Role of Progesterone Receptor Level in Predicting Axillary Lymph Node Metastasis in Clinical T1-T2N0 Luminal Type Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Data Collection and Inclusion Criteria
2.3. Pathological Evaluation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Axillary dissection |
| ER | Estrogen receptor |
| IDC | Invasive ductal carcinoma |
| IHC | Immunohistochemistry |
| ILC | Invasive lobular carcinoma |
| IRB | Institutional Review Board |
| LN | Lymph node |
| LVI | Lymphovascular invasion |
| PNI | Perineural invasion |
| PR | Progesterone receptor |
| SLN | Sentinel lymph node |
| SLNB | Sentinel lymph node biopsy |
References
- Schröder, L.; Fricker, R.; Stein, R.G.; Rink, T.; Fitz, H.; Blasius, S.; Wöckel, A.; Müller, T. Evaluation of Sentinel Lymph Node Biopsy Prior to Axillary Lymph Node Dissection: The Role of Isolated Tumor Cells/Micrometastases and Multifocality/Multicentricity—A Retrospective Study of 1214 Breast Cancer Patients. Arch. Gynecol. Obstet. 2018, 297, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Tanis, P.J.; Nieweg, O.E.; Valdés Olmos, R.A.; Th Rutgers, E.J.; Kroon, B.B. History of Sentinel Node and Validation of the Technique. Breast Cancer Res. 2001, 3, 109. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, S.; Haji, A.; Battoo, A.; Qurieshi, M.; Mir, W.; Shah, M. Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update. J. Breast Cancer 2017, 20, 217. [Google Scholar] [CrossRef]
- Cserni, G.; Bianchi, S.; Vezzosi, V.; Arisio, R.; Bori, R.; Peterse, J.L.; Sapino, A.; Drijkoningen, M.; Kulka, J.; Eusebi, V.; et al. Sentinel Lymph Node Biopsy and Non-Sentinel Node Involvement in Special Type Breast Carcinomas with a Good Prognosis. Eur. J. Cancer 2007, 43, 1407–1414. [Google Scholar] [CrossRef]
