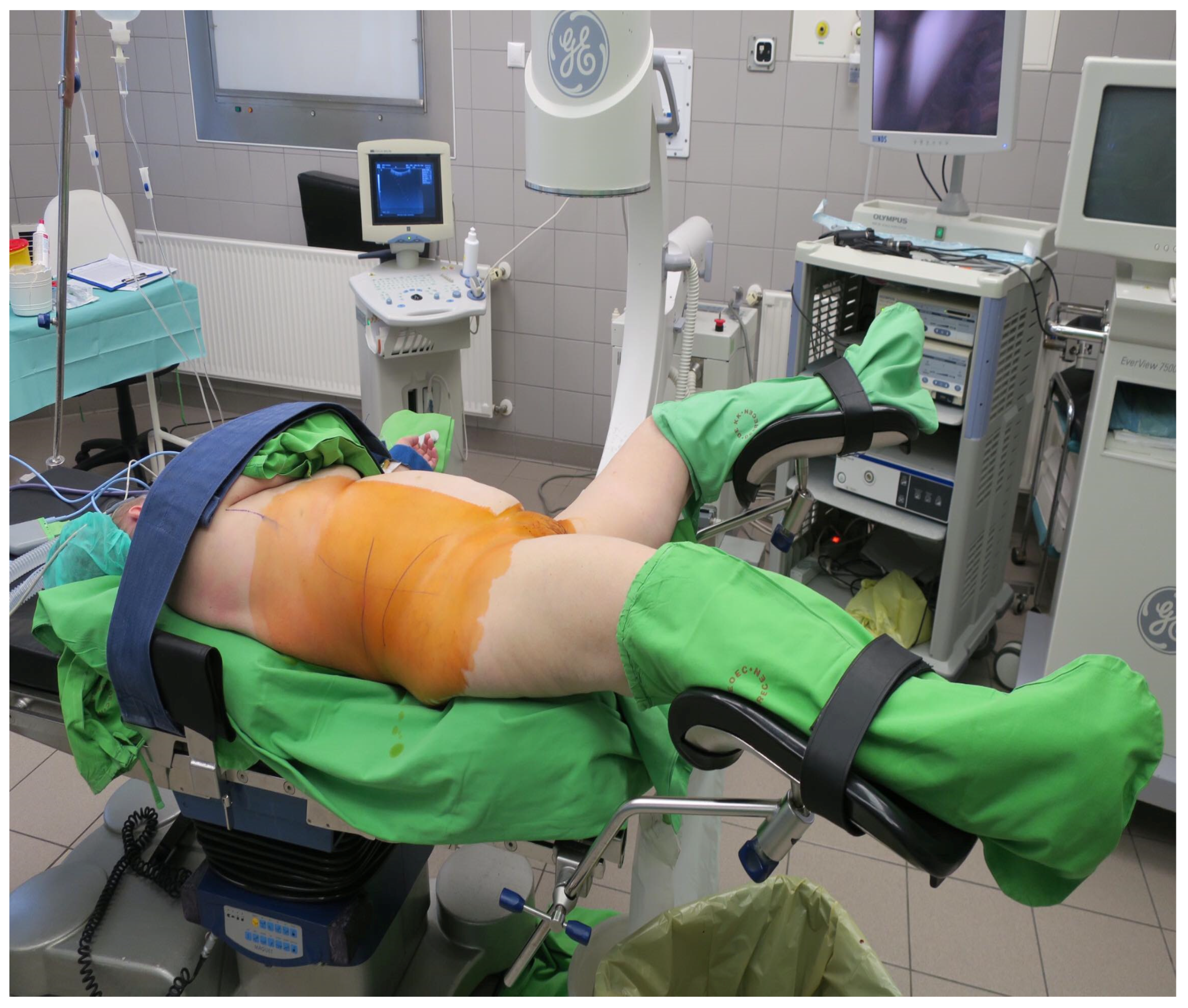
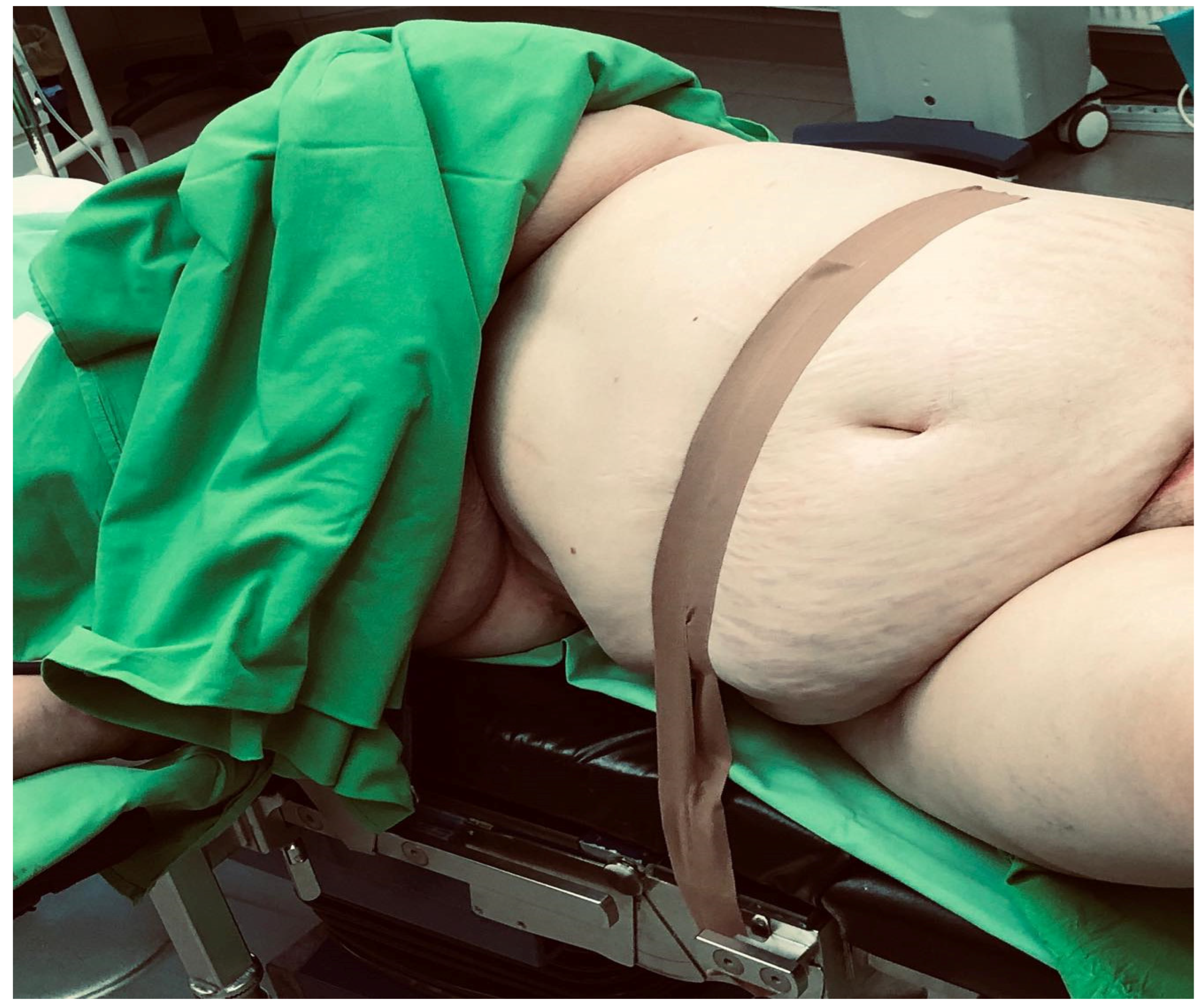
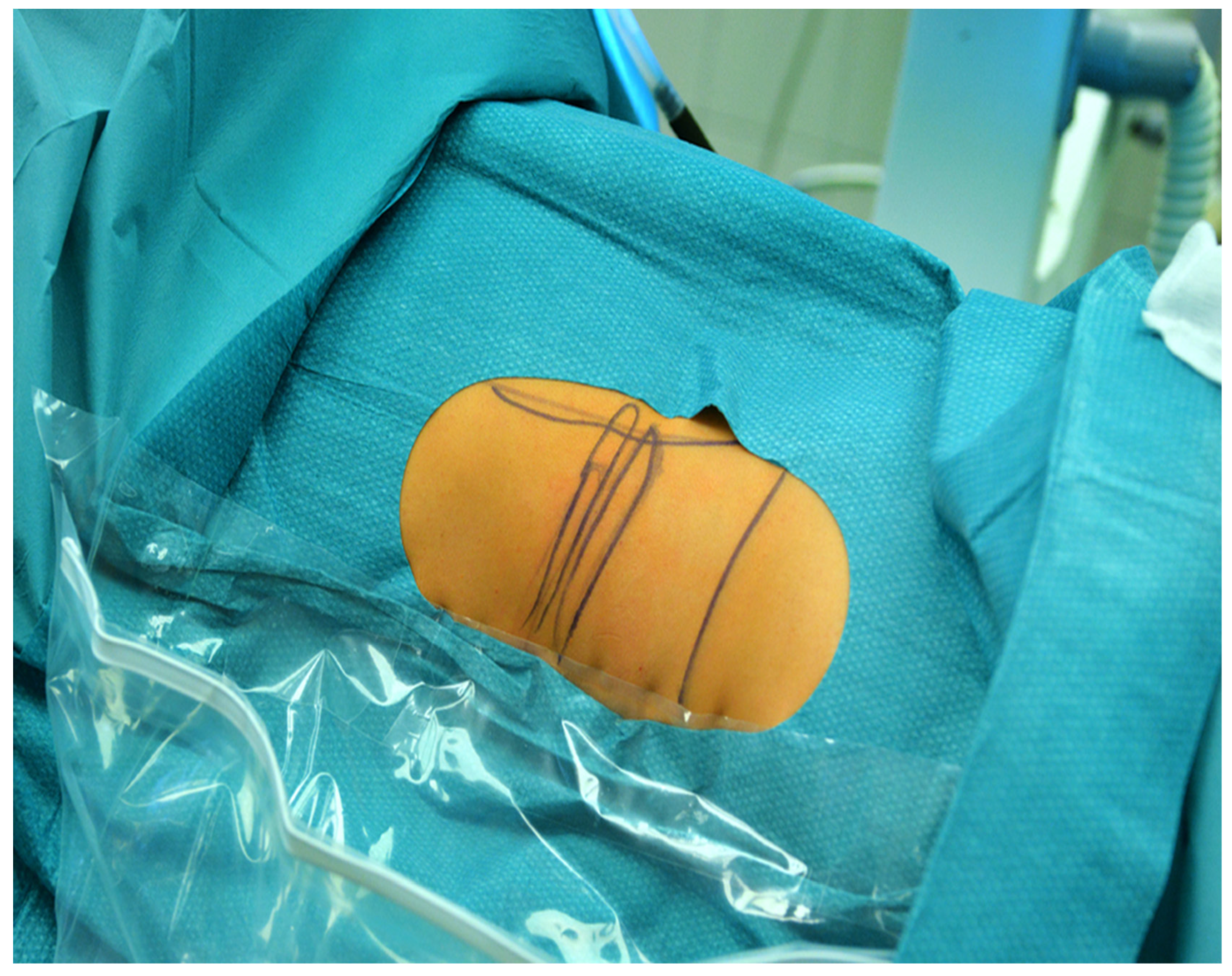
2.1. Surgical Technique
2.2. Outcome Measures
2.3. Statistical Analysis
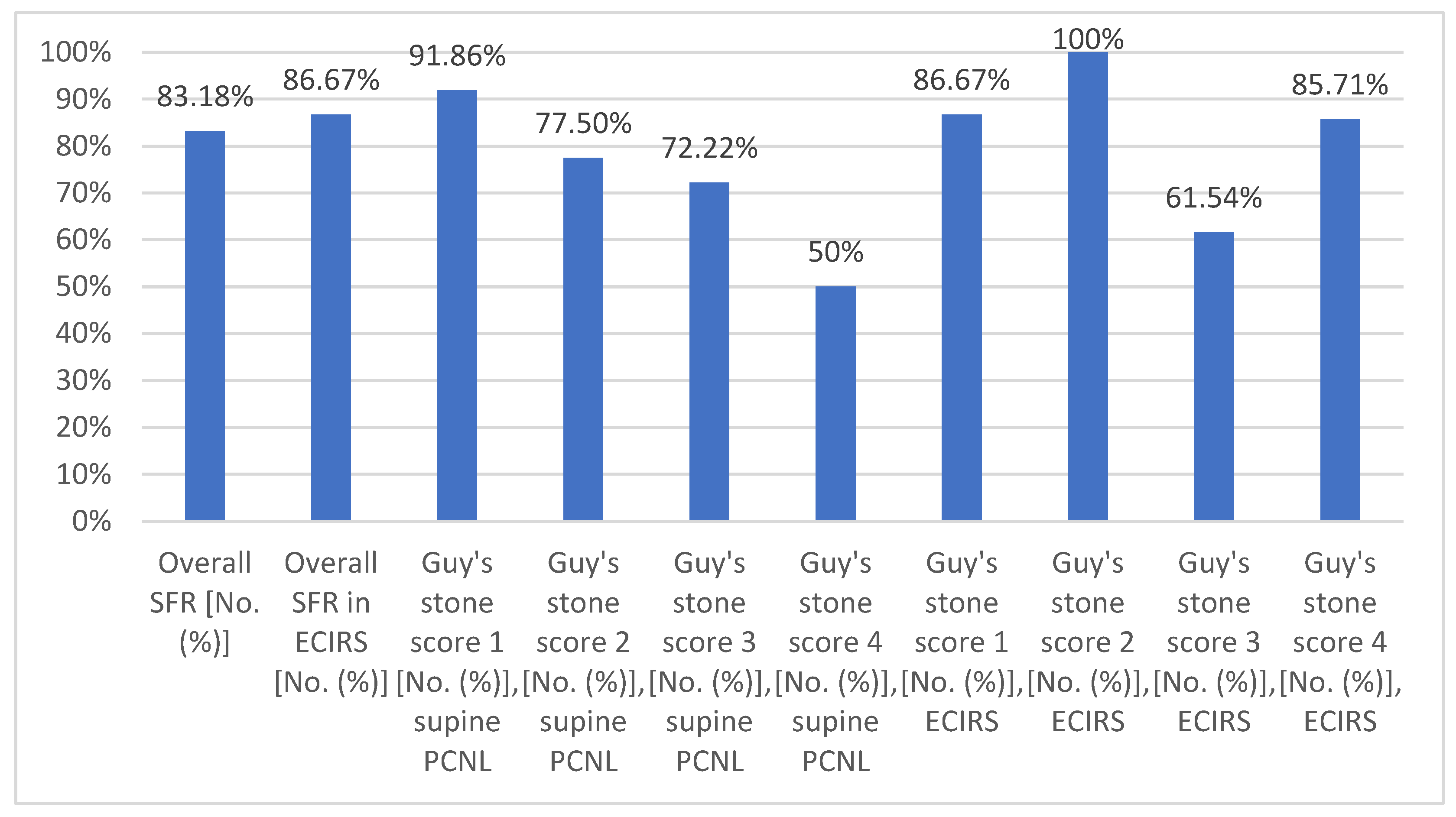
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| BMI | body mass index |
| CT | computed tomography |
| ECIRS | endoscopic combined intrarenal surgery |
| fURS | flexible ureterorenoscopy |
| KUB X-ray | kidney–ureter–bladder X-ray |
| NCCT | noncontrast computed tomography |
| PCNL | percutaneous nephrolithotomy |
| SFR | stone-free rate |
References
- Hoznek, A.; Rode, J.; Ouzaid, I.; Faraj, B.; Kimuli, M.; de la Taille, A.; Salomon, L.; Abbou, C.-C. Modified Supine Percutaneous Nephrolithotomy for Large Kidney and Ureteral Stones: Technique and Results. Eur. Urol. 2012, 61, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bach, C.; Kachrilas, S.; Papatsoris, A.G.; Buchholz, N.; Masood, J. Supine Percutaneous Nephrolithotomy (PCNL): ‘In Vogue’ but in Which Position? BJU Int. 2012, 110, E1018–E1021. [Google Scholar] [CrossRef] [PubMed]
- de la Rosette, J.; Assimos, D.; Desai, M.; Gutierrez, J.; Lingeman, J.; Scarpa, R.; Tefekli, A.; Croes PCNL Study Group. The Clinical Research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study: Indications, Complications, and Outcomes in 5803 Patients. J. Endourol. 