The Role of the Gut Microbiota in Heart Failure: Pathophysiological Insights and Future Perspectives
Abstract
1. Introduction
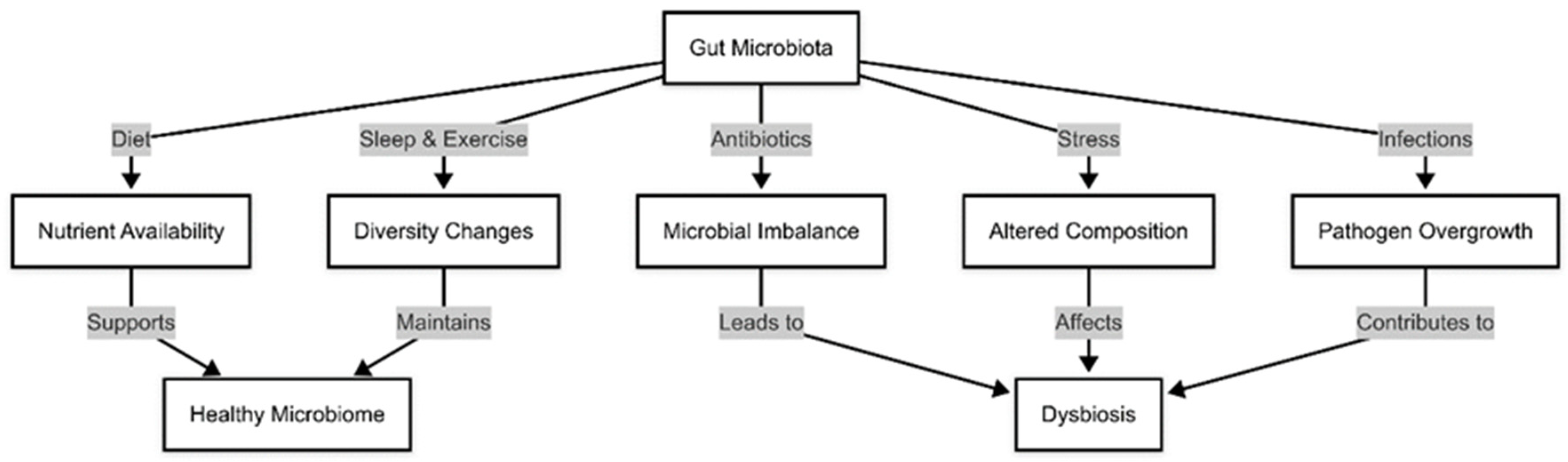
1.1. Factors Influencing Gut Microbiota Composition
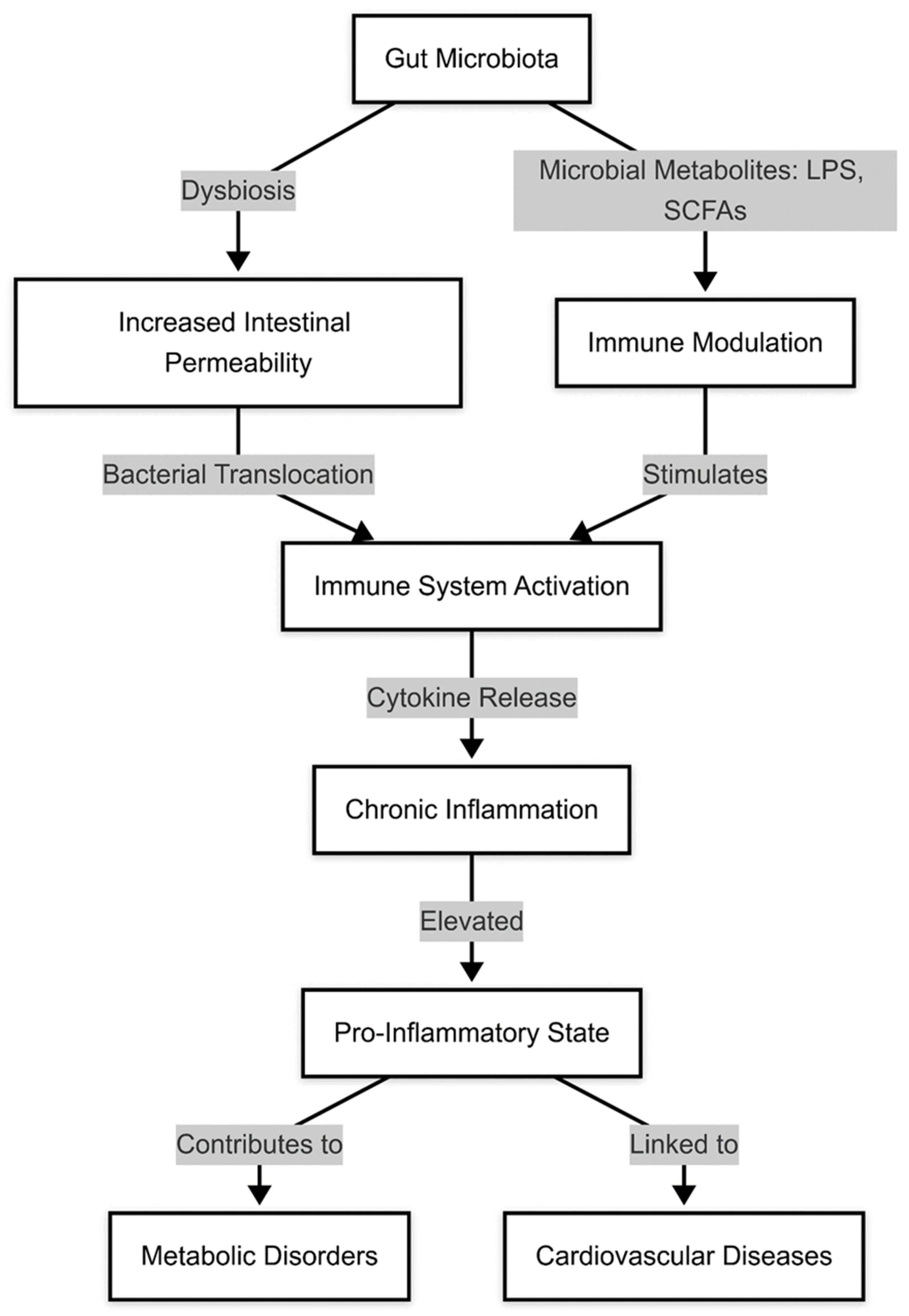
1.2. Diseases Associated with Gut Microbiota Dysregulation
1.3. Gut Microbiota and Hydrosaline Retention
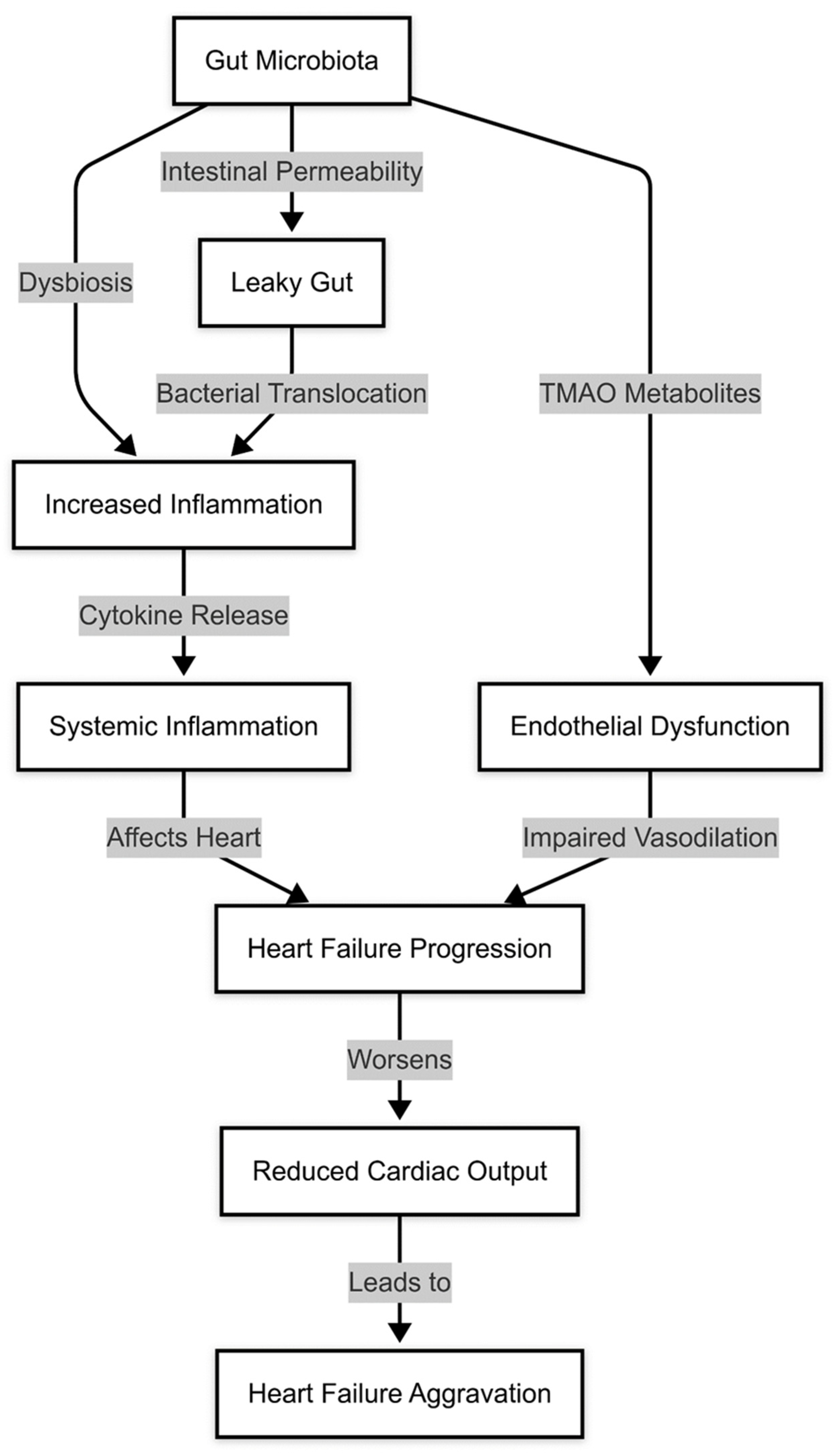
1.4. Gut Microbiota and Endothelial Dysfunction
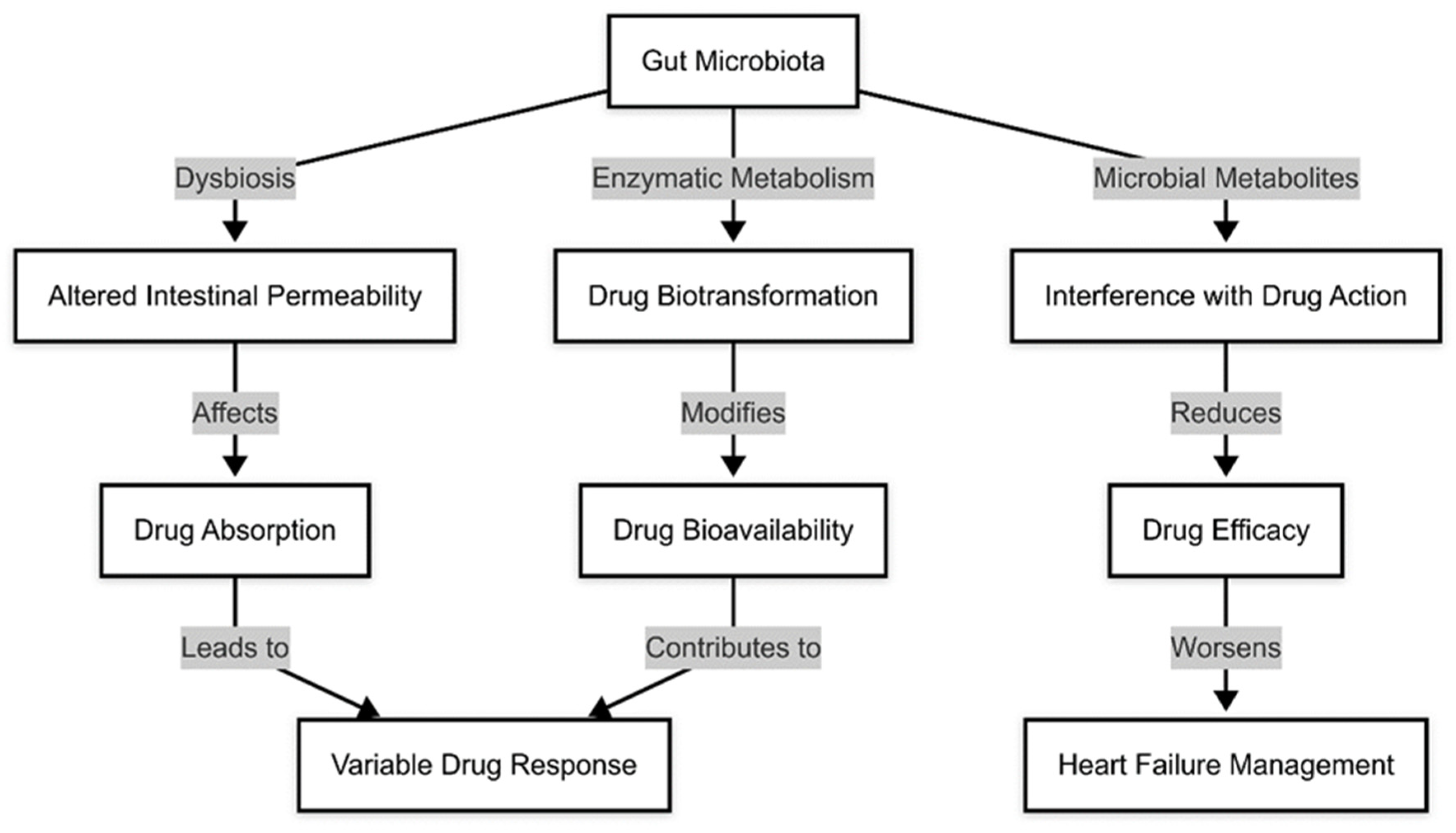
1.5. Gut Microbiota and Pharmacological Treatment in Heart Failure
2. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumari, N.; Kumari, R.; Dua, A.; Singh, M.; Kumar, R.; Singh, P.; Duyar-Ayerdi, S.; Pradeep, S.; Ojesina, A.I.; Kumar, R. From Gut to Hormones: Unraveling the Role of Gut Microbiota in (Phyto)Estrogen Modulation in Health and Disease. Mol. Nutr. Food Res. 2024, 68, e2300688. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Chauhan, N.S. Role of gut-microbiota in disease severity and clinical outcomes. Brief. Funct. Genom. 2024, 23, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Han, T.; Huang, X.; Zhao, Y.; Qian, J.; Zhong, J.; Xie, P.; Liao, L. Exploring the potential causal relationship between gut microbiota and heart failure: A two-sample mendelian randomization study combined with the geo database. Curr. Probl. Cardiol. 2024, 49, 102235. [Google Scholar] [CrossRef] [PubMed]
- Epelde, F. Impact of Exercise on Physiological, Biochemical, and Analytical Parameters in Patients with Heart Failure with Reduced Ejection Fraction. Medicina 2024, 60, 2017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cienkowski, K.; Cienkowska, A.; Kupczynska, K.; Bielecka-Dabrowa, A. The Role of Gut Microbiota and Its Metabolites in Patients with Heart Failure. Biomedicines 2024, 12, 894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petruzziello, C.; Saviano, A.; Manetti, L.L.; Macerola, N.; Ojetti, V. The Role of Gut Microbiota and the Potential Effects of Probiotics in Heart Failure. Medicina 2024, 60, 271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jarmukhanov, Z.; Mukhanbetzhanov, N.; Kozhakhmetov, S.; Nurgaziyev, M.; Sailybayeva, A.; Bekbossynova, M.; Kushugulova, A. The association between the gut microbiota metabolite trimethylamine N-oxide and heart failure. Front. Microbiol. 2024, 15, 1440241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roessler, J.; Zimmermann, F.; Heidecker, B.; Landmesser, U.; Haghikia, A. Gut microbiota-related modulation of immune mechanisms in post-infarction remodelling and heart failure. ESC Heart Fail. 2024, 12, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Marsool, M.D.M.; Goyal, A.; Sulaiman, S.A.; Fatima, L.; Idrees, M.; Sharma, B.; Borra, V.; Gupta, P.; Nadeem, A.; et al. Unveiling the relationship between gut microbiota and heart failure: Recent understandings and insights. Curr. Probl. Cardiol. 2024, 49, 102179. [Google Scholar] [CrossRef] [PubMed]