- Pinder, S.E.; Ellis, I.O.; Galea, M.; O’Rouke, S.; Blamey, R.W.; Elston, C.W. Pathological Prognostic Factors in Breast Cancer. III. Vascular Invasion: Relationship with Recurrence and Survival in a Large Study with Long-Term Follow-Up. Histopathology 1994, 24, 41–47. [Google Scholar] [CrossRef]
- Viale, G.; Zurrida, S.; Maiorano, E.; Mazzarol, G.; Pruneri, G.; Paganelli, G.; Maisonneuve, P.; Veronesi, U. Predicting the Status of Axillary Sentinel Lymph Nodes in 4351 Patients with Invasive Breast Carcinoma Treated in a Single Institution. Cancer 2005, 103, 492–500. [Google Scholar] [CrossRef]
- Yoshihara, E.; Smeets, A.; Laenen, A.; Reynders, A.; Soens, J.; Van Ongeval, C.; Moerman, P.; Paridaens, R.; Wildiers, H.; Neven, P.; et al. Predictors of Axillary Lymph Node Metastases in Early Breast Cancer and Their Applicability in Clinical Practice. Breast 2013, 22, 357–361. [Google Scholar] [CrossRef]
- Alsumai, T.S.; Alhazzaa, N.; Alshamrani, A.; Assiri, S.; Alhefdhi, A. Factors Predicting Positive Sentinel Lymph Node Biopsy in Clinically Node-Negative Breast Cancer. Breast Cancer Targets Ther. 2022, 14, 323–334. [Google Scholar] [CrossRef]
- Paik, S.; Bryant, J.; Park, C.; Fisher, B.; Tan-Chiu, E.; Hyams, D.; Fisher, E.R.; Lippman, M.E.; Wickerham, D.L.; Wolmark, N. ErbB-2 and Response to Doxorubicin in Patients with Axillary Lymph Node-Positive, Hormone Receptor- Negative Breast Cancer. JNCI J. Natl. Cancer Inst. 1998, 90, 1361–1370. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/ Neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.-J. Strategies for Subtypes—Dealing with the Diversity of Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.M.; Osborne, C.K.; McGuire, W.L. Correlations between Estrogen Receptor, Progesterone Receptor, and Patient Characteristics in Human Breast Cancer. J. Clin. Oncol. 1984, 2, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.M.; McGuire, W.L. Steroid Receptors and Other Prognostic Factors in Primary Breast Cancer. Semin. Oncol. 1988, 15, 20–25. [Google Scholar] [PubMed]