2011, 25, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.A.S.; Vicentini, F.C.; Perrella, R.; Murta, C.B.; Claro, J.F.A. Comparative Study of Percutaneous Nephrolithotomy Performed in the Traditional Prone Position and in Three Different Supine Positions. Int. Braz. J. Urol. 2019, 45, 108–117. [Google Scholar] [CrossRef]
- Hosier, G.W.; Visram, K.; McGregor, T.; Steele, S.; Touma, N.J.; Beiko, D. Ambulatory Percutaneous Nephrolithotomy Is Safe and Effective in Patients with Extended Selection Criteria. Can. Urol. Assoc. J. 2022, 16, 89–95. [Google Scholar] [CrossRef]
- Salah, M.; Tallai, B.; Gul, T.; Aboumarzouk, O.; Alrayashi, M.; Abdelkareem, M.; Kamkoum, H.; Ibrahim, M.; Ebrahim, M.; Alnawasra, H.; et al. Percutaneous Nephrolithotomy in Supine Position with Less Than 24-Hour Hospital Stay; a Single-Center Experience. Arab. J. Urol. 2024, 22, 54–60. [Google Scholar] [CrossRef]
- Fernström, I.; Johansson, B. Percutaneous Pyelolithotomy: A New Extraction Technique. Scand. J. Urol. Nephrol. 1976, 10, 257–259. [Google Scholar] [CrossRef]
- Valdivia Uría, J.G.; Lachares Santamaría, E.; Villarroya Rodríguez, S.; Taberner Llop, J.; Abril Baquero, G.; Aranda Lassa, J.M. Percutaneous Nephrolithectomy: Simplified Technic (Preliminary Report), Preliminary report. Arch. Esp. Urol. 1987, 40, 177–180. [Google Scholar]