- Billing, A.M.; Kim, Y.C.; Gullaksen, S.; Schrage, B.; Raabe, J.; Hutzfeldt, A.; Demir, F.; Kovalenko, E.; Lassé, M.; Dugourd, A.; et al. Metabolic Communication by SGLT2 Inhibition. Circulation 2024, 149, 860–884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pitt, B.; Diez, J. Possible Role of Gut Microbiota Alterations in Myocardial Fibrosis and Burden of Heart Failure in Hypertensive Heart Disease. Hypertension 2024, 81, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Liu, Y.B.; Peng, Y.; Peng, J.; Ma, Q.L. Causal effect of air pollution on the risk of cardiovascular and metabolic diseases and potential mediation by gut microbiota. Sci. Total Environ. 2024, 912, 169418. [Google Scholar] [CrossRef] [PubMed]
- Matacchione, G.; Piacenza, F.; Pimpini, L.; Rosati, Y.; Marcozzi, S. The role of the gut microbiota in the onset and progression of heart failure: Insights into epigenetic mechanisms and aging. Clin. Epigenet. 2024, 16, 175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luqman, A.; Hassan, A.; Ullah, M.; Naseem, S.; Ullah, M.; Zhang, L.; Din, A.U.; Ullah, K.; Ahmad, W.; Wang, G. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front. Immunol. 2024, 15, 1321395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yafarova, A.A.; Dementeva, E.V.; Zlobovskaya, O.A.; Sheptulina, A.F.; Lopatukhina, E.V.; Timofeev, Y.S.; Glazunova, E.V.; Lyundup, A.V.; Doludin, Y.V.; Kiselev, A.R.; et al. Gut Microbiota and Metabolic Alterations Associated with Heart Failure and Coronary Artery Disease. Int. J. Mol. Sci. 2024, 25, 11295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Lv, T.; Wang, X.; Wu, M.; Zhang, R.; Yang, X.; Fu, Y.; Liu, Z. Role of the microbiota-gut-heart axis between bile acids and cardiovascular disease. Biomed. Pharmacother. 2024, 174, 116567. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, X.; Jia, Q.; Xu, J.; Shi, J.; Li, X.; Xie, G.; Zhao, X.; He, K. Longitudinal multi-omics analysis uncovers the altered landscape of gut microbiota and plasma metabolome in response to high altitude. Microbiome 2024, 12, 70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahenthiran, A.; Wilcox, J.; Tang, W.H.W. Heart Failure: A Punch from the Gut. Curr. Heart Fail. Rep. 2024, 21, 73–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perticone, M.; Gigliotti, S.; Shehaj, E.; Maio, R.; Suraci, E.; Miceli, S.; Andreozzi, F.; Matera, G.; Perticone, F. Gut Permeability and Immune-Mediated Inflammation in Heart Failure. Biomedicines 2024, 12, 1217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, K.; Taryn, A.; Moon, E.