Effects of Telehealth-Supervised Respiratory Exercise Training on Respiratory Function, Fatigue, Quality of Life, and Functional Capacity of Patients with Multiple Sclerosis
Abstract
1. Introduction
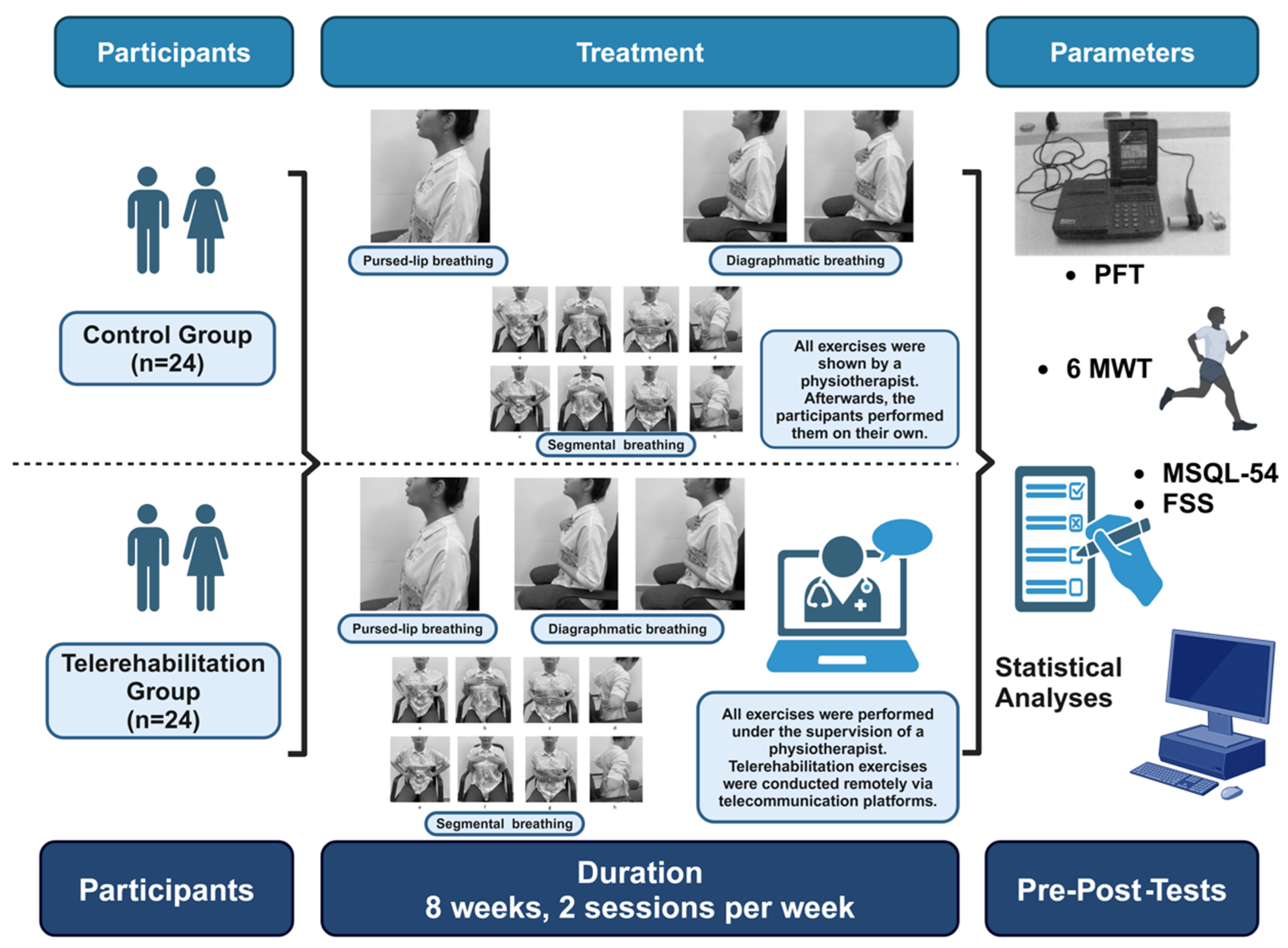
2. Materials and Methods
2.1. Study Design and Participants
2.2. Intervention
2.3. Outcome Measures
2.4. Respiratory Exercise Program
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
3.2. Pretreatment Functional and Pulmonary Assessments
3.3. Pulmonary Function Test Outcomes Posttreatment
3.4. Functional and Quality-of-Life Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- White, L. Prevalence and Severity of MS Across the World: Can New Research Explain the Patterns? MS International Federation [Internet]. [Updated 23 September 2022]. 2022. Available online: https://www.msif.org/news/2022/09/01/prevalence-and-severity-of-ms-across-the-world-can-new-research-explain-the-patterns/ (accessed on 21 April 2024).