- Arpino, G.; Weiss, H.; Lee, A.V.; Schiff, R.; De Placido, S.; Osborne, C.K.; Elledge, R.M. Estrogen Receptor–Positive, Progesterone Receptor–Negative Breast Cancer: Association with Growth Factor Receptor Expression and Tamoxifen Resistance. JNCI J. Natl. Cancer Inst. 2005, 97, 1254–1261. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Yin, X.; Zhang, X.; Huang, J.; Wu, Y.; Wang, M.; Yi, Z.; Li, H.; Li, H.; et al. Clinicopathological Characteristics and Breast Cancer–Specific Survival of Patients with Single Hormone Receptor–Positive Breast Cancer. JAMA Netw. Open 2020, 3, e1918160. [Google Scholar] [CrossRef]
- Li, Z.; Wei, H.; Li, S.; Wu, P.; Mao, X. The Role of Progesterone Receptors in Breast Cancer. Drug Des. Dev. Ther. 2022, 16, 305–314. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Buzdar, A.U. Current Status of Adjuvant Systemic Therapy for Primary Breast Cancer: Progress and Controversy. CA Cancer J. Clin. 1995, 45, 199–226. [Google Scholar] [CrossRef]
- Gajdos, C.; Tartter, P.I.; Bleiweiss, I.J. Lymphatic Invasion, Tumor Size, and Age Are Independent Predictors of Axillary Lymph Node Metastases in Women with T1 Breast Cancers. Ann. Surg. 1999, 230, 692. [Google Scholar] [CrossRef]
- Kuhn, E.; Gambini, D.; Despini, L.; Asnaghi, D.; Runza, L.; Ferrero, S. Updates on Lymphovascular Invasion in Breast Cancer. Biomedicines 2023, 11, 968. [Google Scholar] [CrossRef]
- Zhang, D.; Svensson, M.; Edén, P.; Dihge, L. Identification of Sentinel Lymph Node Macrometastasis in Breast Cancer by Deep Learning Based on Clinicopathological Characteristics. Sci. Rep. 2024, 14, 26970. [Google Scholar] [CrossRef]
- Shahriarirad, R.; Meshkati Yazd, S.M.; Fathian, R.; Fallahi, M.; Ghadiani, Z.; Nafissi, N. Prediction of Sentinel Lymph Node Metastasis in Breast Cancer Patients Based on Preoperative Features: A Deep Machine Learning Approach. Sci. Rep. 2024, 14, 1351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-X.; Liu, Y.