-H.; Peters, B.A.; Solomon, S.D.; Daviglus, M.L.; Kansal, M.M.; Thyagarajan, B.; Gellman, M.D.; Cai, J.; et al. Gut microbiota, blood metabolites, and left ventricular diastolic dysfunction in US Hispanics/Latinos. Microbiome 2024, 12, 85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuzefpolskaya, M.; Bohn, B.; Ladanyi, A.; Pinsino, A.; Braghieri, L.; Carey, M.R.; Clerkin, K.; Sayer, G.T.; Latif, F.; Koji, T.; et al. Alterations in the sarcopenia index are associated with inflammation, gut, and oral microbiota among heart failure, left ventricular assist device, and heart transplant patients. J. Heart Lung Transplant. 2024, 43, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Bratseth, V.; Nendl, A.; Raju, S.C.; Holm, K.; Broch, K.; Hov, J.R.; Seljeflot, I.; Trøseid, M.; Awoyemi, A. Gut dysbiosis and neutrophil extracellular traps in chronic heart failure. Int. J. Cardiol. 2025, 419, 132689. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Bi, Y.; He, X.; Zhang, Y.; Zhao, X. Kidney-tonifying blood-activating decoction delays ventricular remodeling in rats with chronic heart failure by regulating gut microbiota and metabolites and p38 mitogen-activated protein kinase/p65 nuclear factor kappa-B/aquaporin-4 signaling pathway. J. Ethnopharmacol. 2024, 330, 118110. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, Y.; Ding, X.; Lin, S.; Li, X.; Yan, W.; Chen, M. Alteration of the gut microbiome in patients with heart failure: A systematic review and meta-analysis. Microb. Pathog. 2024, 192, 106647. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xiang, S.; Chen, Y.; Liu, Y.; Shen, D.; Yu, X.; Wu, Z.; Sun, Y.; Chen, K.; Luo, J.; et al. Gut microbiota: Implications in pathogenesis and therapy to cardiovascular disease (Review). Exp. Ther. Med. 2024, 28, 427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escobar, C.; Aldeguer, X.; Vivas, D.; Manzano Fernández, S.; Gonzalez Caballero, E.; Garcia Martín, A.; Barrios, V.; Freixa-Pamias, R. The gut microbiota and its role in the development of cardiovascular disease. Expert. Rev. Cardiovasc. Ther. 2025, 23, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Lemaitre, R.N.; Jensen, P.N.; Wang, M.; Wang, Z.; Li, X.S.; Nemet, I.; Lee, Y.; Lai, H.T.; Otto, M.C.d.O.; et al. Trimethylamine N-Oxide and Related Gut Microbe-Derived Metabolites and Incident Heart Failure Development in Community-Based Populations. Circ. Heart Fail. 2024, 17, e011569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, Y.; Wei, H.; Zhou, Z.; Wang, H.; Hang, W.; Wu, J.; Wang, D.W. Gut microbiota-dependent phenylacetylglutamine in cardiovascular disease: Current knowledge and new insights. Front. Med. 2024, 18, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidis, I.; Briasoulis, A.; Tsougos, E. Oral Cardiac Drug-Gut Microbiota Interaction in Chronic Heart Failure Patients: An Emerging Association. Int. J. Mol. Sci. 2024, 25, 1716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shariff, S.; Kwan Su Huey, A.; Parag Soni, N.; Yahia, A.; Hammoud, D.; Nazir, A.; Uwishema, O.; Wojtara, M. Unlocking the gut-heart axis: Exploring the role of gut microbiota in cardiovascular health and disease. Ann. Med. Surg. 2024, 86, 