- Voskuhl, R.R.; Sawalha, A.H.; Itoh, Y. Sex chromosome contributions to sex differences in multiple sclerosis susceptibility and progression. Mult. Scler. J. 2018, 24, 22–31. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Motl, R.; Pilutti, L.A. The benefits of exercise training in multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Enrichi, C.; Regazzetti, M.; Cieślik, B.; Zanetti, C.; D’Imperio, D.; Compagno, E.; Cacciante, L.; Federico, S.; Pregnolato, G.; Zitti, M.; et al. How Lung Volume Recruitment Maneuvers Enhance Respiratory Function in Multiple Sclerosis Patients: A Quasi-Randomized Pilot Study. Medicina 2023, 59, 1896. [Google Scholar] [CrossRef] [PubMed]
- Mutluay, F.K.; Gürses, H.N.; Saip, S. Effects of multiple sclerosis on respiratory functions. Clin. Rehabil. 2005, 19, 426–432. [Google Scholar] [CrossRef]
- Sladeckova, M.; Kocica, J.; Vlckova, E.; Dosbaba, F.; Pepera, G.; Su, J.J.; Batalik, L. Exercise-based telerehabilitation for patients with multiple sclerosis using physical activity: A systematic review. J. Rehabil. Med. 2024, 56, jrm40641. [Google Scholar] [CrossRef]
- Sandroff, B.M.; Dulugonski, D.; Weikert, Y.; Suh, Y.; Balantrapu, R.; Moti, R.W. Physical activity and multiple sclerosis: New insights regarding inactivity. Acta Neurol. Scand. 2012, 126, 256–262. [Google Scholar] [CrossRef]
- Torres-Álamo, L.; López-Liria, R.; Valverde-Martínez, M.Á.; Benzo-Iglesias, M.J.; Rubio-Arias, J.Á. Effectiveness of Respiratory Exercises on Perceived Symptoms of Fatigue among Multiple Sclerosis Patients: A Systematic Review. Sustainability 2023, 15, 12887. [Google Scholar] [CrossRef]
- Laver, K.E.; Adey-Wakeling, Z.; Crotty, M.; Lannin, N.A.; George, S.; Sherrington, C. Telerehabilitation services for stroke. Cochrane Database Syst. Rev. 2020, 1, CD010255. [Google Scholar] [CrossRef]
- Antoniou, V.; Davos, C.H.; Kapreli, E.; Batalik, L.; Panagiotakos, D.B.; Pepera, G. Effectiveness of home-based cardiac rehabilitation, using wearable sensors, as a multicomponent, cutting-edge intervention: A systematic review and meta-analysis. J. Clin. Med. 2022, 11, 3772. [Google Scholar] [CrossRef] [PubMed]
- Cotelli, M.; Manenti, R.; Brambilla, M.; Gobbi, E.; Ferrari, C.; Binetti, G.; Cappa, S.F. Cognitive telerehabilitation in mild cognitive impairment, Alzheimer’s disease, and frontotemporal dementia: A systematic review. J. Telemed. Telecare 2019, 25, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Amatya, B.; Galea, M.P.; Kesselring, J.; Khan, F. Effectiveness of telerehabilitation interventions in persons with multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2015, 4, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Dodakian, L.; Le, V.; See, J.; Augsburger, R.; McKenzie, A.; Zhou, R.J.; Chiu, N.L.; Heckhausen, J.; Cassidy, J.M.; et al. National Institutes of Health StrokeNet Telerehab Investigators. Efficacy of home-based telerehabilitation vs. in clinic therapy for adults after stroke: A randomized clinical trial. JAMA Neurol. 2019, 76, 1079–1087. [Google Scholar] [CrossRef]
- Chan, C.; Yamabayashi, C.; Syed, N.; Kirkham, A.; Camp, P.G. Exercise telemonitoring and telerehabilitation compared with traditional cardiac and pulmonary rehabilitation: A systematic review and meta-analysis. Physiother. Can. 2016, 68, 242–251. [Google Scholar] [CrossRef]