- Karaolides, T.; Moraitis, K.; Bach, C.; Masood, J.; Buchholz, N. Positions for Percutaneous Nephrolithotomy: Thirty-Five Years of Evolution. Arab. J. Urol. 2012, 10, 307–316. [Google Scholar] [CrossRef]
- Hur, K.J.; Moon, H.W.; Kang, S.M.; Kim, K.S.; Choi, Y.S.; Cho, H. Incidence of Posterolateral and Retrorenal Colon in Supine and Prone Position in Percutaneous Nephrolithotomy. Urolithiasis 2021, 49, 585–590. [Google Scholar] [CrossRef]
- Giusti, G.; Pavia, M.P.; Rico, L.; Proietti, S. Percutaneous Nephrolithotomy: Which Position? Supine Position! Eur. Urol. Open Sci. 2021, 35, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, R.M.; Jones, P.; Somani, B.K. Simultaneous Bilateral Endoscopic Surgery (SBES) for Bilateral Urolithiasis: The Future? Evidence from a Systematic Review. Curr. Urol. Rep. 2019, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Cracco, C.M.; Scoffone, C.M. Endoscopic Combined Intrarenal Surgery (ECIRS)—Tips and Tricks to Improve Outcomes: A Systematic Review. Turk. J. Urol. 2020, 46, S46–S57. [Google Scholar] [CrossRef]
- Sabnis, R.; Desai, M.R.; Singh, A. Supine Percutaneous Nephrolithotomy. J. Endourol. 2022, 36, S35–S40. [Google Scholar] [CrossRef]