2752–2758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jama, H.A.; Dona, M.S.; Dinakis, E.; Nakai, M.; Paterson, M.R.; Shihata, W.A.; Krstevski, C.; Cohen, C.D.; Weeks, K.L.; Farrugia, G.E.; et al. Maternal Diet and Gut Microbiota Influence Predisposition to Cardiovascular Disease in Offspring. Circ. Res. 2024, 135, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Nemet, I.; Li, X.S.; Wu, Y.; Haghikia, A.; Witkowski, M.; Koeth, R.A.; Demuth, I.; König, M.; Steinhagen-Thiessen, E.; et al. Prognostic value of gut microbe-generated metabolite phenylacetylglutamine in patients with heart failure. Eur. J. Heart Fail. 2024, 26, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Zhao, L.; Song, Y.; Qu, H.; Du, T.; Shi, L.; Cui, Z.; Jiang, Z.; Gao, Z. Trends in gut-heart axis and heart failure research (1993–2023): A bibliometric and visual analysis. Heliyon 2024, 10, e25995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vacca, A.; Schiattarella, G.G. From Gut to Heart: Role of Indole-3-Propionic Acid in HFpEF. Circ. Res. 2024, 134, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, X.; Hu, M.; Li, T.; Jiang, L.; Zhang, Y. Gut Microbiota as Predictive Biomarker for Chronic Heart Failure in Patients with Different Nutritional Risk. J. Cardiovasc. Transl. Res. 2024, 17, 1240–1257. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kaur, G.I.; Kumar, A.; Kanwal, A.; Singh, S.P. The Role of Gut Microbiota and Associated Compounds in Cardiovascular Health and its Therapeutic Implications. Cardiovasc. Hematol. Agents Med. Chem. 2024, 22, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Guivala, S.J.; Bode, K.A.; Okun, J.G.; Kartal, E.; Schwedhelm, E.; Pohl, L.V.; Werner, S.; Erbs, S.; Thiele, H.; Büttner, P. Interactions between the gut microbiome, associated metabolites and the manifestation and progression of heart failure with preserved ejection fraction in ZSF1 rats. Cardiovasc. Diabetol. 2024, 23, 299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Modrego, J.; Ortega-Hernández, A.; Sánchez-González, S.; Corbatón-Anchuelo, A.; Gómez-Garre, D. Analysis of the gut microbiota profile targeted to multiple hypervariable regions of 16S rRNA in a hypertensive heart failure rat model. Methods Cell Biol. 2024, 188, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, N.; Kobayashi, M.; Ito, M.; Matsui, H.; Ohashi, K.; Murohara, T.; Takeda, J.I.; Ueyama, J.; Hirayama, M.; Ohno, K. Soy protein β-conglycinin ameliorates pressure overload-induced heart failure by increasing short-chain fatty acid (SCFA)-producing gut microbiota and intestinal SCFAs. Clin. Nutr. 2024, 43, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.A.; Puccetti, M.; Costantini, C.; Giovagnoli, S.; Ricci, M.; Garaci, E.; Romani, L. Human and gut microbiota synergy in a metabolically active superorganism: A cardiovascular perspective. Front. Cardiovasc. Med. 2024, 11, 1411306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, Z.; Zang, Q.; Chen, J.; Li, Z.; Yang, D.; Wu, C.; Yang, H.; Guo, N. Whole-body mass spectrometry imaging reveals the systemic metabolic disorder and catecholamines biosynthesis alteration on heart-gut axis in heart failure rat. J. Adv. Res. 2024, in press. [CrossRef] [PubMed]
