Non-Invasive Tests as a Replacement for Liver Biopsy in the Assessment of MASLD
Abstract
1. Introduction
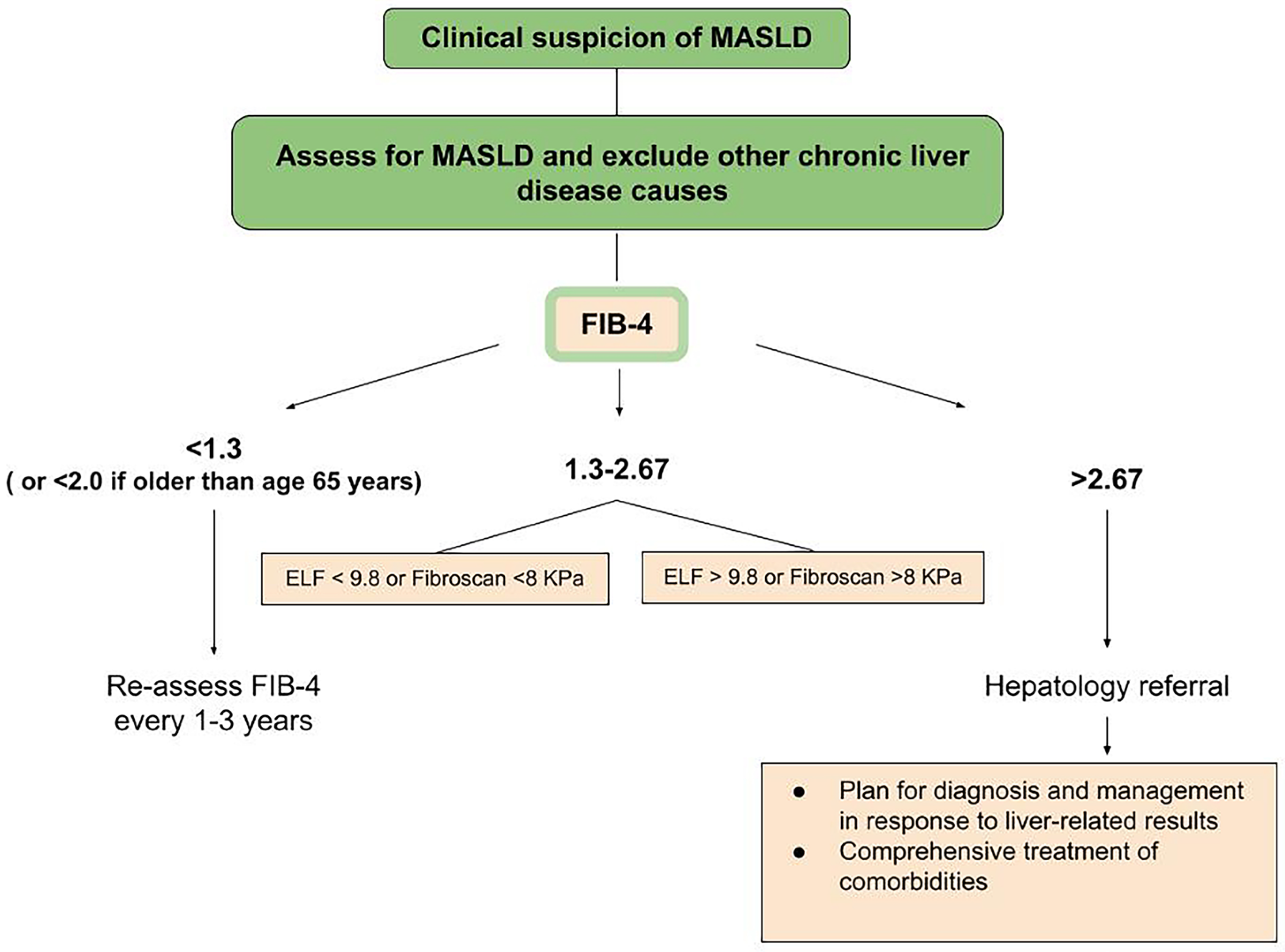
2. Diagnosis Process
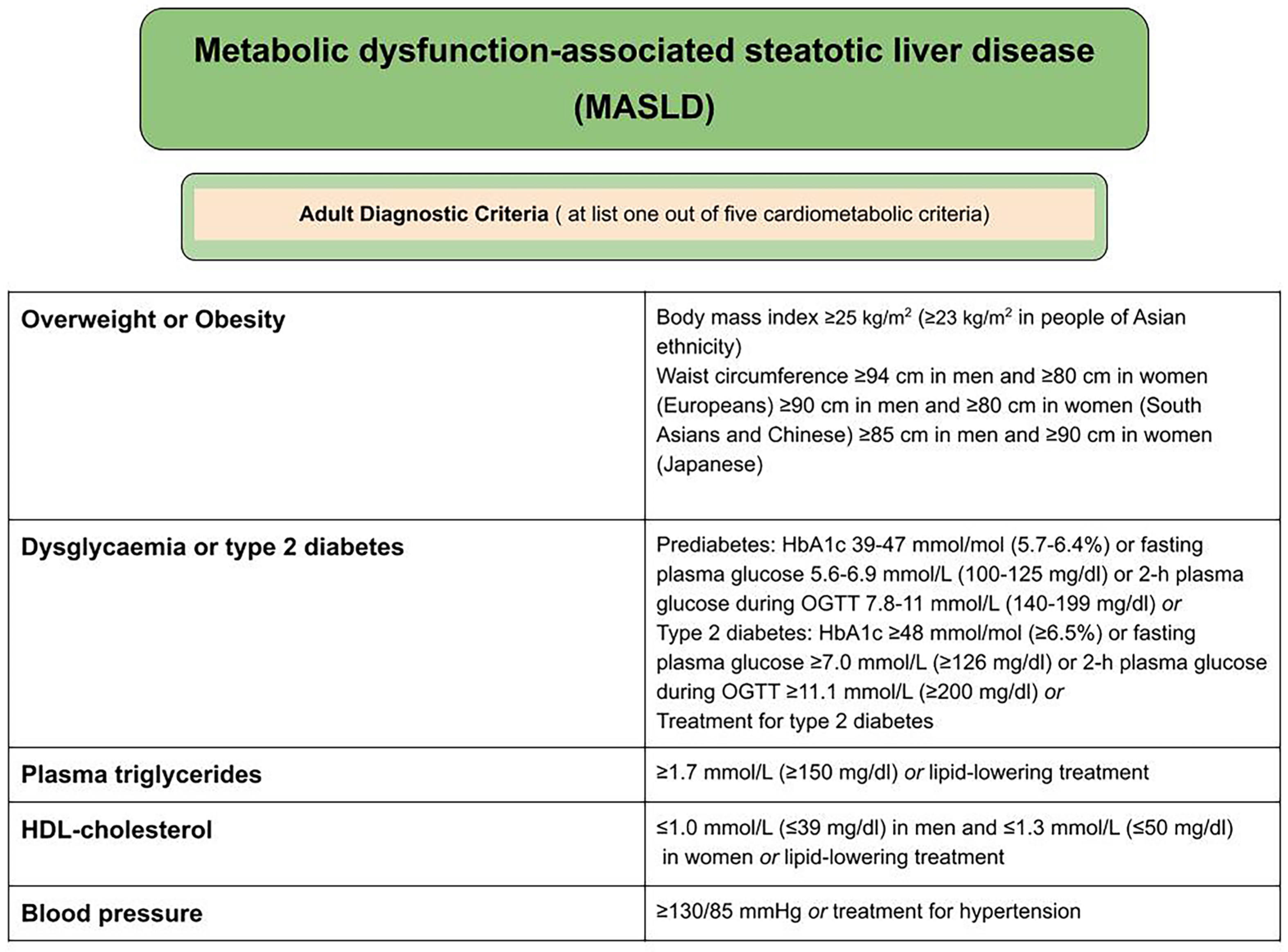
2.1. Criteria for the Diagnosis of MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease)
2.2. Serum Markers
2.3. Elastography
2.4. Liver Biopsy
3. Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Davis, J.L.; Murray, J.F. History and Physical Examination. In Murray and Nadel’s Textbook of Respiratory Medicine; Broaddus, V.C., Mason, R.J., Ernst, J.D., King, T.E., Lazarus, S.C., Murray, J.F., Nadel, J.A., Slutsky, A.S., Gotway, M.B., Eds.; W. B. Saunders: New York, NY, USA, 2016; pp. 263–277.e2. [Google Scholar]
- Kim, D.H.; Kim, S.W.; Hwang, S.H. Efficacy of non-invasive diagnostic methods in the diagnosis and screening of oral cancer and precancer. Braz. J. Otorhinolaryngol. 2022, 88, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- Michalopoulou, E.; Thymis, J.; Lampsas, S.; Pavlidis, G.; Katogiannis, K.; Vlachomitros, D.; Katsanaki, E.; Kostelli, G.; Pililis, S.; Pliouta, L.; et al. The Triad of Risk: Linking MASLD, Cardiovascular Disease and Type 2 Diabetes; From Pathophysiology to Treatment. J. Clin. Med. 2025, 14, 428. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Kaya, E.; Yilmaz, Y. Metabolic-associated Fatty Liver Disease (MAFLD): A Multi-systemic Disease Beyond the Liver. J. Clin. Transl. Hepatol. 2022, 10, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Price, J.K.; Owrangi, S.; Gundu-Rao, N.; Satchi, R.; Paik, J.M. The Global Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among Patients with Type 2 Diabetes. Clin. Gastroenterol. Hepatol. 2024, 22, 1999–2010.e8. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. NAFLD Nomenclature Consensus Group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Solomon, A.; Negrea, M.O.; Cipăian, C.R.; Boicean, A.; Mihaila, R.; Rezi, C.; Cristinescu, B.A.; Berghea-Neamtu, C.S.; Popa, M.L.; Teodoru, M.; et al. Interactions between Metabolic Syndrome, MASLD, and Arterial Stiffening: A Single-Center Cross-Sectional Study. Healthcare 2023, 11, 2696. [Google Scholar] [CrossRef]
- Ozturk, A.; Olson, M.C.; Samir, A.E.; Venkatesh, S.K. Liver fibrosis assessment: MR and US elastography. Abdom. Radiol. 2022, 47, 3037–3050. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Jiang, S.W.; Hu, A.R.; Zhou, A.W.; Hu, T.; Li, H.S.; Fan, Y.; Lin, K. Non-invasive diagnosis of non-alcoholic fatty liver disease: Current status and future perspective. Heliyon 2024, 10, e27325. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- Wattacheril, J.J.; Abdelmalek, M.F.; Lim, J.K.; Sanyal, A.J. AGA Clinical Practice Update on the Role of Noninvasive Biomarkers in the Evaluation and Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2023, 165, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Kaylan, K.B.; Paul, S. NAFLD No More: A Review of Current Guidelines in the Diagnosis and Evaluation of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Curr. Diab Rep. 2024, 25, 5. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Kamada, Y.; Munekage, K.; Nakahara, T.; Fujii, H.; Sawai, Y.; Doi, Y.; Hyogo, H.; Sumida, Y.; Imai, Y.; Miyoshi, E.; et al. The FIB-4 Index Predicts the Development of Liver-Related Events, Extrahepatic Cancers, and Coronary Vascular Disease in Patients with NAFLD. Nutrients 2022, 15, 66. [Google Scholar] [CrossRef]
- Kjaergaard, M.; Lindvig, K.P.; Thorhauge, K.H.; Andersen, P.; Hansen, J.K.; Kastrup, N.; Jensen, J.M.; Hansen, C.D.; Johansen, S.; Israelsen, M.; et al. Using the ELF test, FIB-4 and NAFLD fibrosis score to screen the population for liver disease. J. Hepatol. 2023, 79, 277–286. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Ciba-Stemplewska, A.; Gorczyca-Głowacka, I. Practical aspects of diagnosing metabolic dysfunction-associated fatty liver disease. Lekarz POZ 2023, 9, 155–162. [Google Scholar]
- Drolz, A.; Wolter, S.; Wehmeyer, M.H.; Piecha, F.; Horvatits, T.; Schulze Zur Wiesch, J.; Lohse, A.W.; Mann, O.; Kluwe, J. Performance of non-invasive fibrosis scores in non-alcoholic fatty liver disease with and without morbid obesity. Int. J. Obes. 2021, 45, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yao, X.Y.; Shi, R.X.; Liu, S.F.; Wang, X.Y. A potential link between polycystic ovary syndrome and non-alcoholic fatty liver disease: An update meta-analysis. Reprod. Health 2018, 15, 77. [Google Scholar] [CrossRef]
- Doycheva, I.; Ehrmann, D.A. Nonalcoholic fatty liver disease and obstructive sleep apnea in women with polycystic ovary syndrome. Fertil. Steril. 2022, 117, 897–911. [Google Scholar] [CrossRef]
- Spremović Rađenović, S.; Pupovac, M.; Andjić, M.; Bila, J.; Srećković, S.; Gudović, A.; Dragaš, B.; Radunović, N. Prevalence, Risk Factors, and Pathophysiology of Nonalcoholic Fatty Liver Disease (NAFLD) in Women with Polycystic Ovary Syndrome (PCOS). Biomedicines 2022, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Shengir, M.; Chen, T.; Guadagno, E.; Ramanakumar, A.V.; Ghali, P.; Deschenes, M.; Wong, P.; Krishnamurthy, S.; Sebastiani, G. Non-alcoholic fatty liver disease in premenopausal women with polycystic ovary syndrome: A systematic review and meta-analysis. JGH Open 2021, 5, 434–445. [Google Scholar] [CrossRef]
- Migacz, M. Using non-invasive indicators to screen the PCOS population for liver disease—A single-centre study. Endokrynol. Pol. 2025, 76, 94–99. [Google Scholar] [CrossRef]