- Masarwe, I.; Savin, Z.; Rabinowich, A.; Lifshitz, K.; Herzberg, H.; Marom, R.; Croitoru, S.; Mano, R.; Yossepowitch, O.; Aviram, G.; et al. Querying the Significance of Patient Position During Computerized Tomography on the Reliability of Pre-percutaneous Nephrolithotomy Planning. World J. Urol. 2022, 40, 1553–1560. [Google Scholar] [CrossRef]
- Tzou, D.T.; Tailly, T.O.; Stern, K.L. Ultrasound-Guided PCNL—Why Are We Still Performing Exclusively Fluoroscopic Access? Curr. Urol. Rep. 2023, 24, 335–343. [Google Scholar] [CrossRef]
- Pulido-Contreras, E.; Garcia-Padilla, M.A.; Medrano-Sanchez, J.; Leon-Verdin, G.; Primo-Rivera, M.A.; Sur, R.L. Percutaneous Nephrolithotomy with Ultrasound-Assisted Puncture: Does the Technique Reduce Dependence on Fluoroscopic Ionizing Radiation? World J. Urol. 2021, 39, 3579–3585. [Google Scholar] [CrossRef]
- Seitz, C.; Desai, M.; Häcker, A.; Hakenberg, O.W.; Liatsikos, E.; Nagele, U.; Tolley, D. Incidence, Prevention, and Management of Complications Following Percutaneous Nephrolitholapaxy. Eur. Urol. 2012, 61, 146–158. [Google Scholar] [CrossRef]
- Al-Dessoukey, A.A.; Moussa, A.S.; Abdelbary, A.M.; Zayed, A.; Abdallah, R.; Elderwy, A.A.; Massoud, A.M.; Aly, A.H. Percutaneous Nephrolithotomy in the Oblique Supine Lithotomy Position and Prone Position: A Comparative Study. J. Endourol. 2014, 28, 1058–1063. [Google Scholar] [CrossRef]