- Huang, Y.J.; Ferrari, M.W.; Lin, S.; Wang, Z.H. Recent advances on the Role of Gut Microbiota in the Development of Heart Failure by Mediating Immune Metabolism. Curr. Probl. Cardiol. 2024, 49, 102128. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ye, J.; Zhao, Z.; Hu, S.; Liang, H.; Ouyang, J.; Hu, Z. Shenfu injection improves isoproterenol-induced heart failure in rats by modulating co-metabolism and regulating the trimethylamine-N-oxide-inflammation axis. Front. Pharmacol. 2024, 15, 1412300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ranjan, P.; Goswami, S.K.; Dutta, R.K.; Colin, K.; Pal, H.C.; Zhang, Q.; Lal, H.; Prasad, R.; Verma, S.K. Hypertrophic heart failure promotes gut dysbiosis and gut leakage in interleukin 10-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H447–H459. [Google Scholar] [CrossRef] [PubMed]
- Castells-Nobau, A.; Moreno-Navarrete, J.M.; de la Vega-Correa, L.; Puig, I.; Federici, M.; Sun, J.; Burcelin, R.; Guzylack-Piriou, L.; Gourdy, P.; Cazals, L.; et al. Multiomics of the intestine-liver-adipose axis in multiple studies unveils a consistent link of the gut microbiota and the antiviral response with systemic glucose metabolism. Gut 2025, 74, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xie, S.; Liu, Y.; Yue, W.; Wang, L.; Liang, Y.; Chen, Y.; Yuan, H.; Yu, J. Gut microbiota profiling reflects the renal dysfunction and psychological distress in patients with diabetic kidney disease. Front. Endocrinol. 2024, 15, 1410295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, X.; Chen, Z.; Gao, C.; Zhang, L.; Liu, Y.; Lin, M.; Zhu, P.; Yang, J.; Wang, Z.; Zhang, J.; et al. 20S-O-Glc-DM treats metabolic syndrome-induced heart failure through regulating gut flora. Eur. J. Pharmacol. 2024, 982, 176946. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.P.; Gogonea, V.; Sweet, W.; Mohan, M.L.; Singh, K.D.; Anderson, J.T.; Mallela, D.; Witherow, C.; Kar, N.; Stenson, K.; et al. Gut microbe-generated phenylacetylglutamine is an endogenous allosteric modulator of β2-adrenergic receptors. Nat. Commun. 2024, 15, 6696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Wit, S.; Geerlings, L.; Shi, C.; Dronkers, J.; Schouten, E.M.; Blancke, G.; Andries, V.; Yntema, T.; Meijers, W.C.; Koonen, D.P.Y.; et al. Heart failure-induced microbial dysbiosis contributes to colonic tumour formation in mice. Cardiovasc. Res. 2024, 120, 612–622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yi, B.; Zhang, P.; Chen, J.; Fang, Z.; Yang, X.; Yang, D.; Zang, Q.; Xu, J.; Ren, T.; Yang, H.; et al. Dengzhanshengmai capsule alleviates heart failure and concomitantly decreases phenylacetylglutamine level, interacting with the intestinal microflora in rats. Microb. Biotechnol. 