A Comparison of the Safety and Efficacy of Remimazolam and Dexmedetomidine for Sedation in Surgical Patients Under Regional Anesthesia: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligible Criteria
2.2. Search Strategy
2.3. Study Selection and Data Extraction
2.4. Outcome Measures
2.5. Assessment of Risk of Bias and Quality of Evidence
2.6. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Characteristics of Study and Participant
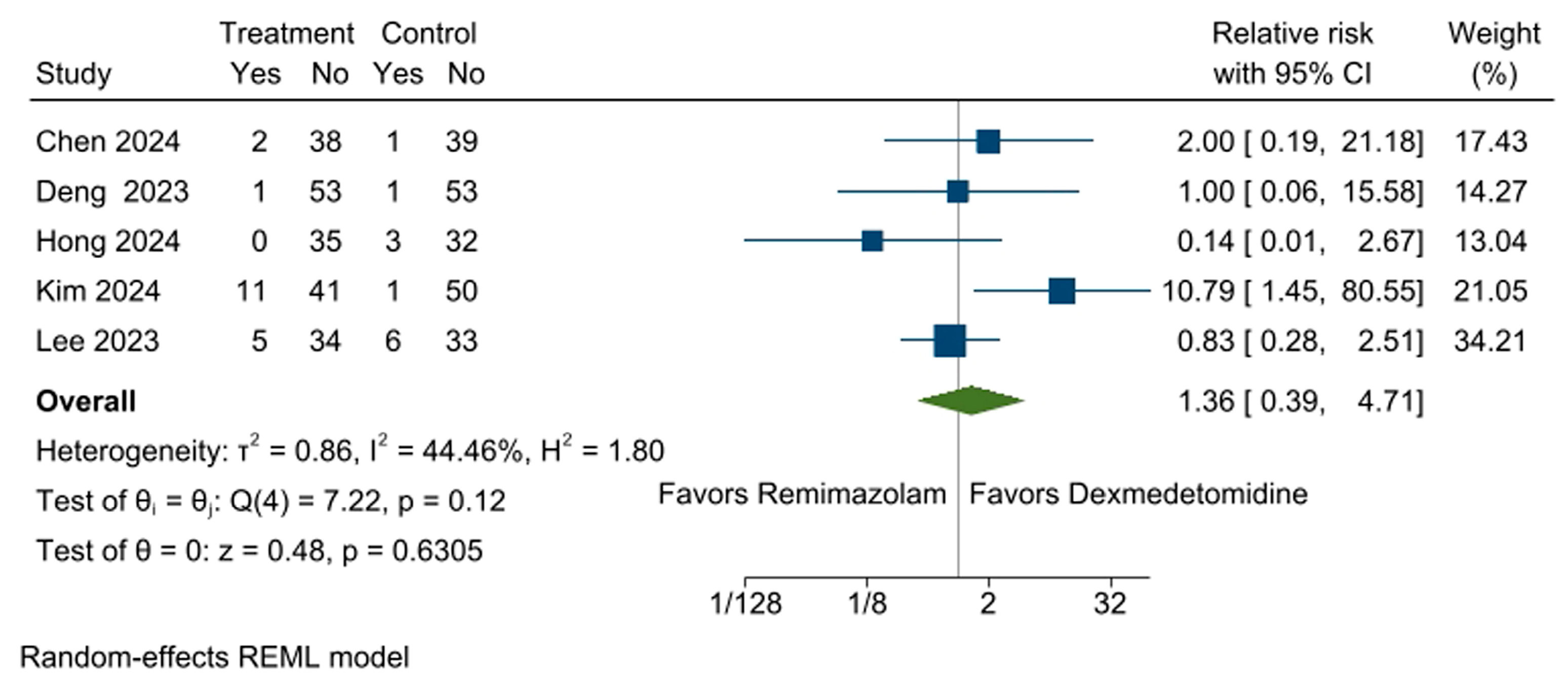
3.3. Incidence of Respiratory Depression
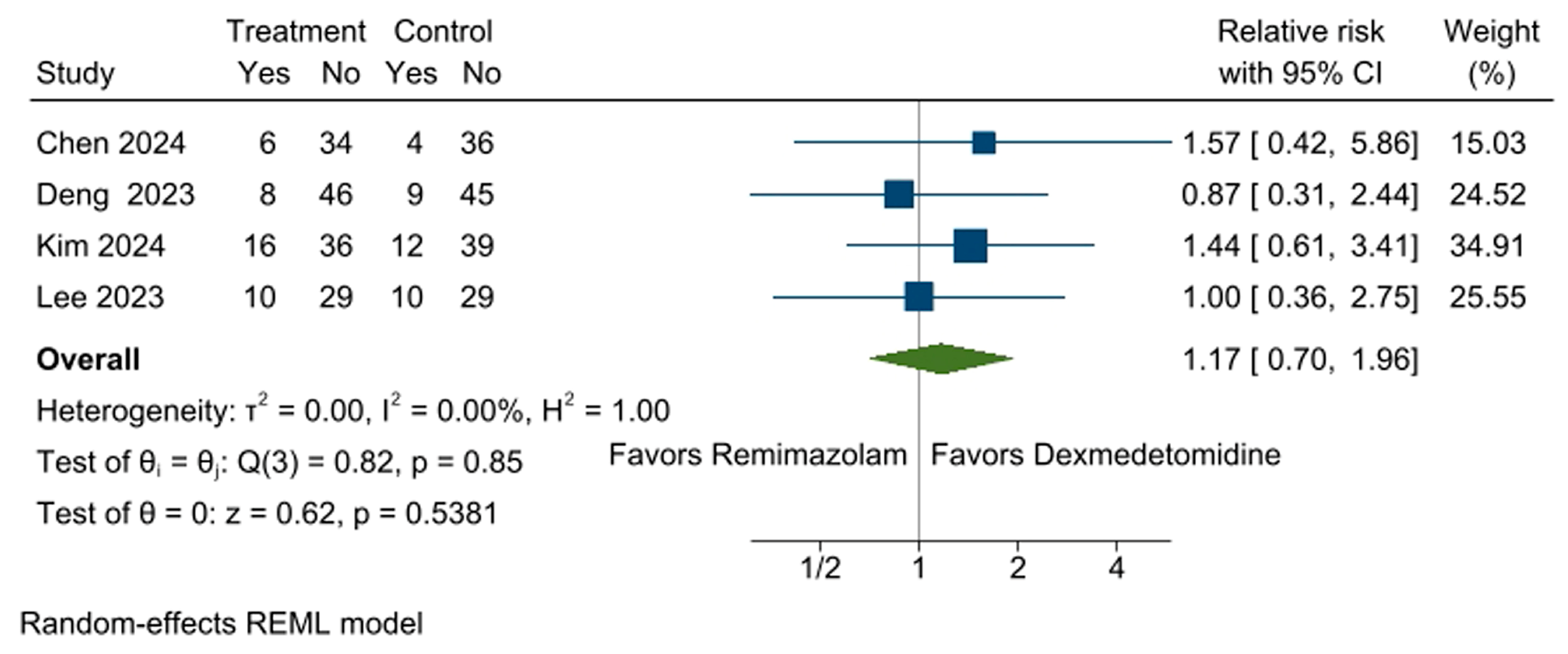
3.4. Incidence of Bradycardia
3.5. Incidence of Hypotension
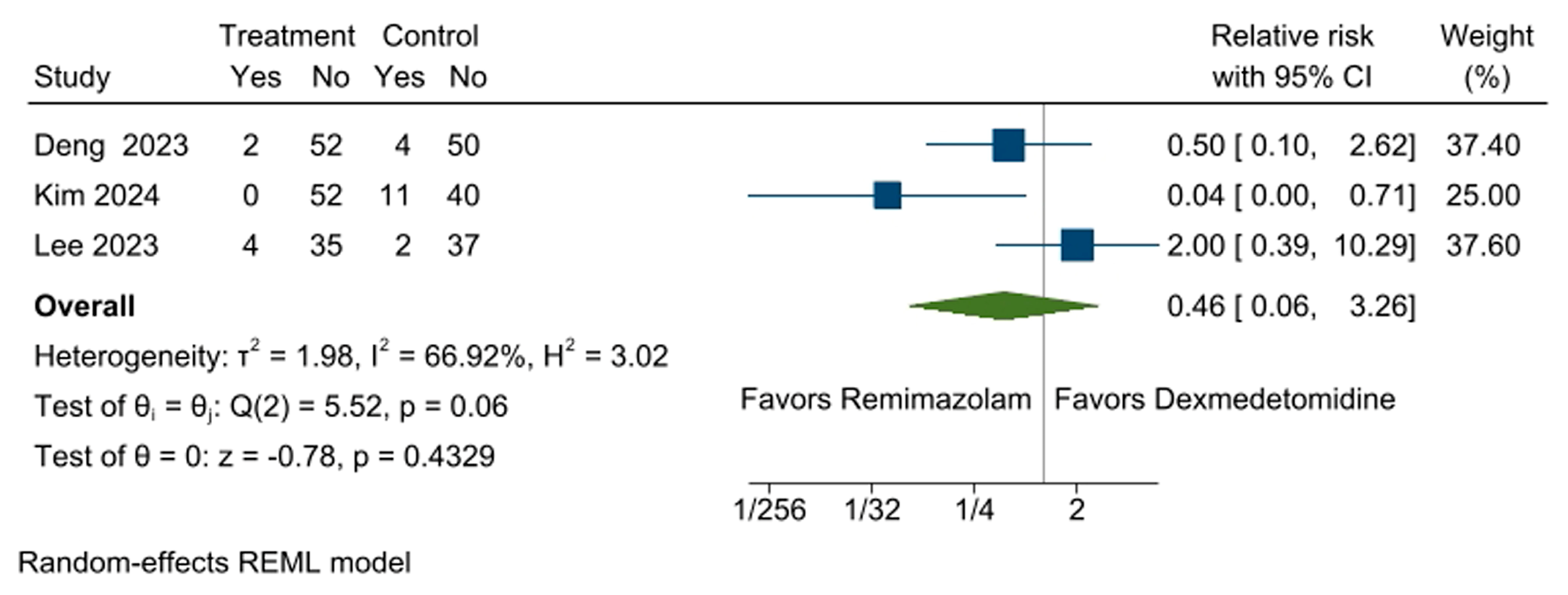
3.6. Incidence of Hypertension
3.7. Respiratory Rates During Sedation
3.8. Heart Rates During Sedation
3.9. Mean Arterial Pressure During Sedation
3.10. Time to Target Sedation Depth
3.11. Emergence Time from Sedation
3.12. Incidence of PONV
3.13. Risk of Bias
3.14. Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hohener, D.; Blumenthal, S.; Borgeat, A. Sedation and regional anaesthesia in the adult patient. Br. J. Anaesth. 2008, 100, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Attri, J.P.; Gupta, K.K.; Khetarpal, R. Emerging trends of sedation during regional anesthesia. Anesth. Pain Intensive Care 2015, 19, 527–532. [Google Scholar]
- Barends, C.R.M.; Absalom, A.R.; Struys, M. Drug selection for ambulatory procedural sedation. Curr. Opin. Anaesthesiol. 2018, 31, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Fechner, J. Remimazolam—Current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J. Anesthesiol. 2022, 75, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Dexmedetomidine: Present and future directions. Korean J. Anesthesiol. 2019, 72, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Eccles, M.; Flottorp, S.; Guyatt, G.H.; Henry, D.; Hill, S.; Liberati, A.; O’Connell, D.; Oxman, A.D.; Phillips, B.; et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv. Res. 2004, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, version 6.4; Cochrane: Chichester, UK, 2023. [Google Scholar]