-R.; Xie, S.; Jiang, Y.-Z.; Shao, Z.-M. A Nomogram Predicting Lymph Node Metastasis in T1 Breast Cancer Based on the Surveillance, Epidemiology, and End Results Program. J. Cancer 2019, 10, 2443–2449. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, L.; Chen, Y.; Mao, L.; Xie, H.; Wang, S.; Guan, X. Development and Validation of a Nomogram for Prediction of Lymph Node Metastasis in Early-Stage Breast Cancer. Gland Surg. 2021, 10, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, Q.; Wang, J.; Yang, X. A Nomogram Model for Predicting the Risk of Axillary Lymph Node Metastasis in Patients with Early Breast Cancer and CN0 Status. Oncol. Lett. 2024, 28, 345. [Google Scholar] [CrossRef]
- Bevilacqua, J.L.B.; Kattan, M.W.; Fey, J.V.; Cody, H.S.; Borgen, P.I.; Van Zee, K.J. Doctor, What Are My Chances of Having a Positive Sentinel Node? A Validated Nomogram for Risk Estimation. J. Clin. Oncol. 2007, 25, 3670–3679. [Google Scholar] [CrossRef]
- Veerapong, J.; Boughey, J.; Mittendorf, E.; Harrel, R.; Bassett, R.; Ross, M. A Validated Risk Assessment of Sentinel Lymph Node Involvement in Breast Cancer Patients. Ann. Surg. Oncol. 2011, 18 (Suppl. S1), S1–S144. [Google Scholar]
- CAP-College of American Pathologists. Protocol for the Examination of Resection Specimens from Patients with Invasive Carcinoma of the Breast, Version: 4.10.0.0; CAP-College of American Pathologists: Northfield, IL, USA, 2024. [Google Scholar]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Rakha, E.A.; El-Sayed, M.E.; Green, A.R.; Paish, E.C.; Powe, D.G.; Gee, J.; Nicholson, R.I.; Lee, A.H.S.; Robertson, J.F.R.; Ellis, I.O. Biologic and Clinical Characteristics of Breast Cancer with Single Hormone Receptor–Positive Phenotype. J. Clin. Oncol. 2007, 25, 4772–4778. [Google Scholar] [CrossRef]
- Cui, X.; Schiff, R.; Arpino, G.; Osborne, C.K.; Lee, A.V. Biology of Progesterone Receptor Loss in Breast Cancer and Its Implications for Endocrine Therapy. J. Clin. Oncol. 2005, 23, 7721–7735. [Google Scholar] [CrossRef]