- Vassilatou, E. Nonalcoholic fatty liver disease and polycystic ovary syndrome. World J. Gastroenterol. 2014, 20, 8351–8363. [Google Scholar] [CrossRef]
- Gürkan, E. Evaluation of Liver Fibrosis in Polycystic Ovary Syndrome by Shear Wave Elastography, FIB-4 Score, and Serum Periostin Levels. Endocrinol. Res. Pract. 2023, 27, 78–84. [Google Scholar] [CrossRef]
- Elhoseeny, M.M.; Abdulaziz, B.A.; Mohamed, M.A. Fetuin-A: A relevant novel serum biomarker for non-invasive diagnosis of metabolic dysfunction-associated steatotic liver disease (MASLD): A retrospective case-control study. BMC Gastroenterol. 2024, 24, 226. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Wang, Z.; Chen, S.; Ni, Y.; Jiang, D. Higher non-HDL-cholesterol to HDL-cholesterol ratio linked with increased nonalcoholic steatohepatitis. Lipids Health Dis. 2018, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Nouso, K.; Matsushita, H.; Kariyama, K.; Sakurai, T.; Takahashi, Y.; Chiba, H.; Hui, S.P.; Ito, Y.; Ohta, M.; et al. Low-Density Lipoprotein (LDL)-Triglyceride and Its Ratio to LDL-Cholesterol as Diagnostic Biomarkers for Nonalcoholic Steatohepatitis. J. Appl. Lab. Med. 2020, 5, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Jiang, L. Serum Cytokeratin-18 levels as a prognostic biomarker in advanced liver disease: A comprehensive meta-analysis. Clin. Exp. Med. 2024, 24, 160. [Google Scholar] [CrossRef]
- Albeltaji, A.; Al-qatati, A.; Alzaharna, M. Serum Cytokeratin-18 as a Non-invasive Biomarker and its Association with Biochemical Parameters in the Diagnosis of Non-alcoholic Fatty Liver Disease. Jordan Med. J. 2024, 80. [Google Scholar] [CrossRef]
- Zeng, Y.; He, H.; An, Z. Advance of Serum Biomarkers and Combined Diagnostic Panels in Nonalcoholic Fatty Liver Disease. Dis. Markers 2022, 2022, 1254014. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, L.; Mak, A.L.; van Koppen, A. Development of a novel non-invasive biomarker panel for hepatic fibrosis in MASLD. Nat. Commun. 2024, 15, 4564. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, M.-H.; Liu, D.; Lin, Y.; Song, S.J.; Chu, E.S.-H.; Liu, D.; Singh, S.; Berman, M.; Lau, H.C.-H.; et al. A blood-based biomarker panel for non-invasive diagnosis of metabolic dysfunction-associated steatohepatitis. Cell Metab. 2023, 37, 59–68.e3. [Google Scholar] [CrossRef]
- Huang, Z.H.; Deng, M.Q.; Lin, Y.; Ye, C.H.; Zheng, M.H.; Zheng, Y.P. Body posture can modulate liver stiffness measured by transient elastography: A prospective observational study. BMC Gastroenterol. 2024, 24, 1386. [Google Scholar] [CrossRef] [PubMed]
- Taru, M.-G.; Neamti, L.; Taru, V.; Procopciuc, L.M.; Procopet, B.; Lupsor-Platon, M. How to Identify Advanced Fibrosis in Adult Patients with Non-Alcoholic Fatty Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH) Using Ultrasound Elastography-A Review of the Literature and Proposed Multistep Approach. Diagnostics 2023, 13, 4788. [Google Scholar] [CrossRef]