- Karami, H.; Mohammadi, R.; Lotfi, B. A Study on Comparative Outcomes of Percutaneous Nephrolithotomy in Prone, Supine, and Flank Positions. World J. Urol. 2013, 31, 1225–1230. [Google Scholar] [CrossRef]
- Keller, E.X.; DE Coninck, V.; Proietti, S.; Talso, M.; Emiliani, E.; Ploumidis, A.; Mantica, G.; Somani, B.; Traxer, O.; Scarpa, R.M.; et al. European Association of Urology—European Society of Residents in Urology (EAU-ESRU). Prone Versus Supine Percutaneous Nephrolithotomy: A Systematic Review and Meta-analysis of Current Literature. Minerva. Urol. Nephrol. 2021, 73, 50–58. [Google Scholar] [CrossRef]
- Curry, D.; Srinivasan, R.; Kucheria, R.; Goyal, A.; Allen, D.; Goode, A.; Yu, D.; Ajayi, L. Supine Percutaneous Nephrolithotomy in the Galdako-Modified Valdivia Position: A High-Volume Single Center Experience. J. Endourol. 2017, 31, 1001–1006. [Google Scholar] [CrossRef]
| Variables | |
|---|---|
| Patients (n) | 208 |
| Treated renal units (n) | 220 |
| Number of ECIRS procedures, [No. (%)] | 60 [27.27] |
| Age (years), mean ± SD | 52.21 ± 15.55 |
| Sex | |
| male [No. (%)] | 108 [49.09] |
| female [No. (%)] | 112 [50.91] |
| Numbers of PCNL/year | |
| 2019 | 15 |
| 2020 | 32 |
| 2021 | 23 |
| 2022 | 55 |