- Osborne, C.K.; Schiff, R.; Arpino, G.; Lee, A.S.; Hilsenbeck, V.G. Endocrine Responsiveness: Understanding How Progesterone Receptor Can Be Used to Select Endocrine Therapy. Breast 2005, 14, 458–465. [Google Scholar] [CrossRef]
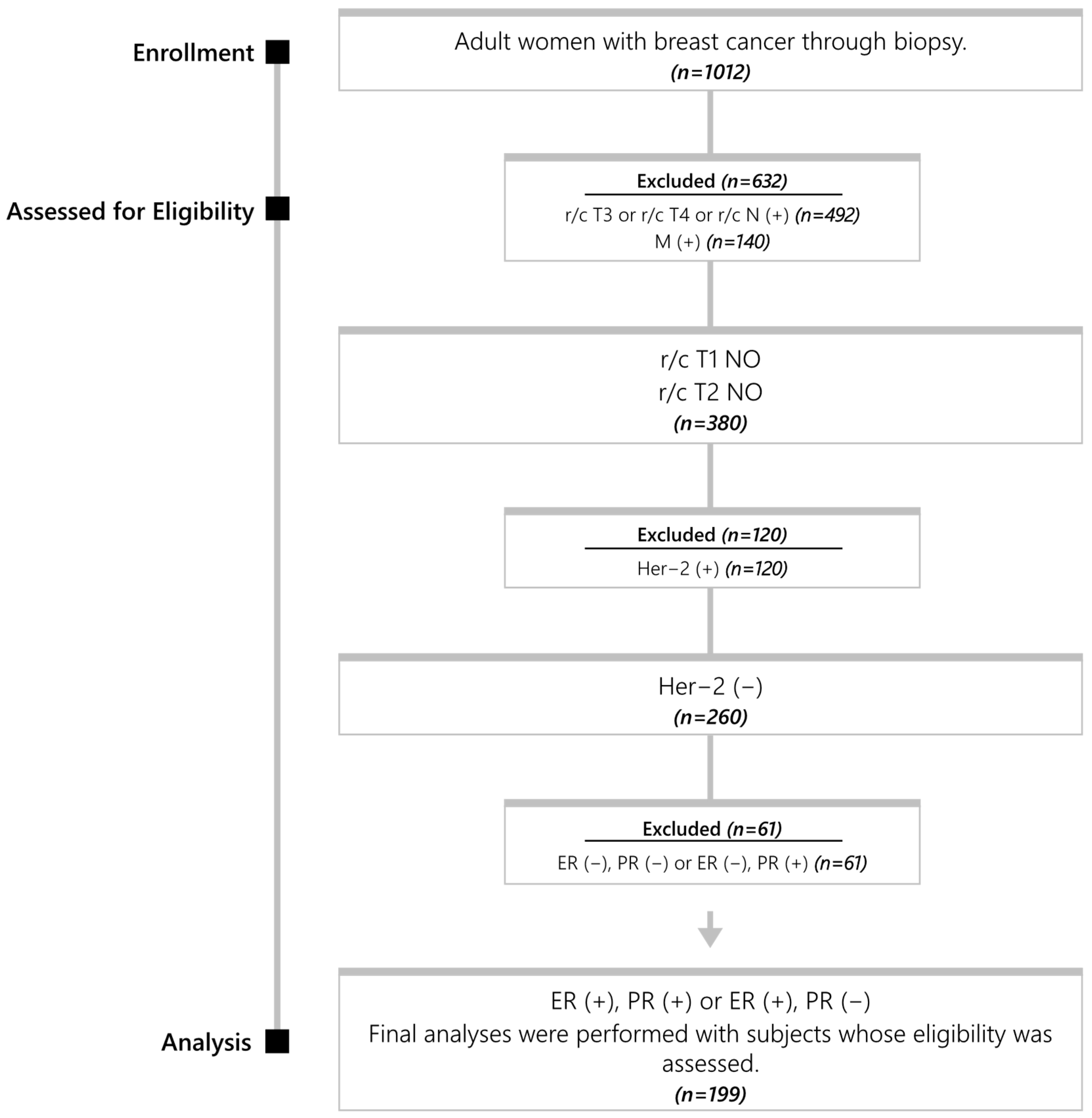
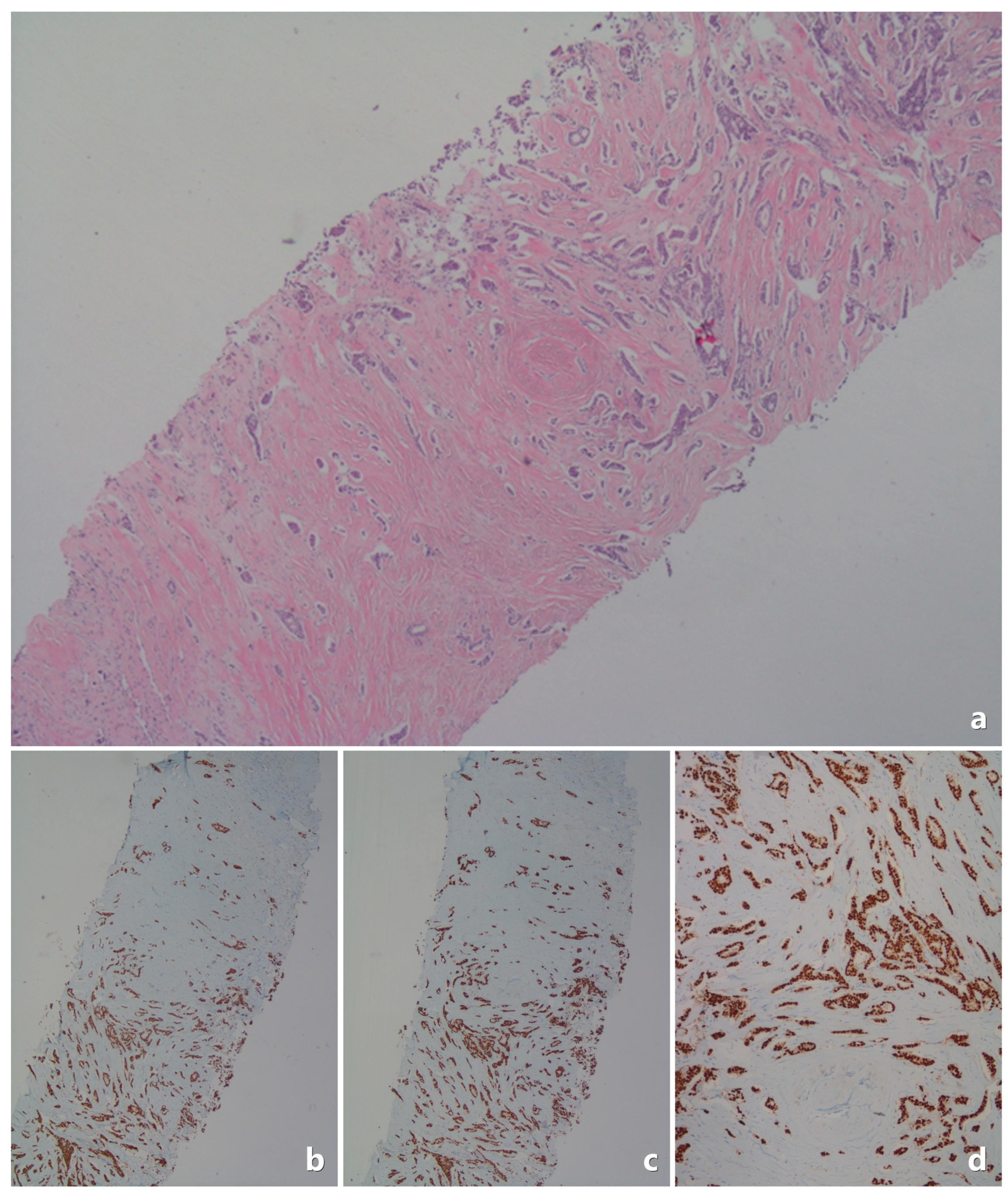
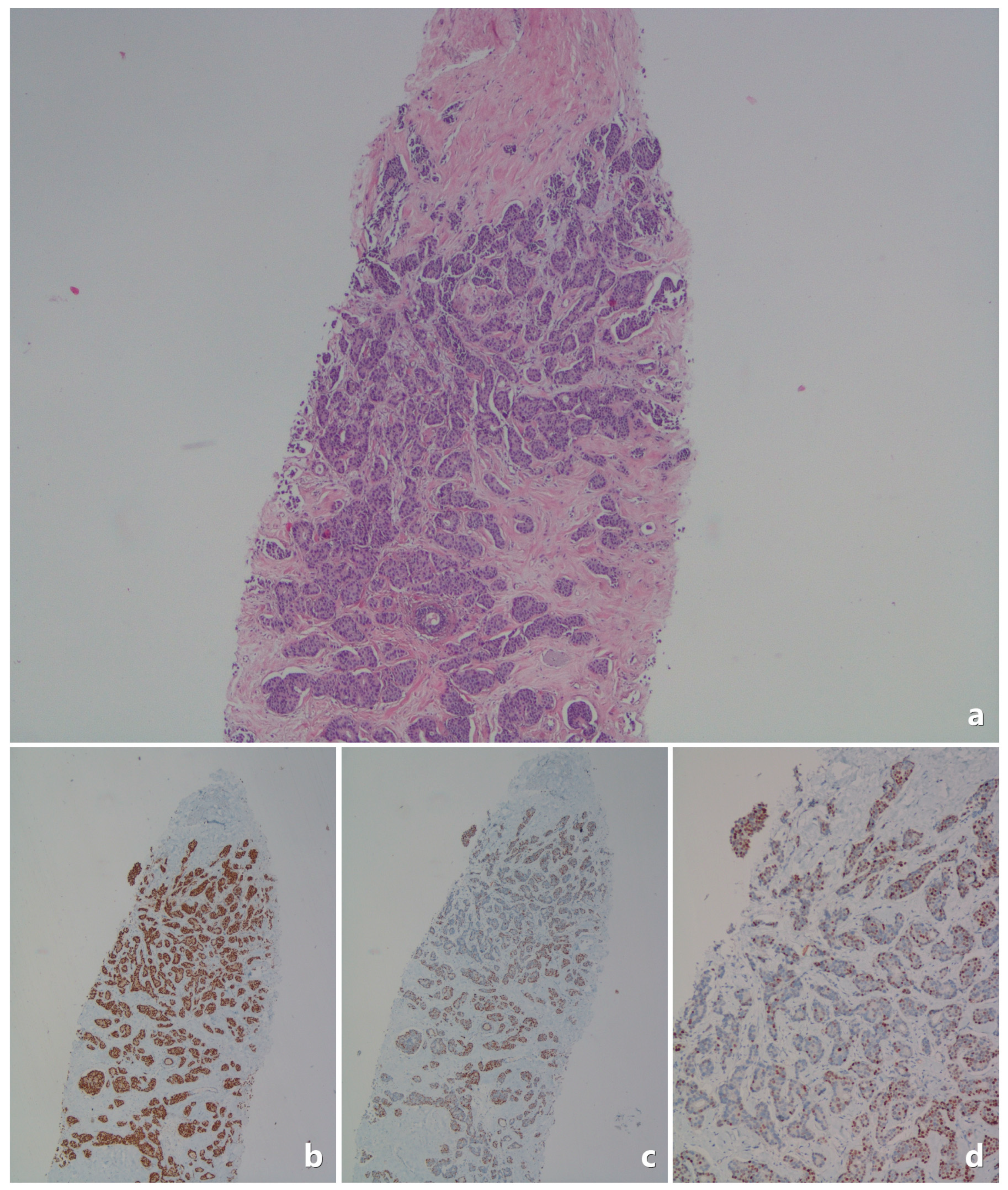
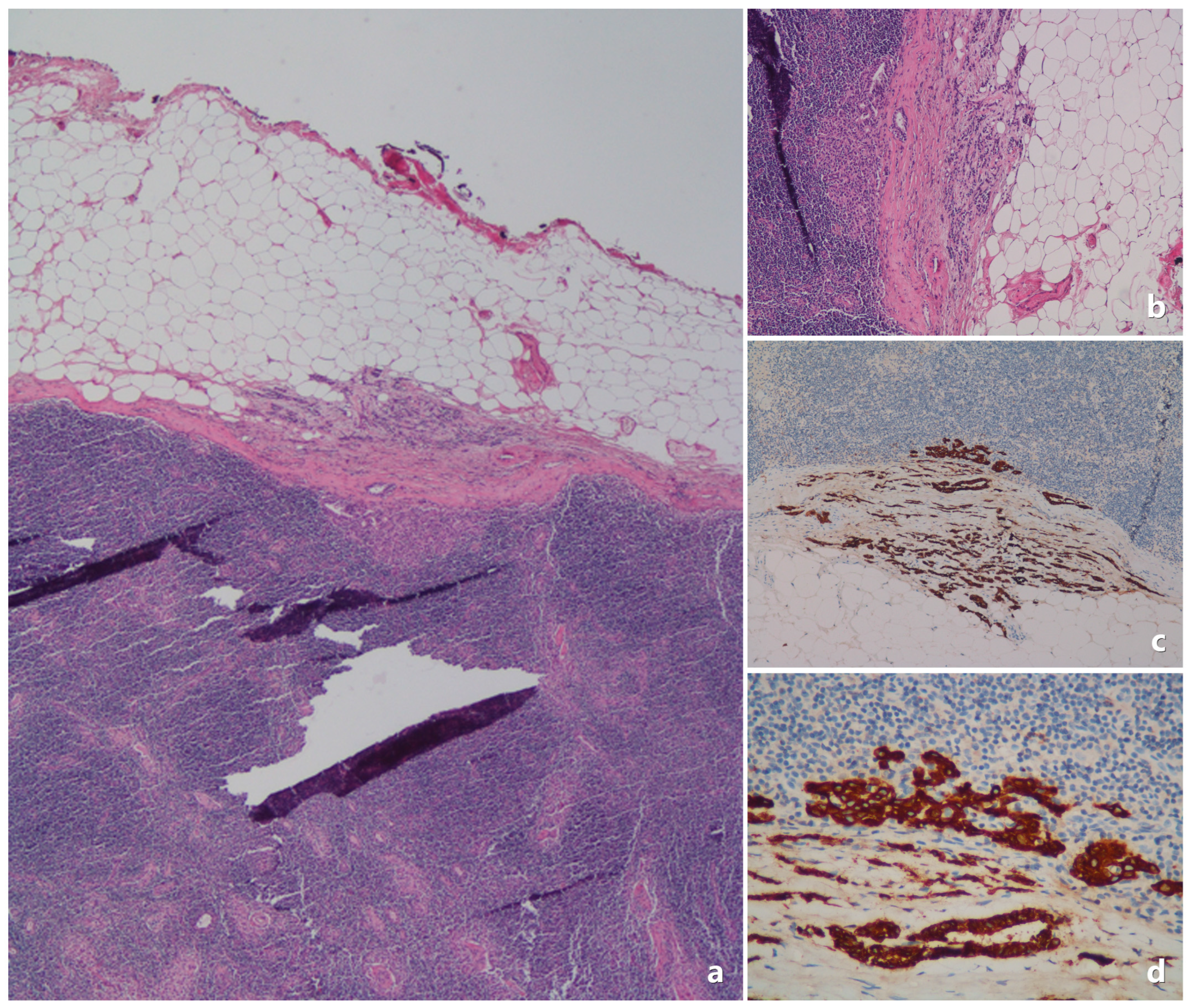
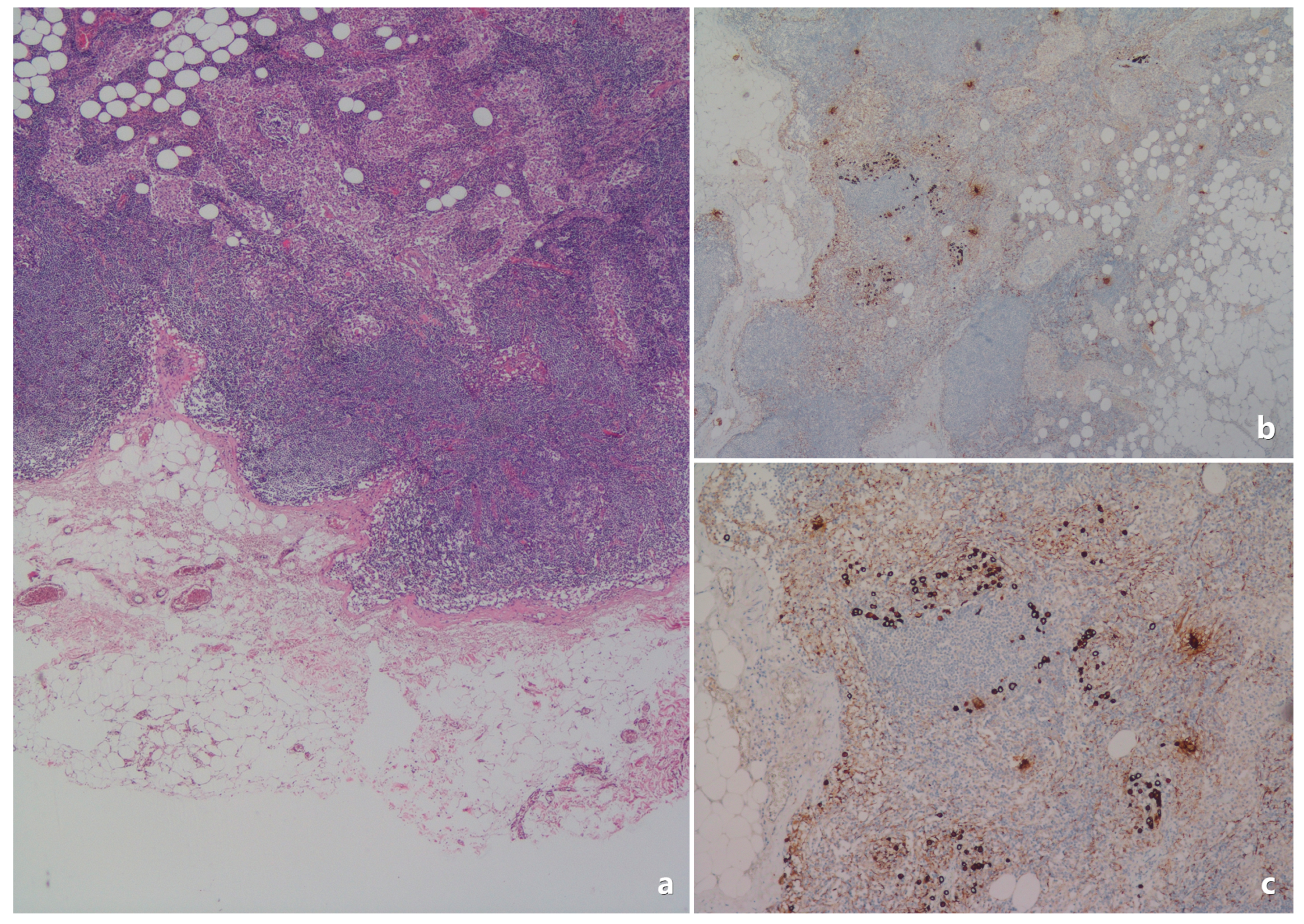
| Category | Feature | Value |
|---|---|---|
| Patient Demographics | Age (Median) | 56 years [35–86 years] |
| Age Group | ≤40 years: 9 (4.5%) >40 years: 190 (95.5%) | |
| Menopausal Status | Premenopausal: 80 (40.2%) Postmenopausal: 119 (59.8%) | |
| Tumor Characteristics | Tumor Location | Right-sided: 96 (48.2%) Left-sided: 103 (51.8%) |
| Number of Tumors | Unifocal: 185 (93%) Multifocal: 11 (5.5%) Multicentric: 3 (1.5%) | |
| Histological Type | IDC: 179 (89.9%) ILC: 5 (2.5%) Mixed type: 2 (1%) Others: 13 (6.5%) | |
| Tumor Grade | Grade I: 25 (12.6%) Grade II: 114 (57.3%) Grade III: 10 (5%) Unknown: 50 (25.1%) | |
| Lymphovascular Invasion | Present: 59 (29.6%) Absent: 140 (70.4%) | |
| Perineural Invasion | Present: 56 (28.1%) Absent: 143 (71.9%) | |
| Extracapsular Invasion | Present: 25 (12.6%) Absent: 174 (87.4%) | |
| Tumor Size (median) | 20 mm (3–50) | |
| T1 Tumor | 101 (50.8%) T1a: 9 (4.6%), T1b: 12 (6%) T1c: 80 (40.2%) | |
| T2 Tumor | 98 (49.2%) | |
| Pathological Nodal Status | Nodal Status | N0: 140 (70.4%) N1: 45 (22.6%) N2: 9 (4.5%) N3: 5 (2.5%) |
| Surgical Intervention | Type of Surgery | Mastectomy: 16 (8%) Breast-conserving surgery: 183 (92%) |
| Axillary Surgery | SLNB: 146 (73.4%) AD: 53 (26.6%) 37 SLNB + complementary AD, 16 due to undetectable SLN | |
| Lymph Node Status | LN Metastasis in SLNB | Positive: 52 (28.4%) Negative: 131 (71.6%) |