- Yeroushalmi, S.; Maloni, H.; Costello, K.; Wallin, M.T. Telemedicine and multiple sclerosis: A comprehensive literature review. J. Telemed. Telecare 2020, 26, 400–413. [Google Scholar] [CrossRef]
- Golbus, J.R.; Lopez-Jimenez, F.; Barac, A.; Cornwell, W.K., 3rd; Dunn, P.; Forman, D.E.; Martin, S.S.; Schorr, E.N.; Supervia, M.; Exercise, Cardiac Rehabilitation and Secondary Prevention Committee of the Council on Clinical Cardiology; et al. Digital technologies in cardiac rehabilitation: A science advisory from the American Heart Association. Circulation 2023, 148, 95–107. [Google Scholar] [CrossRef]
- Pepera, G.; Antoniou, V.; Su, J.J.; Lin, R.; Batalik, L. Comprehensive and personalized approach is a critical area for developing remote cardiac rehabilitation programs. World J. Clin. Cases 2024, 12, 2009–2015. [Google Scholar] [CrossRef]
- Calvache-Mateo, A.; Heredia-Ciuró, A.; Martín-Núñez, J.; Hernández-Hernández, S.; Reychler, G.; López-López, L.; Valenza, M.C. Efficacy and safety of respiratory telerehabilitation in patients with Long COVID-19: A systematic review and meta-analysis. Healthcare 2023, 11, 2519. [Google Scholar] [CrossRef]
- Nazem, A.; ShahAli, S.; Dadgoo, M.; Mohsenifar, H.; Ebrahimi Takamjani, I.; Abadi Marand, L. Effectiveness of breathing exercises on urinary symptoms, muscle activity, and strength in women with multiple sclerosis and urinary incontinence-a study protocol for a randomized controlled trial study. Trials 2025, 26, 18. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardization of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Armutlu, K.; Korkmaz, N.C.; Keser, I.; Sumbuloglu, V.; Akbiyik, D.I.; Guney, Z.; Karabudak, R. The validity and reliability of the Fatigue Severity Scale in Turkish multiple sclerosis patients. Int. J. Rehabil. Res. 2007, 30, 81–85. [Google Scholar] [CrossRef]
- Idiman, E.; Uzunel, F.; Ozakbas, S.; Yozbatiran, N.; Oguz, M.; Callioglu, B.; Gokce, N.; Bahar, Z. Cross-cultural adaptation and validation of multiple sclerosis quality of life questionnaire (MSQOL-54) in a Turkish multiple sclerosis sample. J. Neurol. Sci. 2006, 240, 77–80. [Google Scholar]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117, Erratum in Am. J. Respir. Crit. Care Med. 2016, 193, 1185. [Google Scholar] [CrossRef] [PubMed]
- Fregonezi, G.d.F.; Resqueti, V.; Rous, R.G.J. Pursed lips breathing. Rev. Bras. Fisioter. 2004, 40, 279–282. [Google Scholar]
- Stock, M.C.; Downs, J.B.; Gauer, P.K.; Alster, J.M.; Imrey, P.B. Prevention of postoperative pulmonary complications with CPAP, incentive spirometry, and conservative therapy. Chest 1985, 87, 151–157. [Google Scholar]
- Jallouli, S.; Maaloul, R.; Ghroubi, S.; Kammoun, R.; Damak, M.; Sakka, S.; Driss, T.; de Marco, G.; Mhiri, C.; Elleuch, M.H.; et al. Benefits of self-paced concurrent training on lung function, cardiopulmonary fitness and fatigue perception in patients with multiple sclerosis. Neurodegener. Dis. Manag. 2024, 14, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Chiara, T.; Martin, A.D.; Davenport, P.W.; Bolser, D.C. Expiratory muscle strength training in persons with multiple sclerosis having mild to moderate disability: Effect on maximal expiratory pressure, pulmonary function, and maximal voluntary cough. Arch. Phys. Med. Rehabil. 2006, 87, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.D.; Udhoji, S.; Mashtare, T.L.; Fisher, N.M. A combined inspiratory and expiratory muscle training program improves respiratory muscle strength and fatigue in multiple sclerosis. Arch. Phys. Med. Rehabil. 2013, 94, 1964–1970. [Google Scholar] [CrossRef]