- Selvaraj, E.A.; Mózes, F.E.; Jayaswal, A.N.A.; Zafarmand, M.H.; Vali, Y.; Lee, J.A.; Levick, C.K.; Young, L.A.J.; Palaniyappan, N.; Liu, C.-H.; et al. Diagnostic accuracy of elastography and magnetic resonance imaging in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 2021, 75, 770–785. [Google Scholar] [CrossRef]
- Marti-Aguado, D.; Carot-Sierra, J.M.; Villalba-Ortiz, A.; Siddiqi, H.; Vallejo-Vigo, R.M.; Lara-Romero, C.; Martín-Fernández, M.; Fernández-Patón, M.; Alfaro-Cervello, C.; Crespo, A.; et al. Identification of Candidates for MASLD Treatment with Indeterminate Vibration-Controlled Transient Elastography. Clin. Gastroenterol. Hepatol. 2024, in press. [CrossRef] [PubMed]
- Gidener, T.; Dierkhising, R.A.; Mara, K.C.; Therneau, T.M.; Venkatesh, S.K.; Ehman, R.L.; Yin, M.; Allen, A.M. Change in serial liver stiffness measurement by magnetic resonance elastography and outcomes in NAFLD. Hepatology 2023, 77, 268–274. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Cusi, K.; Das, S.R.; Gibbons, C.H.; et al. Introduction and Methodology: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S1–S4. [Google Scholar] [CrossRef]
- Ahmed, N.; Kumari, A.; Murty, R.S. FibroScan’s evolution: A critical 20-year review. J. Ultrasound 2024. online ahead of print. [Google Scholar] [CrossRef]
- Cazac, G.-D.; Lăcătușu, C.-M.; Mihai, C.; Grigorescu, E.-D.; Onofriescu, A.; Mihai, B.-M. Ultrasound-Based Hepatic Elastography in Non-Alcoholic Fatty Liver Disease: Focus on Patients with Type 2 Diabetes. Biomedicines 2022, 10, 2375. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ganie, M.A.; Masoodi, I.; Jana, M.; Shalimar; Gupta, N.; Sofi, N.Y. Fibroscan as a non-invasive predictor of hepatic steatosis in women with polycystic ovary syndrome. Indian. J. Med. Res. 2020, 151, 333–341. [Google Scholar] [CrossRef]
- Xuan, Y.; Zhu, M.; Xu, L.; Huangfu, S.; Li, T.; Liu, C.; Zhou, D. Elevated non-HDL-C to HDL-C ratio as a marker for NAFLD and liver fibrosis risk: A cross-sectional analysis. Front. Endocrinol. 2024, 15, 1457589. [Google Scholar] [CrossRef]
- Pierce, T.T.; Samir, A.E. Liver Fibrosis: Point-Ultrasound Elastography Is a Safe, Widely Available, Low-Cost, Noninvasive Biomarker of Liver Fibrosis That Is Suitable for Broad Community Use. AJR Am. J. Roentgenol. 2022, 219, 382–383. [Google Scholar] [CrossRef]
- Patel, K.; Sebastiani, G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020, 2, 100067. [Google Scholar] [CrossRef]
- Fraquelli, M.; Rigamonti, C.; Casazza, G.; Conte, D.; Donato, M.F.; Ronchi, G.; Colombo, M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007, 56, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y.; Qiu, S.; Ma, S.; Yan, F.; Yang, G.-Z.; Li, R.; Feng, Y. A Comparative Study of Three Systems for Liver Magnetic Resonance Elastography. J. Magn. Reson. Imaging 2024, 60, 2472–2484. [Google Scholar] [CrossRef] [PubMed]