| LN Metastasis in AD | Positive: 26 (49%) Negative: 27 (51%) | |
| LN total | Positive: 59 (29.6%) Negative: 140 (70.4%) | |
| Molecular and Hormonal Status | Ki-67 Index | <14%: 75 (37.7%) ≥14%: 112 (56.3%) Unknown: 12 (6%) |
| Molecular Subtype | Luminal A: 66 (33.2%) Luminal B: 121 (60.8%) Unknown: 12 (6%) | |
| Immunohistochemistry | ER+/PR+: 185 (93%) ER+/PR−: 14 (7%) | |
| Progesterone Receptor | <10%: 21 (10.6%) ≥10%: 178 (89.4%) <20%: 29 (14.6%) ≥20%: 170 (85.4%) <50%: 46 (23.1%) ≥50%: 153 (76.9%) <80%: 71 (35.7%) ≥80%: 128 (64.3%) |
| Variable | Lymph Node-Negative n (%) | Lymph Node-Positive n (%) | Total N | Chi-Square Value (df) | p-Value |
|---|---|---|---|---|---|
| Age Group | 0.249 (1) | 0.618 | |||
| ≤40 years | 7 (77.8) | 2 (22.2) | 9 | ||
| >40 years | 133 (70.0) | 57 (30.0) | 190 | ||
| Postmenopausal | 0.522 (1) | 0.470 | |||
| Yes | 86 (72.3) | 33 (27.7) | 119 | ||
| No | 54 (67.5) | 26 (32.5) | 80 | ||
| Localization | 0.206 (1) | 0.650 | |||
| Right | 69 (71.9) | 27 (28.1) | 96 | ||
| Left | 71 (68.9) | 32 (31.1) | 103 | ||
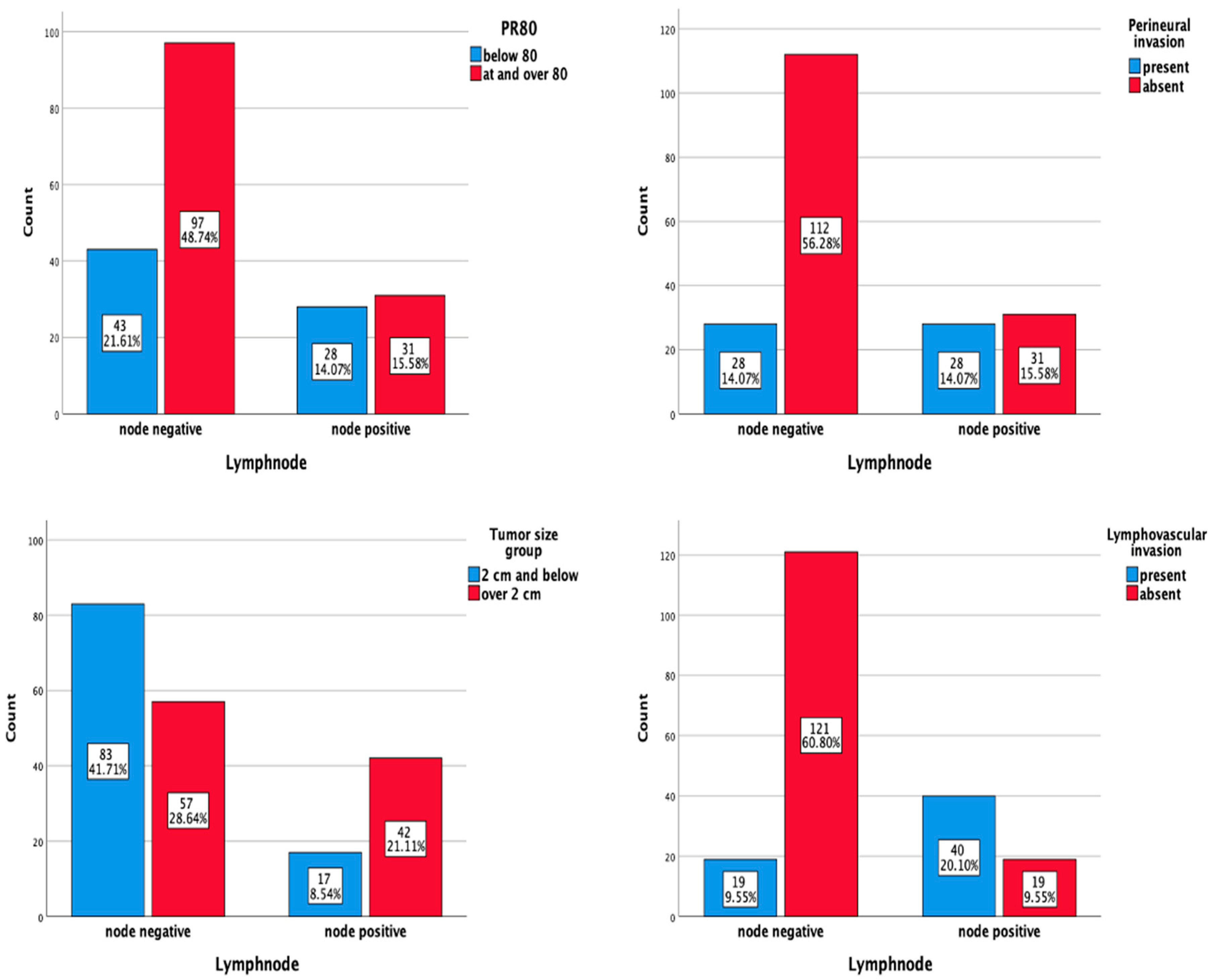
| Tumor size group | 15.417 (1) | <0.001 | |||
| ≤2 cm | 83 (83.0) | 17 (17.0) | 100 | ||
| >2 cm | 57 (57.6) | 42 (42.4) | 99 | ||
| Pathological T | 19.630 (4) | <0.001 | |||
| T1a | 7 (77.8) | 2 (22.2) | 9 | ||
| T1b | 12 (100.0) | 0 (0.0) | 12 | ||
| T1c | 65 (81.3) | 15 (18.8) | 80 | ||
| T2 | 56 (57.7) | 41 (42.3) | 97 | ||
| T3 | 0 (0.0) | 1 (100.0) | 1 | ||
| Number of Foci | 5.624 (2) | 0.060 | |||
| Unifocal | 134 (72.4) | 51 (27.6) | 185 | ||
| Multifocal | 5 (45.5) | 6 (54.5) | 11 | ||
| Multicentric | 1 (33.3) | 2 (66.7) | 3 | ||
| Histological Type | 8.8189 (1) | 0.42 | |||
| IDC | 126 (70.4) | 53 (29.6) | 179 | ||
| ILC | 1 (20) | 4 (80) | 5 | ||
| Others | 11 (84.6) | 2 (15.4) | 13 | ||
| Mixed | 2 (100) | 2 | |||
| Lymphovascular Invasion | 58.513 (1) | <0.001 | |||