- Chen, Y.; Cai, Y.; Yu, G.; Zhang, X.; Hu, T.; Xue, R. Safety and effcacy of remimazolam tosilate for sedation during combined spinal-epidural anesthesia for orthopedic procedures: A randomized controlled trial. BMC Anesthesiol. 2024, 24, 75. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.M.; Meng, Z.T.; Yang, J.; Zhang, C.J.; Lu, M.; Wang, Y.X.; Mu, D.L. Effect of intraoperative remimazolam on postoperative sleep quality in elderly patients after total joint arthroplasty: A randomized control trial. J. Anesth. 2023, 37, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Park, J.Y.; Rhee, K.Y.; Kim, S.H. Comparison emergence of sedation, using dexmedetomidine and remimazolam, in spinal anaesthesia-double blinded randomized controlled trial. Int. J. Med. Sci. 2024, 21, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Bae, J.; Yoo, S.; Lim, Y.J.; Kim, J.T. Comparison of remimazolam and dexmedetomidine for intraoperative sedation in patients undergoing lower extremity surgery under spinal anesthesia: A randomized clinical trial. Reg. Anesth. Pain. Med. 2023, 49, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.; Kang, H.Y.; Choi, J.H.; Kim, M.K.; You, A.H. Comparison of oxygen reserve index according to the remimazolam or dexmedetomidine for intraoperative sedation under regional anesthesia-A single-blind randomized controlled trial. Front. Med. 2023, 10, 1288243. [Google Scholar] [CrossRef] [PubMed]
- Terres, M.T.; Assis, M.L.; DA Silveira, C.B.; Amaral, S. Remimazolam versus propofol for endoscopy sedation in elderly patients: A systematic review, meta-analysis and trial sequential analysis. Minerva Anestesiol. 2024, 90, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.E. Postoperative nausea and vomiting. Korean J. Anesthesiol. 