Aggressive Squamoid Eccrine Ductal Carcinoma of the Face: A Rare and Challenging Diagnosis—Case Report and Literature Review
Abstract
1. Introduction
2. Case Report
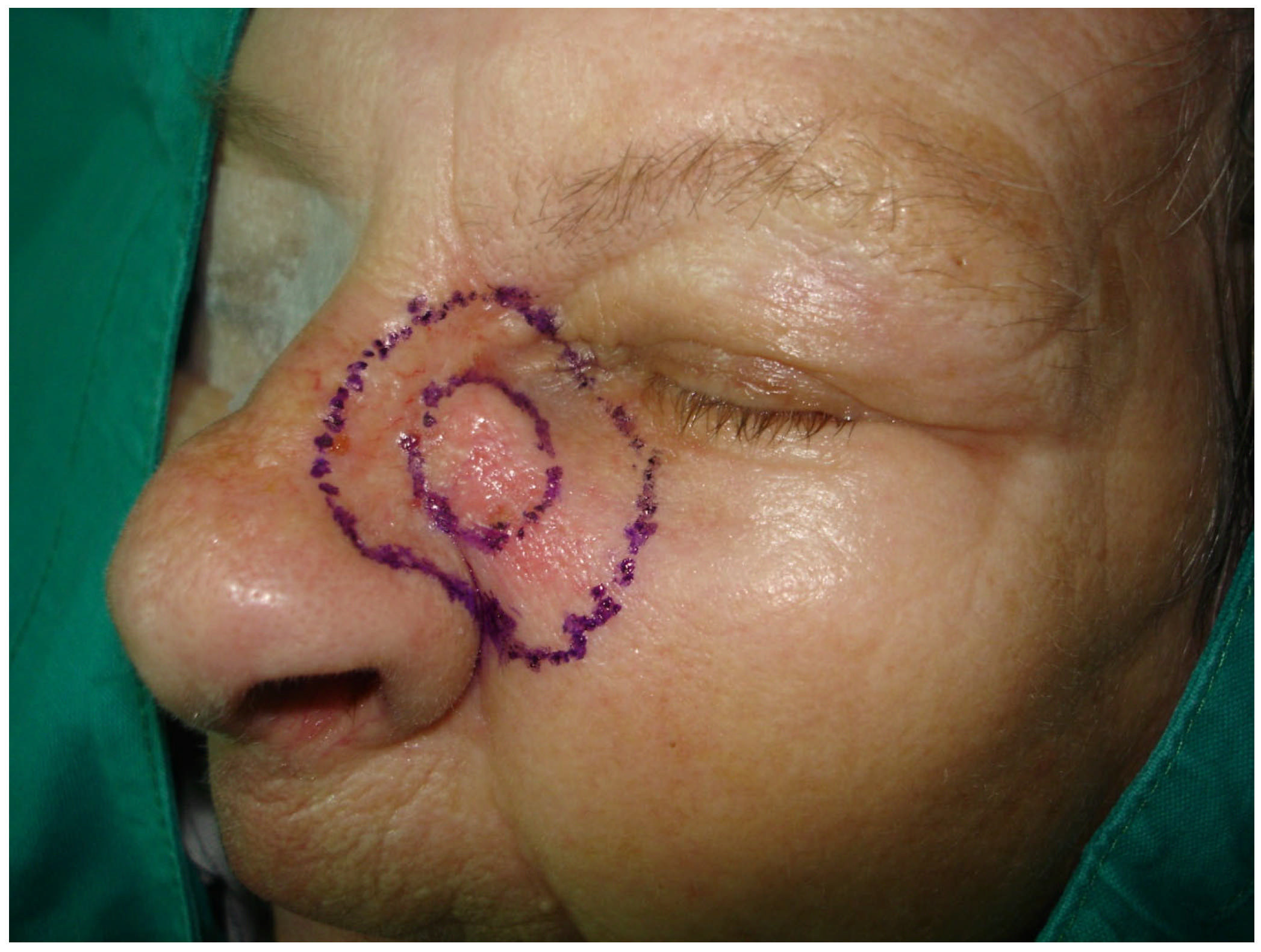
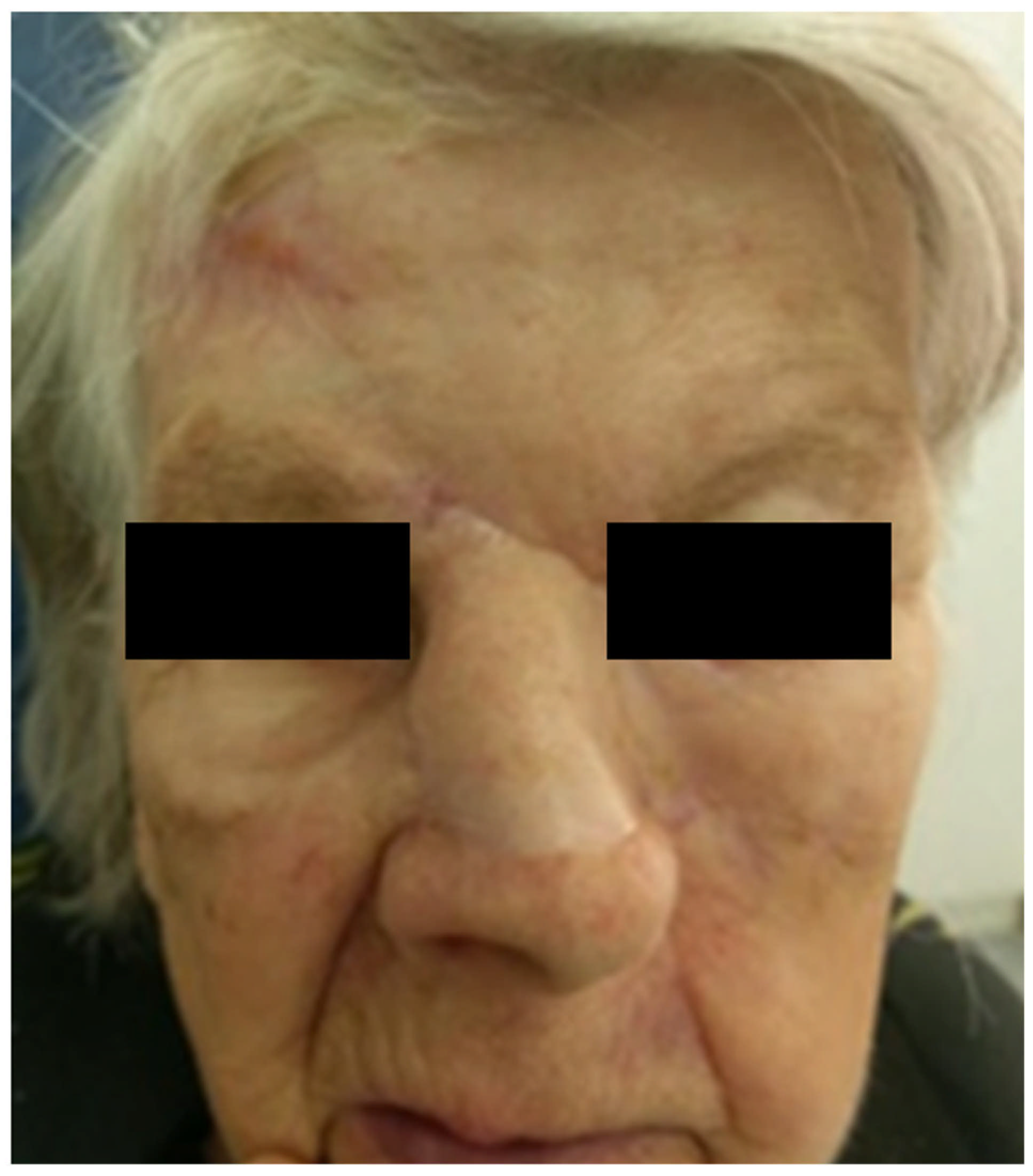