| Yes | 19 (32.2) | 40 (67.8) | 59 | ||
| No | 121 (86.4) | 19 (13.6) | 140 | ||
| Grade | 2.852 | 0.415 | |||
| 1 | 21 (84.0) | 4 (16.0) | 25 | ||
| 2 | 78 (69.0) | 35 (31.0) | 113 | ||
| 3 | 6 (60.0) | 4 (40.0) | 10 | ||
| Unknown | 35 (70.0) | 15 (30.0) | 50 | ||
| Ki67 Level | 1.270 (1) | 0.260 | |||
| <14% | 56 (74.7) | 19 (25.3) | 75 | ||
| ≥14% | 75 (67.0) | 37 (33.0) | 112 | ||
| PR Level (10) | 0.803 (1) | 0.370 | |||
| <10% | 13 (61.9) | 8 (38.1) | 21 | ||
| ≥10% | 127 (71.3) | 51 (28.7) | 178 | ||
| PR Level (20) | 0.380 (1) | 0.537 | |||
| <20% | 19 (65.5) | 10 (34.5) | 29 | ||
| ≥20% | 121 (71.2) | 49 (28.8) | 170 | ||
| PR Level (50) | 1.532 (1) | 0.216 | |||
| <50% | 29 (63) | 17 (37) | 46 | ||
| ≥50% | 111(72.5) | 42 (27.5) | 153 | ||
| PR Level (80) | 5.070 (1) | 0.024 | |||
| <80% | 43 (60.6) | 28 (39.4) | 71 | ||
| ≥80% | 97 (75.8) | 31 (24.2) | 128 | ||
| IHC | 0.266 (1) | 0.606 | |||
| ER+PR+ | 131 (70.8) | 54 (29.2) | 185 | ||
| ER+PR− | 9 (64.3) | 5 (35.7) | 14 | ||
| Perineural invasion | 15.475 (1) | <0.001 | |||
| Present | 28 (50.0) | 28 (50.0) | 56 | ||
| Absent | 112 (78.3) | 31 (21.7) | 143 |
| Variable | Beta (B) | Std. Error (S.E.) | Wald | df | Sig. (p-Value) | Odds Ratio (Exp(B)) | 95%CI | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Age Group | 0.405 | 0.817 | 0.246 | 1 | 0.620 | 1.500 | 0.302 | 7.443 |
| Postmenopausal Status | −0.227 | 0.315 | 0.521 | 1 | 0.471 | 1.254 | 0.677 | 2.325 |
| Tumor Size Group | −1.280 | 0.335 | 14.605 | 1 | <0.001 | 3.597 | 1.865 | 6.944 |
| Lymphovascular Invasion | −2.596 | 0.372 | 48.641 | 1 | <0.001 | 13.333 | 6.451 | 27.777 |
| Ki67 Level | −0.374 | 0.333 | 1.264 | 1 | 0.261 | 1.453 | 0.757 | 2.703 |
| PR10 | 0.427 | 0.479 | 0.794 | 1 | 0.373 | 1.531 | 0.599 | 3.921 |
| PR20 | −0.265 | 0.426 | 0.379 | 1 | 0.538 | 1.300 | 0.564 | 2.994 |
| PR50 | −0.438 | 0.355 | 1.520 | 1 | 0.218 | 1.550 | 0.772 | 3.105 |