- Kharat, A.; Vanpully, N.S.; Jeeson, J.C. Simplified Guide to MR Elastography in Early Detection of Hepatic Fibrosis with Case Reports: The New Norm in Assessing Liver Health. Indian. J. Radiol. Imaging 2021, 31, 644–652. [Google Scholar] [CrossRef]
- Sarkar Das, T.; Meng, X.; Abdallah, M.; Bilal, M.; Sarwar, R.; Shaukat, A. An Assessment of the Feasibility, Patient Acceptance, and Performance of Point-of-Care Transient Elastography for Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD): A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 2478. [Google Scholar] [CrossRef]
- Srivastava, A.; Jong, S.; Gola, A.; Srivastava, A.; Jong, S.; Gola, A.; Gailer, R.; Morgan, S.; Sennett, K.; Tanwar, S.; et al. Cost-comparison analysis of FIB-4, ELF and fibroscan in community pathways for non-alcoholic fatty liver disease. BMC Gastroenterol. 2019, 19, 122. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.-T.; Liang, L.Y.; Wong, G.L.-H. Noninvasive tests for liver fibrosis in 2024: Are there different scales for different diseases? Gastroenterol. Rep. 2024, 12, goae024. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Wang, L.-K.; Cai, S.-Y.; Chen, H.-X.; Zhou, Y.; Cheng, L.-K.; Lin, Y.-W.; Zheng, M.-H.; Zheng, Y.-P. Palm-Sized Wireless Transient Elastography System with Real-Time B-Mode Ultrasound Imaging Guidance: Toward Point-of-Care Liver Fibrosis Assessment. Diagnostics 2024, 14, 189. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.-S.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef]
- Arieira, C.; Monteiro, S.; Xavier, S.; Dias de Castro, F.; Magalhães, J.; Marinho, C.; Pinto, R.; Costa, W.; Pinto Correia, J.; Cotter, J. Transient elastography: Should XL probe be used in all overweight patients? Scand. J. Gastroenterol. 2019, 54, 1022–1026. [Google Scholar] [CrossRef]
- Wang, T.J.; Jirapinyo, P.; Shah, R.; Schuster, K.; Papke, D.J.; Thompson, C.C.; Doyon, L.; Lautz, D.B.; Ryou, M. EUS-guided shear wave elastography for fibrosis screening in patients with obesity and metabolic dysfunction-associated steatotic liver disease: A pilot study (with video). Gastrointest. Endosc. 2025, 101, 456–462. [Google Scholar] [CrossRef]
- Bastard, C.; Audière, S.; Foucquier, J.; Lorée, H.; Miette, V.; Bronowicki, J.-P.; Stern, C.; Caussy, C.; Sandrin, L. Guided-VCTE: An Enhanced FibroScan Examination With Improved Guidance and Applicability. Ultrasound Med. Biol. 2025, 51, 628–637. [Google Scholar] [CrossRef]