2.1. Clinical Examination
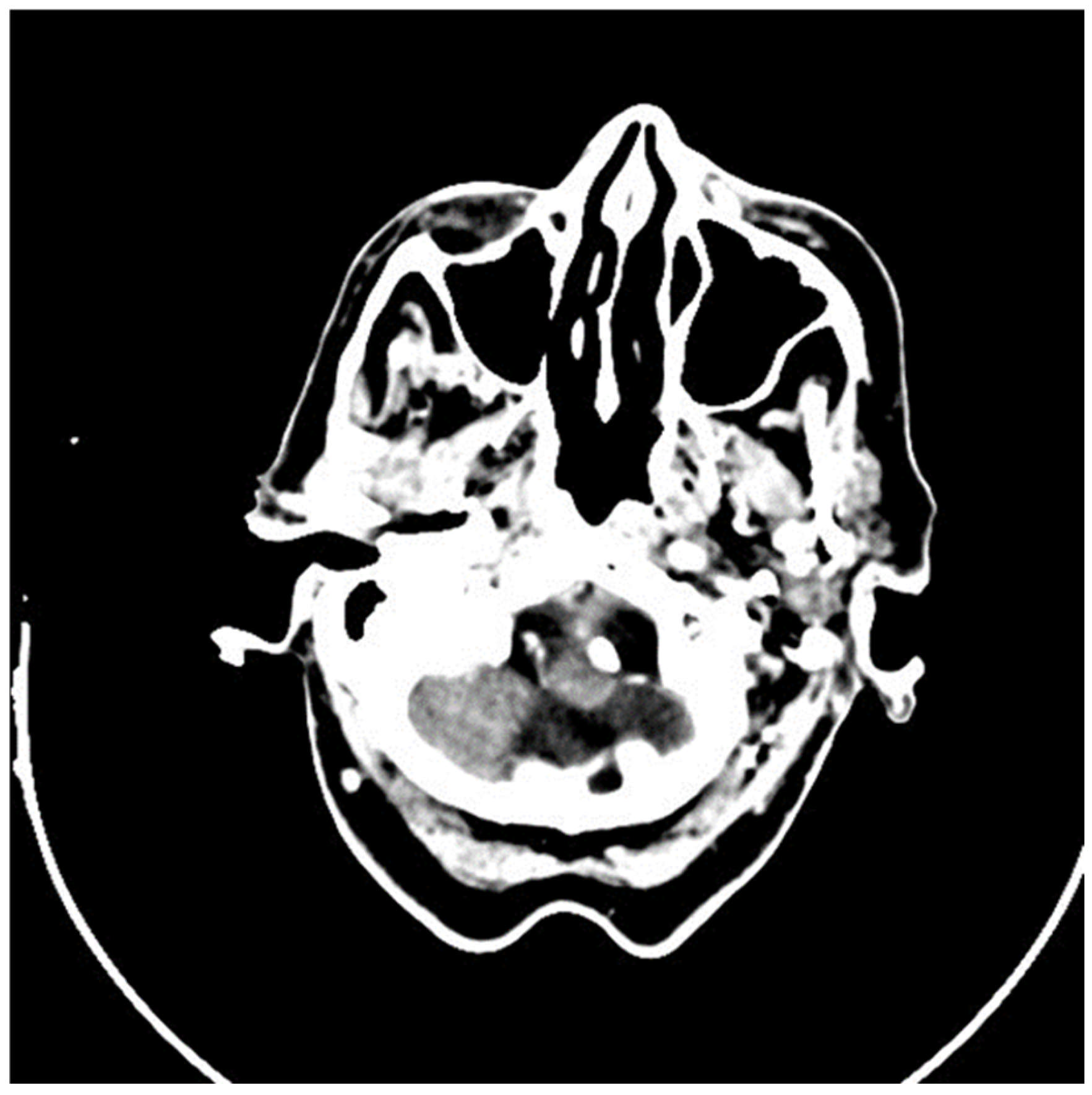
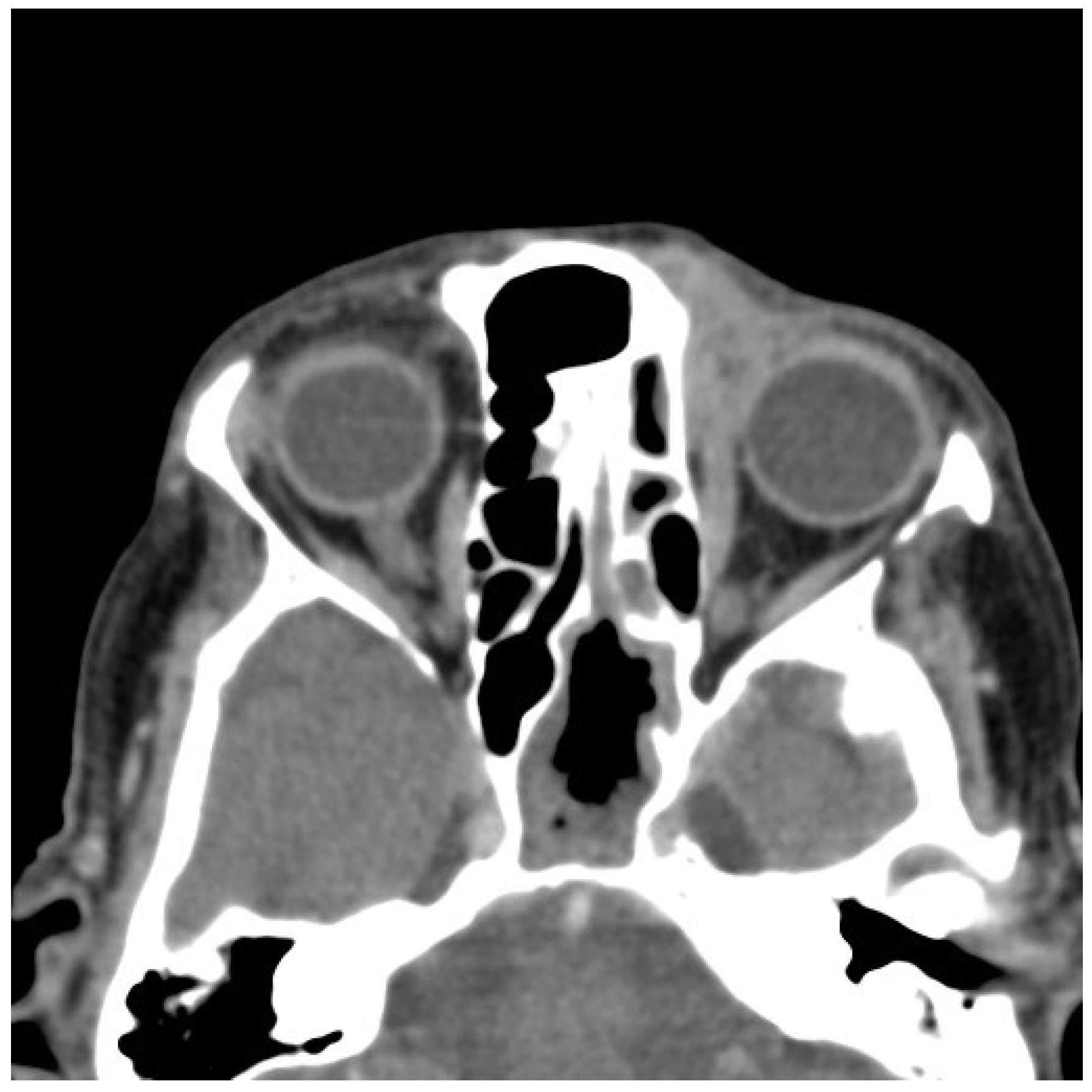
2.2. Imaging and Preoperative Diagnostics
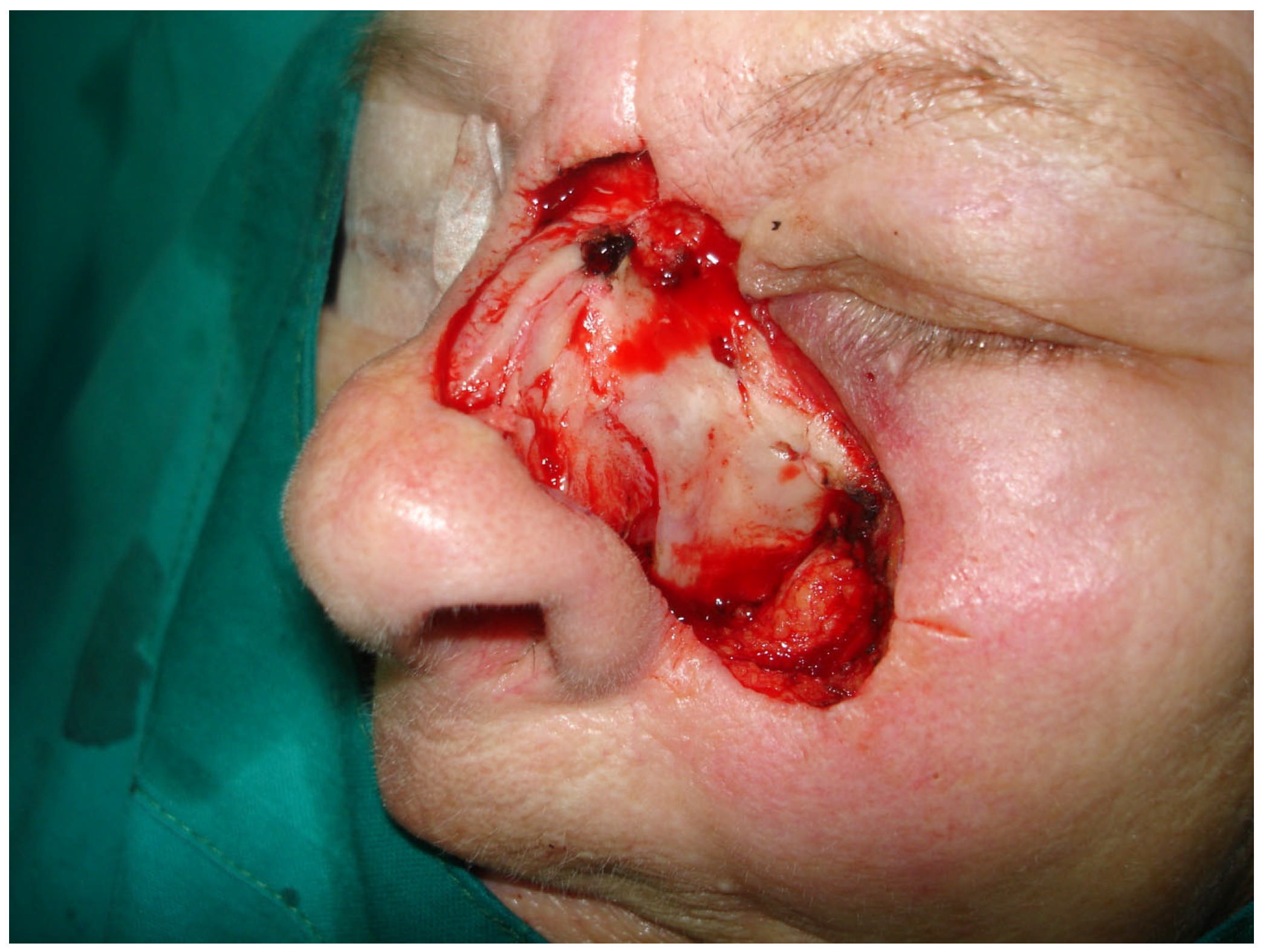
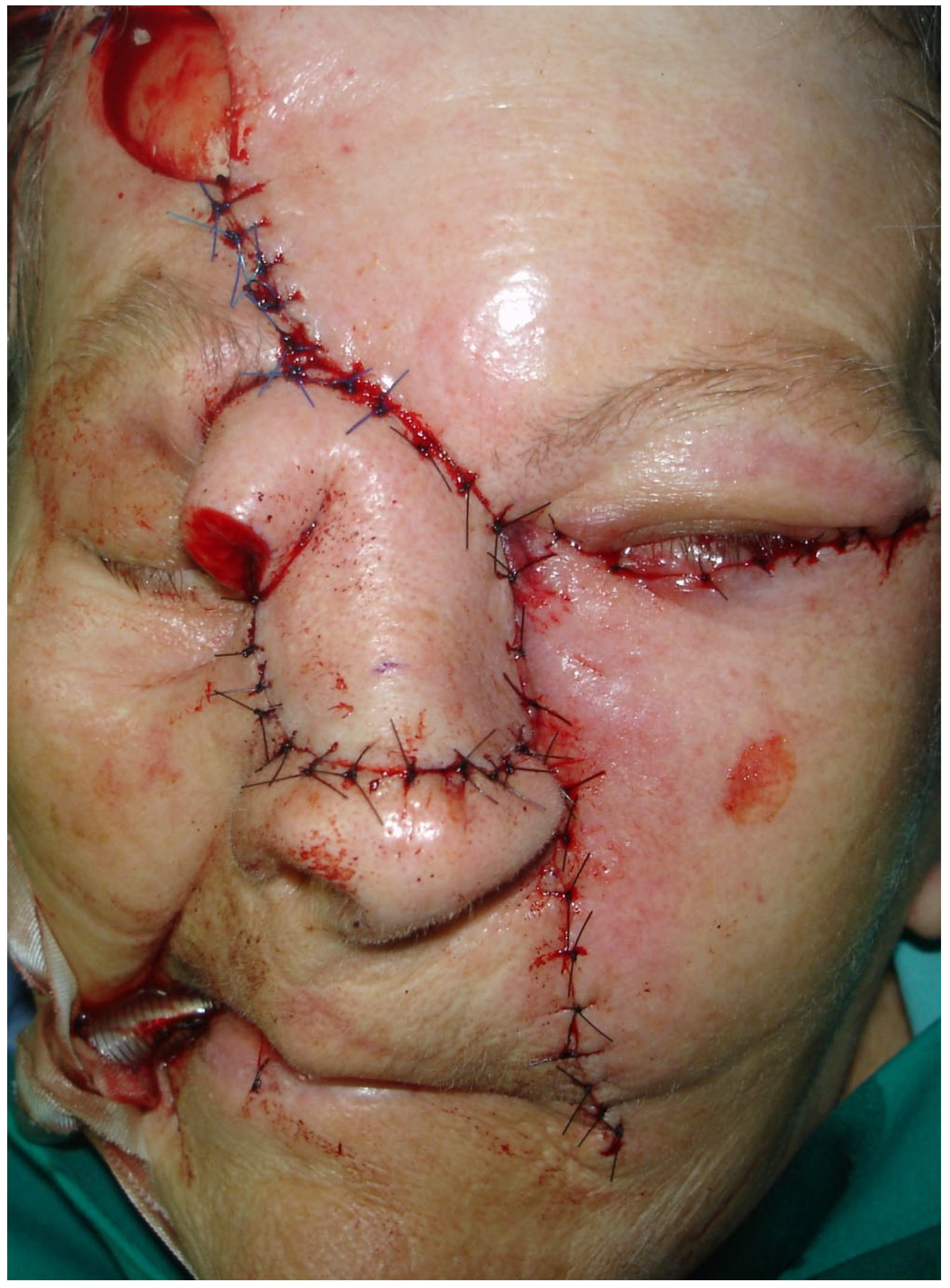