2024, 17, e14365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, Z.; Fu, L.; Wang, Y.; Tai, S. Impact of gut microbiota on cardiac aging. Arch. Gerontol. Geriatr. 2025, 128, 105639. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Jing, W.; Qian, W.; Fan, L.; Cheng, J. Association between dietary patterns and cardiovascular diseases: A review. Curr. Probl. Cardiol. 2024, 49, 102412. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.C.; Molinaro, A.; Awoyemi, A.; Jørgensen, S.F.; Braadland, P.R.; Nendl, A.; Seljeflot, I.; Ueland, P.M.; McCann, A.; Aukrust, P.; et al. Microbial-derived imidazole propionate links the heart failure-associated microbiome alterations to disease severity. Genome Med. 2024, 16, 27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zagorul’ko, A.A.; Dadashov, M.S.; Kosmina, K.O.; Babanazarova, A.B.; Gubaydulina, E.I.; Rasulov, M.N.; Raevskii, K.P. Intestinal microbiota and chronic heart failure: Association and targets of influence of therapy. Adv. Gerontol. 2024, 37, 373–382. (In Russian) [Google Scholar] [PubMed]
- Usman, I.; Anwar, A.; Shukla, S.; Pathak, P. Mechanistic Review on the Role of Gut Microbiota in the Pathology of Cardiovascular Diseases. Cardiovasc. Hematol. Disord. Drug Targets. 2024, 24, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peña-Ocaña, B.A.; Silva-Flores, M.; Shotaro, T.; García-Gálvez, L.; Hernández-Esquivel, L.; Robledo-Cadena, D.X.; Barrera-Oviedo, D.; Pérez-Torres, I.; Tostado-Islas, O.; Maeda, T.; et al. Transplant of gut microbiota ameliorates metabolic and heart disorders in rats fed with a hypercaloric diet by modulating microbial metabolism and diversity. Biomed. Pharmacother. 2024, 181, 117667. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tavares, L.C.; Lee, H.-C.; Steele, J.R.; Ribeiro, R.V.; Beale, A.L.; Yiallourou, S.; Carrington, M.J.; Kaye, D.M.; Head, G.A.; et al. Faecal metaproteomics analysis reveals a high cardiovascular risk profile across healthy individuals and heart failure patients. Gut Microbes 2025, 17, 2441356. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.W.; Capaldi, E.; Smith, T.; Verma, P.; Varga, J.; Ho, K.J. Trimethylamine N-oxide: A meta-organismal axis linking the gut and fibrosis. Mol. Med. 2024, 30, 128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jing, L.; Zhang, H.; Xiang, Q.; Hu, H.; Zhai, C.; Xu, S.; Tian, H. Role of Trimethylamine N-Oxide in Heart Failure. Rev. Cardiovasc. Med. 2024, 25, 240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, L.; Li, Y.; Chen, M.; Xue, L.; Wang, J.; Ding, Y.; Gu, Q.; Zhang, J.; Zhao, H.; Xie, X.; et al. Therapeutic applications of gut microbes in cardiometabolic diseases: Current state and perspectives. Appl. Microbiol. Biotechnol. 