- Fry, D.K.; Pfalzer, L.A.; Chokshi, A.R.; Wagner, M.T.; Jackson, E.S. Randomized control trial of effects of a 10-week inspiratory muscle training program on measures of pulmonary function in persons with multiple sclerosis. J. Neurol. Phys. Ther. 2007, 31, 162–172. [Google Scholar]
- Jeong, I.C.; Karpatkin, H.; Finkelstein, J. Physical telerehabilitation improves quality of life in patients with multiple sclerosis. Stud. Health Technol. Inform. 2021, 284, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Fjeldstad-Pardo, C.; Thiessen, A.; Pardo, G. Telerehabilitation in multiple sclerosis: Results of a randomized feasibility and efficacy pilot study. Int. J. Telerehabil. 2018, 10, 55–64. [Google Scholar] [CrossRef]
- Westerdahl, E.; Jonsson, M.; Emtner, M. Pulmonary function and health-related quality of life 1-year follow up after cardiac surgery. J. Cardiothorac. Surg. 2016, 11, 99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seron, P.; Oliveros, M.J.; Gutierrez-Arias, R.; Fuentes-Aspe, R.; Torres-Castro, R.C.; Merino-Osorio, C.; Nahuelhual, P.; Inostroza, J.; Jalil, Y.; Solano, R.; et al. Effectiveness of telerehabilitation in physical therapy: A rapid overview. Phys. Ther. 2021, 101, pzab053. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Amatya, B.; Kesselring, J.; Galea, M. Telerehabilitation for persons with multiple sclerosis. Cochrane Database Syst. Rev. 2015, 2015, CD010508. [Google Scholar] [CrossRef] [PubMed]

| Variables | Telerehabilitation Group (n = 26) | Control Group (n = 26) | Test Value | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Women | 18 (69.2%) | 17 (65.4%) | χ2 = 0.087 | 1.000 |
| Men | 8 (30.8%) | 9 (34.6%) | ||
| Smoking Status | ||||
| Non-smoker | 18 (69.2%) | 11 (42.3%) | χ2 = 4.182 | 0.117 |
| Smoker | 7 (26.9%) | 11 (42.3%) | ||
| Former smoker | 1 (3.8%) | 4 (15.4%) | ||
| Age (years) ± SD | 33.08 ± 9.38 | 33.85 ± 9.36 | t = 0.296 | 0.768 |
| BMI (kg/m2) ± SD | 25.57 ± 4.97 | 25.81 ± 4.06 | t = 0.188 | 0.851 |
| EDSS Score ± SD | 1.98 ± 1.69 | 2.31 ± 1.35 | t = 0.773 | 0.443 |
| Telerehabilitation Group (n = 24) | Control Group (n = 24) | Inter-Group | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | t (p) | |
| FVC (L) | |||
| Before | 3.95 ± 0.96 | 3.99 ± 1.23 | −0.12 (0.903) |
| After | 4.01 ± 0.89 | 3.97 ± 1.21 | 0.14 (0.888) |
| Intra-group t (p) | −0.99 (0.330) | 0.33 (0.745) | |
| FVC (% Predicted) | |||
| Before | 101.62 ± 12.70 | 95.96 ± 18.58 | 1.28 (0.206) |
| After | 103.65 ± 11.42 | 97.31 ± 17.34 | 1.56 (0.125) |
| Intra-group t (p) | −1.17 (0.255) | −1.00 (0.326) | |
| FEV1 (L) | |||
| Before | 3.17 ± 0.83 | 3.25 ± 0.95 | −0.32 (0.750) |
| After | 3.39 ± 0.76 | 3.36 ± 1.03 | 0.14 (0.889) |
| Intra-group t (p) | −3.61 (0.001 *) | −2.24 (0.034 *) | |
| FEV1 (% Predicted) | |||
| Before | 94.81 ± 15.75 | 93.04 ± 15.69 | 0.41 (0.687) |
| After | 97.54 ± 15.62 | 94.04 ± 14.21 | 0.85 (0.402) |
| Intra-group t (p) | −3.57 (0.001 *) | −1.30 (0.206) | |
| PEF (L) | |||
| Before | 5.32 ± 0.96 | 5.80 ± 2.20 | −1.02 (0.314) |
| After | 5.58 ± 0.95 | 5.93 ± 2.21 | −0.74 (0.464) |
| Intra-group t (p) | −5.18 (0.000 *) | −3.27 (0.003 *) | |
| PEF (% Predicted) | |||
| Before | 73.43 ± 16.52 | 73.51 ± 19.84 | −0.15 (0.988) |
| After | 76.54 ± 15.10 | 76.12 ± 17.35 | 0.10 (0.925) |