2.3. Surgical Treatment
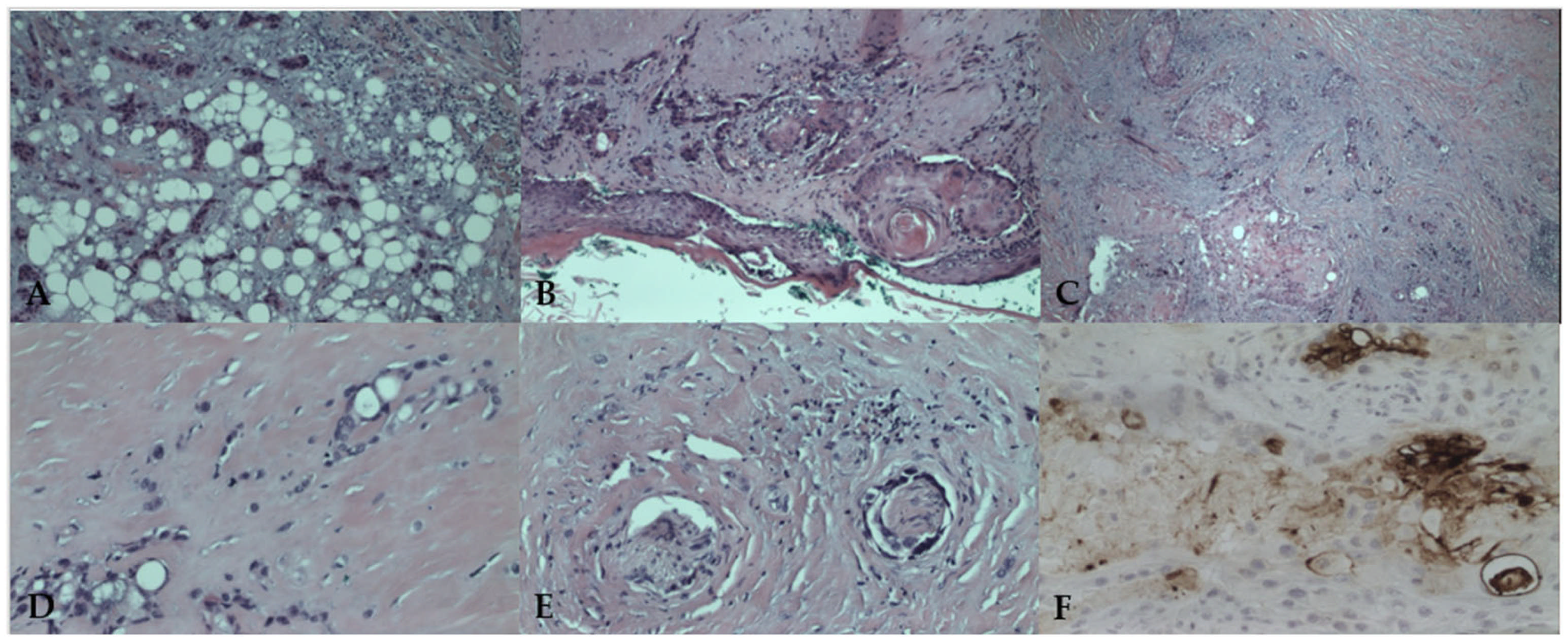
2.4. Histopathological Findings
2.5. Postoperative Course and Follow-Up
3. Discussion
3.1. Literature Review
3.2. Histopathological and Immunohistochemical Features