2014, 67, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.W.; Choi, Y.H.; Kim, J.Y.; Choi, J.B.; Lee, S.K.; Youn, E.J.; Lee, J.S. Efficacy of the bispectral index and Observer’s Assessment of Alertness/Sedation Scale in monitoring sedation during spinal anesthesia: A randomized clinical trial. J. Int. Med. Res. 2020, 48, 300060519893165. [Google Scholar] [CrossRef] [PubMed]
- Shehabi, Y.; Howe, B.D.; Bellomo, R.; Arabi, Y.M.; Bailey, M.; Bass, F.E.; Bin Kadiman, S.; McArthur, C.J.; Murray, L.; Reade, M.C.; et al. Early Sedation with Dexmedetomidine in Critically Ill Patients. N. Engl. J. Med. 2019, 380, 2506–2517. [Google Scholar] [CrossRef] [PubMed]
- Shehabi, Y.; Serpa Neto, A.; Howe, B.D.; Bellomo, R.; Arabi, Y.M.; Bailey, M.; Bass, F.E.; Kadiman, S.B.; McArthur, C.J.; Reade, M.C.; et al. Early sedation with dexmedetomidine in ventilated critically ill patients and heterogeneity of treatment effect in the SPICE III randomised controlled trial. Intensive Care Med. 2021, 47, 455–466. [Google Scholar] [CrossRef] [PubMed]


| Author | Year | Procedure | Sample Size | Age | Assessment Tools | Induction | Maintenance | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | A1 | A2 | Remimazolam | Dexmedetomidine | Remimazolam | Dexmedetomidine | ||||
| Chen [10] | 2024 | Orthopedic surgery | 40 | 40 | 58.8 | 61.7 | Ramsay score of 2–5 and BIS 60–80 | 0.03 mg/kg/min | 0.3 µg/kg | 0.2–0.5 mg/kg/h | 0.2–1.0 µg/kg/h |
| Deng [11] | 2023 | Total joint arthroplasty | 54 | 54 | 70.8 | 71.8 | BIS 70–80 | 0.025–0.1 mg/kg | 0.2–0.7 µg/kg/h | 0.1–1.0 mg/kg/h | 0.2–0.7 µg/kg/h |
| Hong [12] | 2024 | Orthopedic surgery | 35 | 35 | 39.0 | 38.0 | MOAA/S 3 | 6 mg/kg/h | 6 µg/kg/h | 1 mg/kg/h | 1 µg/kg/h |
| Kim [13] | 2024 | Orthopedic surgery | 52 | 51 | 52.0 | 54.0 | MOAA/S 3 or 4 | 0.075 mg/kg | 1 µg/kg | 0.5–1 mg/kg/h | 0.2–0.7 µg/kg/h |