| 2023 | 45 |
| 2024 | 50 |
| BMI (kg/m2), mean ± SD | 29.36 ± 6.86 |
| ASA score 1 [No. (%)] | 34 [15.45] |
| ASA score 2 [No. (%)] | 117 [53.18] |
| ASA score 3 [No. (%)] | 64 [29.09] |
| ASA score 4 [No. (%)] | 5 [2.27] |
| Laterality | |
| left side [No. (%)] | 112 [50.91] |
| right side [No. (%)] | 96 [43.64] |
| bilateral [No. (%)] | 12 [5.45] |
| Guy’s stone score distribution in supine PCNL | |
| Guy’s stone score 1 [No. (%)] | 86 [53.75] |
| Guy’s stone score 2 [No. (%)] | 40 [25] |
| Guy’s stone score 3 [No. (%)] | 18 [11.25] |
| Guy’s stone score 4 [No. (%)] | 16 [10] |
| Guy’s stone score distribution in ECIRS | |
| Guy’s stone score 1 [No. (%)] | 15 [25] |
| Guy’s stone score 2 [No. (%)] | 25 [41.67] |
| Guy’s stone score 3 [No. (%)] | 13 [21.67] |
| Guy’s stone score 4 [No. (%)] | 7 [11.67] |
| Presence of horseshoe kidney [No. (%)] | 5 [2.27] |
| Solitary kidney [No. (%)] | 2 [0.91] |
| Diverticulum stone [No. (%)] | 1 [0.45] |
| Previous PCNL on the intended side [No. (%)] | 31 [14.09] |
| Maximum diameter of the stones (mm), mean ± SD | 35.10 ± 20.38 |
| Stone volume (mm3), mean ± SD | 45,852.70 ± 96,753.46 |
| Stone density (HU), mean ± SD | 890.92 ± 295.70 |
| Preoperative urine culture positivity [No. (%)] | 81 [36.82] |
| Preoperative creatinine (µmol), mean ± SD | 84.56 ± 29.05 |