- Chang, P.E.; Goh, G.B.; Ngu, J.H.; Tan, H.K.; Tan, C.K. Clinical applications, limitations and future role of transient elastography in the management of liver disease. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Y.; Liao, M.; Zheng, J.; Huang, Z.P.; Xie, S.D. Two-dimensional shear wave elastography utilized in patients with ascites: A more reliable method than transient elastography for noninvasively detecting the liver stiffness-an original study with 170 patients. Ann. Transl. Med. 2023, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Cain, O.; Chauhan, A.; Webb, G.J. Medical liver biopsy: Background, indications, procedure and histopathology. Frontline Gastroenterol. 2020, 11, 40–47. [Google Scholar] [CrossRef]
- Sharma, S.; Khalili, K.; Nguyen, G.C. Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J. Gastroenterol. 2014, 20, 16820–16830. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.B.; Mehta, K.J. Liver biopsy for assessment of chronic liver diseases: A synopsis. Clin. Exp. Med. 2023, 23, 273–285. [Google Scholar] [CrossRef]
- Reddy, K.R. Liver biopsy: Archaic but resilient and many roads lead to Rome. Clin. Liver Dis. 2024, 23, e0247. [Google Scholar] [CrossRef]
- Chan, M.; Navarro, V.J. Percutaneous Liver Biopsy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Aljawad, M.; Yoshida, E.M.; Uhanova, J.; Marotta, P.; Chandok, N. Percutaneous liver biopsy practice patterns among Canadian hepatologists. Can. J. Gastroenterol. 2013, 27, e31–e34. [Google Scholar] [CrossRef]
- González-Mateo, E.; Camarena, F.; Jiménez, N. Real-time ultrasound shear wave elastography using a local phase gradient. Comput. Methods Programs Biomed. 2025, 260, 108529. [Google Scholar] [CrossRef]
- Congly, S.E.; Shaheen, A.A.; Swain, M.G. Modelling the cost effectiveness of non-alcoholic fatty liver disease risk stratification strategies in the community setting. PLoS ONE 2021, 16, e0251741. [Google Scholar] [CrossRef]
- Noureddin, M.; Jones, C.; Alkhouri, N.; Gomez, E.V.; Dieterich, D.T.; Rinella, M.E.; Therapondos, G.; Girgrah, N.; Mantry, P.S.; Sussman, N.L.; et al. Screening for Nonalcoholic Fatty Liver Disease in Persons with Type 2 Diabetes in the United States Is Cost-effective: A Comprehensive Cost-Utility Analysis. Gastroenterology 2021, 160, 2226. [Google Scholar] [CrossRef] [PubMed]
- Crossan, C.; Majumdar, A.; Srivastava, A.; Thorburn, D.; Rosenberg, W.; Pinzani, M.; Longworth, L.; Tsochatzis, E.A. Referral pathways for patients with NAFLD based on non-invasive fibrosis tests: Diagnostic accuracy and cost analysis. Liver Int. 2019, 39, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Johansen, P.; Howard, D.; Bishop, R.; Moreno, S.I.; Buchholtz, K. Systematic Literature Review and Critical Appraisal of Health Economic Models Used in Cost-Effectiveness Analyses in Non-Alcoholic Steatohepatitis: Potential for Improvements. Pharmacoeconomics 2020, 38, 485–497. [Google Scholar] [CrossRef]