2024, 108, 156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarlak, Z.; Naderi, N.; Amidi, B.; Ghorbanzadeh, V. Sodium Butyrate, a Gut Microbiota Derived Metabolite, in Type 2 Diabetes Mellitus and Cardiovascular Disease: A Review. Cardiovasc. Hematol. Agents Med. Chem. 2024, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.; Zain, Z.; Bokhari, S.F.H.; Waheed, S.; Reza, T.; Eze-Odurukwe, A.; Patel, M.; Almadhoun, M.K.I.K.; Hussain, A.; Reyaz, I. Navigating the Gut-Cardiac Axis: Understanding Cardiovascular Complications in Inflammatory Bowel Disease. Cureus 2024, 16, e55268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, H.; Li, O.; Kim, D.; Xue, M.; Bao, Z.; Yang, F. Aged microbiota exacerbates cardiac failure by PPARα/PGC1α pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167271. [Google Scholar] [CrossRef] [PubMed]
- Semo, D.; Reinecke, H.; Godfrey, R. Gut microbiome regulates inflammation and insulin resistance: A novel therapeutic target to improve insulin sensitivity. Signal Transduct. Target. Ther. 2024, 9, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, L.; Yang, L.; Liu, M. The effect of high-fiber diet based on gut microbiota in patients with chronic heart failure. Physiol. Genom. 2025, 57, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, D.; Reddy, S.T.; Fogelman, A.M. Evidence further linking the intestine to cardiovascular disease. Curr. Opin. Lipidol. 2024, 35, 223–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cruz, C.S.; Huerta, A.R.; Barrientos, J.L.; Rodriguez, C.; Devani, A.; Boosahda, V.; Mareddy, N.S.R.; Silva, G.B.; Miranda, J.C.D.C.; Gochi, K.A.R.; et al. Inflammatory Bowel Disease and Cardiovascular Disease: An Integrative Review With a Focus on the Gut Microbiome. Cureus 2024, 16, e65136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chong-Nguyen, C.; Yilmaz, B.; Coles, B.; Sokol, H.; MacPherson, A.; Siepe, M.; Reineke, D.; Mosbahi, S.; Tomii, D.; Nakase, M.; et al. A scoping review evaluating the current state of gut microbiota and its metabolites in valvular heart disease physiopathology. Eur. J. Clin. Investig. 2025, e14381. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, H.; Xia, H.; Wang, Z.; Li, B.; Xue, H.; Jin, S.; Xiao, L.; Wu, Y.; Guo, Q. Butyrate attenuates sympathetic activation in rats with chronic heart failure by inhibiting microglial inflammation in the paraventricular nucleus. Acta Biochim. Biophys. Sin. 2024, 56, 1823–1832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mousavi Ghahfarrokhi, S.S.; Mohamadzadeh, M.; Samadi, N.; Fazeli, M.R.; Khaki, S.; Khameneh, B.; Khameneh Bagheri, R. Management of Cardiovascular Diseases by Short-Chain Fatty Acid Postbiotics. Curr. Nutr. Rep. 2024, 13, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Asano, R.; Mori, A.; Ishibashi, T.; Motooka, D.; Nakai, M.; Nakaoka, Y. Gut Dysbiosis in Patients With Fontan Circulation. J. Am. Heart Assoc. 2024, 13, e034538. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Valasciuc, E.; Costea, C.F.; Scripcariu, D.V.; Ouatu, A.; Hurjui, L.L.; Tarniceriu, C.C.; Floria, D.E.; Ciocoiu, M.; Baroi, L.G.; et al. Duality of Branched-Chain Amino Acids in Chronic Cardiovascular Disease: Potential Biomarkers versus Active Pathophysiological Promoters. Nutrients 2024, 16, 1972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, W.X.; Wang, T.; Zhang, Y.N.; Chen, Q.; Wang, Y.; Xing, Y.Q.; Zheng, J.; Duan, C.C.; Chen, L.J.; Zhao, H.J.; et al. Molecular Mechanism of Polysaccharides Extracted from Chinese Medicine Targeting Gut Microbiota for Promoting Health. Chin. J. Integr. Med. 2024, 30, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Camargo Tavares, L.; Marques, F.Z. Clinical Trial Evidence of the Gut Microbial Metabolite Butyrate in Hypertension. Hypertension 2024, 81, 2137–2139. [Google Scholar] [CrossRef] [PubMed]
- Gavzy, S.J.; Kensiski, A.; Saxena, V.; Lakhan, R.; Hittle, L.; Wu, L.; Iyyathurai, J.; Dhakal, H.; Lee, Z.L.B.; Li, L.; et al. Early Immunomodulatory Program Triggered by Protolerogenic Bifidobacterium pseudolongum Drives Cardiac Transplant Outcomes. Transplantation 2024, 108, e91–e105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rohmann, N.; Stürmer, P.; Geisler, C.; Schlicht, K.; Knappe, C.; Hartmann, K.; Türk, K.; Hollstein, T.; Beckmann, A.; Seoudy, A.K.; et al. Effects of lifestyle and associated diseases on serum CC16 suggest complex interactions among metabolism, heart and lungs. J. Adv. Res. 2024, 59, 161–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Latif, F.; Mubbashir, A.; Khan, M.S.; Shaikh, Z.; Memon, A.; Alvares, J.; Azhar, A.; Jain, H.; Ahmed, R.; Kanagala, S.G. Trimethylamine N-oxide in cardiovascular disease: Pathophysiology and the potential role of statins. Life Sci. 2025, 361, 123304. [Google Scholar] [CrossRef] [PubMed]
- Muralitharan, R.R.; E Nakai, M.; Snelson, M.; Zheng, T.; Dinakis, E.; Xie, L.; Jama, H.; Paterson, M.; Shihata, W.; Wassef, F.; et al. Influence of angiotensin II on the gut microbiome: Modest effects in comparison to experimental factors. Cardiovasc. Res. 2024, 120, 1155–1163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epelde, F. The Role of the Gut Microbiota in Heart Failure: Pathophysiological Insights and Future Perspectives. Medicina 2025, 61, 720. https://doi.org/10.3390/medicina61040720
Epelde F. The Role of the Gut Microbiota in Heart Failure: Pathophysiological Insights and Future Perspectives. Medicina. 2025; 61(4):720. https://doi.org/10.3390/medicina61040720
Chicago/Turabian StyleEpelde, Francisco. 2025. "The Role of the Gut Microbiota in Heart Failure: Pathophysiological Insights and Future Perspectives" Medicina 61, no. 4: 720. https://doi.org/10.3390/medicina61040720
APA StyleEpelde, F. (2025). The Role of the Gut Microbiota in Heart Failure: Pathophysiological Insights and Future Perspectives. Medicina, 61(4), 720. https://doi.org/10.3390/medicina61040720