| Intra-group t (p) | −4.74 (0.000 *) | −2.78 (0.010 *) |
| Variable | Telerehabilitation Group (n = 24) | Control Group (n = 24) | Intergroup (t, p) |
|---|---|---|---|
| FSS Scores | |||
| Before | 3.67 ± 1.80 | 3.95 ± 1.66 | −0.59 (0.561) |
| After | 3.23 ± 1.58 | 3.64 ± 1.57 | −1.73 (0.91) |
| Intra-group (t, p) | 2.45 (0.022 *) | −2.25 (0.045 *) | |
| MSQL-54 | |||
| PCS Before | 56.42 ± 21.28 | 52.74 ± 21.10 | 0.63 (0.534) |
| PCS After | 63.60 ± 19.66 | 57.13 ± 19.99 | 1.18 (0.245) |
| Intra-group (t, p) | −5.46 (0.000 *) | −4.23 (0.000 *) | |
| MCS Before | 57.83 ± 19.97 | 52.14 ± 24.49 | 0.92 (0.363) |
| MCS After | 62.41 ± 19.93 | 56.73 ± 22.72 | 0.96 (0.342) |
| Intra-group (t, p) | −2.21 (0.036 *) | −3.59 (0.001 *) | |
| 6MWT | |||
| Heart Rate Pre-test | |||
| Before | 87.00 ± 12.24 | 83.15 ± 11.17 | 1.18 (0.242) |
| After | 85.15 ± 6.93 | 81.69 ± 6.83 | 1.81 (0.076) |
| Intra-group (t, p) | 0.89 (0.001 *) | 0.76 (0.455) | |
| Heart Rate Post-test | |||
| Before | 95.77 ± 12.97 | 93.23 ± 11.65 | 0.74 (0.461) |
| After | 86.96 ± 7.15 | 84.50 ± 6.73 | 1.28 (0.207) |
| Intra-group (t, p) | 3.90 (0.001 *) | 3.77 (0.001 *) | |
| 6MWT Distance (m) | |||
| Before | 317.69 ± 78.08 | 338.46 ± 67.18 | −1.03 (0.309) |
| After | 330.96 ± 74.32 | 351.92 ± 58.84 | −1.13 (0.265) |
| Intra-group (t, p) | −2.75 (0.011 *) | −2.58 (0.016 *) | |
| Change in Heart Rate | |||
| Before | 8.77 ± 7.35 | 10.08 ± 10.46 | −0.52 (0.604) |
| After | 1.81 ± 2.91 | 3.00 ± 5.03 | −1.05 (0.302) |
| Intra-group (t, p) | 5.08 (0.000 *) | 3.67 (0.001 *) | |
| Distance (m) | |||
| Before | 317.69 ± 78.08 | 338.46 ± 67.18 | −1.03 (0.309) |
| After | 330.96 ± 74.32 | 351.92 ± 58.84 | −1.13 (0.265) |
| Intra-group (t, p) | −2.75 (0.011 *) | 2.58 (0.016 *) | |
| Saturation Pre-test (%) | |||
| Before | Median: 98 (95–99) | Median: 98 (95–99) | 333.0 (0.921) |
| After | Median: 98 (85–99) | Median: 98 (85–99) | 314.5 (0.652) |
| Intra-group (z, p) | 0.00 (1.00) | 1.10 (0.270) | |
| Saturation Post-test (%) | |||
| Before | Median: 98 (97–99) | Median: 98 (82–101) | 335.0 (0.953) |
| After | Median: 98 (92–99) | Median: 98 (92–99) | 325.0 (0.803) |
| Intra-group (z, p) | −1.90 (0.058) | −0.14 (0.888) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayçiçek, Ş.Ö.; Tunç, A.; Bağcı, C. Effects of Telehealth-Supervised Respiratory Exercise Training on Respiratory Function, Fatigue, Quality of Life, and Functional Capacity of Patients with Multiple Sclerosis. Medicina 2025, 61, 651. https://doi.org/10.3390/medicina61040651
Ayçiçek ŞÖ, Tunç A, Bağcı C. Effects of Telehealth-Supervised Respiratory Exercise Training on Respiratory Function, Fatigue, Quality of Life, and Functional Capacity of Patients with Multiple Sclerosis. Medicina. 2025; 61(4):651. https://doi.org/10.3390/medicina61040651
Chicago/Turabian StyleAyçiçek, Şeyda Öznur, Abdulkadir Tunç, and Cahit Bağcı. 2025. "Effects of Telehealth-Supervised Respiratory Exercise Training on Respiratory Function, Fatigue, Quality of Life, and Functional Capacity of Patients with Multiple Sclerosis" Medicina 61, no. 4: 651. https://doi.org/10.3390/medicina61040651
APA StyleAyçiçek, Ş. Ö., Tunç, A., & Bağcı, C. (2025). Effects of Telehealth-Supervised Respiratory Exercise Training on Respiratory Function, Fatigue, Quality of Life, and Functional Capacity of Patients with Multiple Sclerosis. Medicina, 61(4), 651. https://doi.org/10.3390/medicina61040651