- Hudson, D.; Afzaal, T.; Bualbanat, H.; AlRamdan, R.; Howarth, N.; Parthasarathy, P.; AlDarwish, A.; Stephenson, E.; Almahanna, Y.; Hussain, M.; et al. Modernizing metabolic dysfunction-associated steatotic liver disease diagnostics: The progressive shift from liver biopsy to noninvasive techniques. Therap. Adv. Gastroenterol. 2024, 17, 17562848241276334. [Google Scholar] [CrossRef] [PubMed]
| Aspect | Liver Biopsy | Liver Elastography |
|---|---|---|
| Invasiveness | Invasive, risk of complications | Non-invasive, safe, no known adverse effects |
| Diagnostic Value | Gold standard, detailed histology | High accuracy for fibrosis and steatosis assessment |
| Patient Contraindications | Absolute: bleeding risk, uncooperative patients, vascular tumors; relative: obesity, ascites | TE less effective in patients with ascites or obesity (solved via XL probe, Guided-VCTE, or 2D-SWE) |
| Procedure Time | Time-consuming, requires monitoring | Quick (under 5–10 min), immediate results |
| Risks and Complications | Pain, bleeding, hypotension, visceral injury, pneumothorax, rare mortality | Painless, no known adverse bioeffects; minor limitations in obese patients or with ascites |
| Repeatability | Limited due to risks | Easily repeatable, suitable for monitoring |
| Screening Use | Not suitable for mass screening | Ideal for large-scale screening (e.g., diabetes, obesity) |
| Cost & Accessibility | Expensive, hospital-based | Cost-effective, widely available (outpatient/bedside) |
| Technological Advances | Minimal improvements | Continuous innovation (AI, portable devices, Guided-VCTE) |
| Sampling Coverage | Small liver fragment, risk of sampling error | Assesses larger liver area, less variability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frączek, J.; Sowa, A.; Agopsowicz, P.; Migacz, M.; Dylińska-Kala, K.; Holecki, M. Non-Invasive Tests as a Replacement for Liver Biopsy in the Assessment of MASLD. Medicina 2025, 61, 736. https://doi.org/10.3390/medicina61040736
Frączek J, Sowa A, Agopsowicz P, Migacz M, Dylińska-Kala K, Holecki M. Non-Invasive Tests as a Replacement for Liver Biopsy in the Assessment of MASLD. Medicina. 2025; 61(4):736. https://doi.org/10.3390/medicina61040736
Chicago/Turabian StyleFrączek, Julia, Aleksandra Sowa, Paulina Agopsowicz, Maciej Migacz, Katarzyna Dylińska-Kala, and Michał Holecki. 2025. "Non-Invasive Tests as a Replacement for Liver Biopsy in the Assessment of MASLD" Medicina 61, no. 4: 736. https://doi.org/10.3390/medicina61040736
APA StyleFrączek, J., Sowa, A., Agopsowicz, P., Migacz, M., Dylińska-Kala, K., & Holecki, M. (2025). Non-Invasive Tests as a Replacement for Liver Biopsy in the Assessment of MASLD. Medicina, 61(4), 736. https://doi.org/10.3390/medicina61040736