| Lee [14] | 2023 | Surgery under regional anesthesia | 39 | 39 | 61.1 | 57.3 | MOAA/S 3 | 2.5 mg | 1 µg/kg | 0.1–1.0 mg/kg/h | 0.2–0.7 µg/kg/h |
| No. of Studies | No. of Participants | MD (95% CI) | p-Value | I2 | |
|---|---|---|---|---|---|
| Respiratory rates | 3 | 228 | –0.36 [–0.95, 0.24] | 0.2390 | 0% |
| Heart rates | 3 | 228 | 8.17 [6.32, 10.03] | 0.0000 | 0% |
| Mean arterial pressure | 3 | 228 | 9.01 [–9.97, 27.99] | 0.3522 | 99% |
| Time to target sedation depth | 3 | 253 | –6.04 [–6.99, –5.09] | 0.0000 | 68% |
| Emergence time from sedation | 3 | 253 | –11.84 [–25.87, 2.19] | 0.0981 | 99% |
| PONV | 4 | 359 | 1.27 [0.68, 2.37] | 0.4535 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, H.-S.; Park, S.-H.; Koo, B.-W.; Bang, S.; Shin, H.-J. A Comparison of the Safety and Efficacy of Remimazolam and Dexmedetomidine for Sedation in Surgical Patients Under Regional Anesthesia: A Meta-Analysis of Randomized Controlled Trials. Medicina 2025, 61, 726. https://doi.org/10.3390/medicina61040726
Na H-S, Park S-H, Koo B-W, Bang S, Shin H-J. A Comparison of the Safety and Efficacy of Remimazolam and Dexmedetomidine for Sedation in Surgical Patients Under Regional Anesthesia: A Meta-Analysis of Randomized Controlled Trials. Medicina. 2025; 61(4):726. https://doi.org/10.3390/medicina61040726
Chicago/Turabian StyleNa, Hyo-Seok, Sang-Hi Park, Bon-Wook Koo, Seunguk Bang, and Hyun-Jung Shin. 2025. "A Comparison of the Safety and Efficacy of Remimazolam and Dexmedetomidine for Sedation in Surgical Patients Under Regional Anesthesia: A Meta-Analysis of Randomized Controlled Trials" Medicina 61, no. 4: 726. https://doi.org/10.3390/medicina61040726
APA StyleNa, H.-S., Park, S.-H., Koo, B.-W., Bang, S., & Shin, H.-J. (2025). A Comparison of the Safety and Efficacy of Remimazolam and Dexmedetomidine for Sedation in Surgical Patients Under Regional Anesthesia: A Meta-Analysis of Randomized Controlled Trials. Medicina, 61(4), 726. https://doi.org/10.3390/medicina61040726