| Preoperative Hgb (g/L), mean ± SD | 139.44 ± 18.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, Z.; Drabik, G.; Murányi, M.; Nagy, A.; Goumas, I.K.; Flaskó, T. Tubeless Percutaneous Nephrolithotomy in the Barts ‘Flank-Free’ Modified Supine Position with 24-Hour Discharge: A Single-Center Experience. Medicina 2025, 61, 748. https://doi.org/10.3390/medicina61040748
Kiss Z, Drabik G, Murányi M, Nagy A, Goumas IK, Flaskó T. Tubeless Percutaneous Nephrolithotomy in the Barts ‘Flank-Free’ Modified Supine Position with 24-Hour Discharge: A Single-Center Experience. Medicina. 2025; 61(4):748. https://doi.org/10.3390/medicina61040748
Chicago/Turabian StyleKiss, Zoltán, Gyula Drabik, Mihály Murányi, Attila Nagy, Ioannis Kartalas Goumas, and Tibor Flaskó. 2025. "Tubeless Percutaneous Nephrolithotomy in the Barts ‘Flank-Free’ Modified Supine Position with 24-Hour Discharge: A Single-Center Experience" Medicina 61, no. 4: 748. https://doi.org/10.3390/medicina61040748
APA StyleKiss, Z., Drabik, G., Murányi, M., Nagy, A., Goumas, I. K., & Flaskó, T. (2025). Tubeless Percutaneous Nephrolithotomy in the Barts ‘Flank-Free’ Modified Supine Position with 24-Hour Discharge: A Single-Center Experience. Medicina, 61(4), 748. https://doi.org/10.3390/medicina61040748