| PR80 | −0.712 | 0.319 | 4.989 | 1 | 0.026 | 2.036 | 1.090 | 3.802 |
| Perineural Invasion | −1.285 | 0.336 | 14.651 | 1 | <0.001 | 3.610 | 1.872 | 6.993 |
| IHC | −0.298 | 0.581 | 0.264 | 1 | 0.607 | 1.347 | 0.431 | 4.201 |
| Variable | Beta (B) | Std. Error (S.E.) | Wald | df | Sig. (p-Value) | Odds Ratio (Exp(B)) | 95%CI | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Tumor Size Group | −1.173 | 0.399 | 8.646 | 1 | 0.003 | 3.236 | 1.479 | 7.042 |
| LVI | −2.412 | 0.405 | 35.470 | 1 | <0.001 | 1.111 | 2.439 | 5.050 |
| PNI | −0.695 | 0.417 | 2.777 | 1 | 0.096 | 2.004 | 0.884 | 4.545 |
| PR80 | −0.837 | 0.402 | 4.344 | 1 | 0.037 | 2.309 | 1.051 | 5.076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdogan, M.; Kelten Talu, C.; Guzeloz, Z.; Demir, G.; Eyiler, F.; Akay, S.; Yilmaz, E.; Unal, O.U. Role of Progesterone Receptor Level in Predicting Axillary Lymph Node Metastasis in Clinical T1-T2N0 Luminal Type Breast Cancer. Medicina 2025, 61, 710. https://doi.org/10.3390/medicina61040710
Erdogan M, Kelten Talu C, Guzeloz Z, Demir G, Eyiler F, Akay S, Yilmaz E, Unal OU. Role of Progesterone Receptor Level in Predicting Axillary Lymph Node Metastasis in Clinical T1-T2N0 Luminal Type Breast Cancer. Medicina. 2025; 61(4):710. https://doi.org/10.3390/medicina61040710
Chicago/Turabian StyleErdogan, Mihriban, Canan Kelten Talu, Zeliha Guzeloz, Gonul Demir, Ferhat Eyiler, Seval Akay, Ezgi Yilmaz, and Olcun Umit Unal. 2025. "Role of Progesterone Receptor Level in Predicting Axillary Lymph Node Metastasis in Clinical T1-T2N0 Luminal Type Breast Cancer" Medicina 61, no. 4: 710. https://doi.org/10.3390/medicina61040710
APA StyleErdogan, M., Kelten Talu, C., Guzeloz, Z., Demir, G., Eyiler, F., Akay, S., Yilmaz, E., & Unal, O. U. (2025). Role of Progesterone Receptor Level in Predicting Axillary Lymph Node Metastasis in Clinical T1-T2N0 Luminal Type Breast Cancer. Medicina, 61(4), 710. https://doi.org/10.3390/medicina61040710