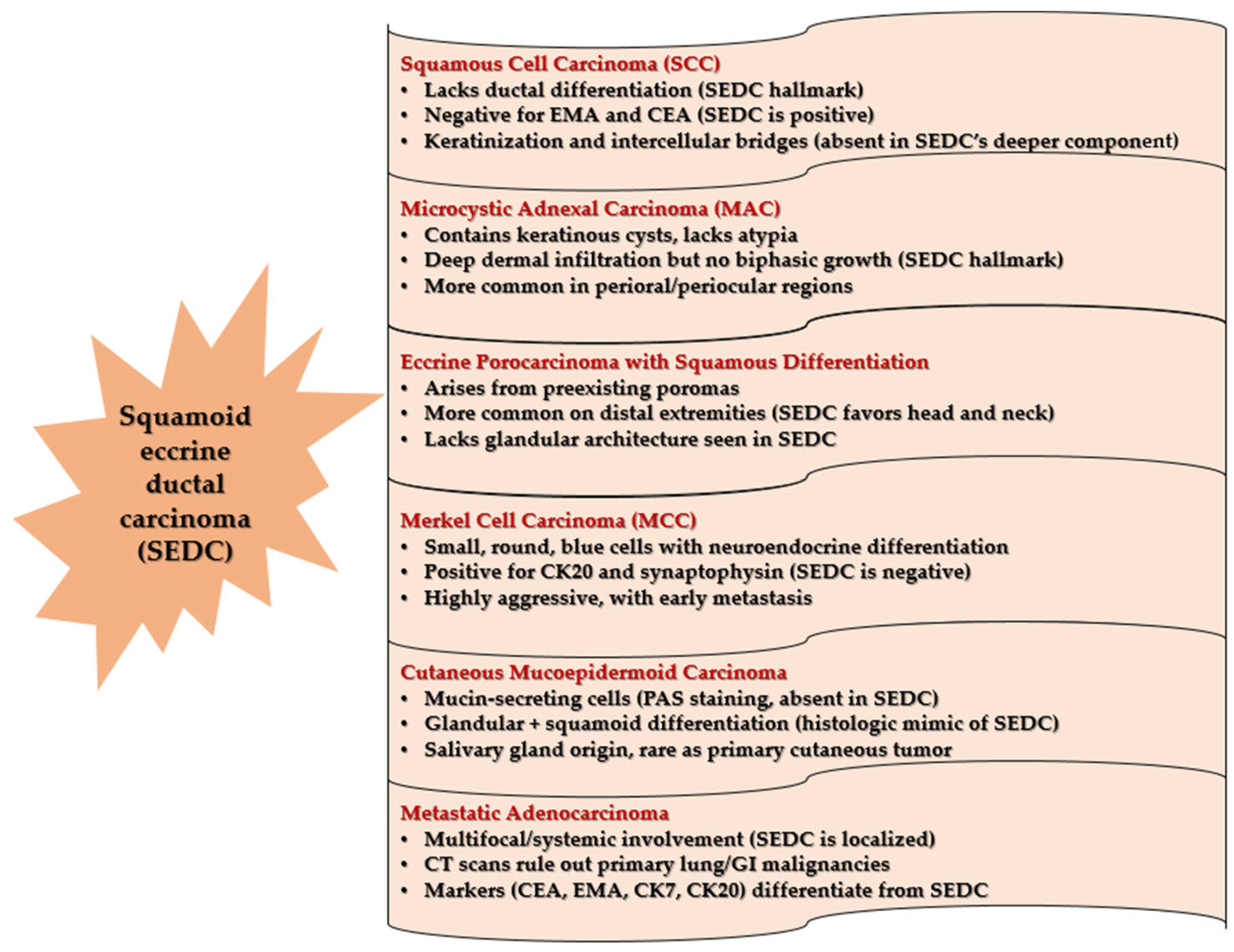
3.3. Differential Diagnosis
3.4. Diagnostics, Treatment, and Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SEDC | Squamoid eccrine ductal carcinoma. |
| SCC | Squamous cell carcinoma. |
| EMA | Epithelial membrane origin. |
| CEA | Carcinogenic antigen. |
| MMS | Mohs micrographic surgery. |
| PNI | Perineural invasion. |
| CT | Computed tomography. |
| LVI | Lymphovascular invasion. |
| D2-40 | Podoplanin. |
| AR | Androgen receptor. |
| NGS | Next-generation sequencing. |
| MCC | Merkel cell carcinoma. |
| PAS | Periodic acid-Schiff. |
| MCPyV | Merkel cell polyomavirus. |
| MAC | Mycrocystic adnexal carcinoma. |
| MRI | Magnetic resonance imaging. |
References
- Wick, M.R.; Swanson, P.E. Cutaneous Adnexal Tumors. In A Guide to Pathological Diagnosis; American Society of Clinical Pathologists: Chicago, IL, USA, 1991; pp. 10–13. [Google Scholar]
- Wong, T.Y.; Suster, S.; Mihm, M.C. Squamoid Eccrine Ductal Carcinoma. Histopathology 1997, 30, 288–293. [Google Scholar] [PubMed]
- Brenn, T. Malignant Sweat Gland Tumors: An Update. Adv. Anat. Pathol. 2015, 22, 242–253. [Google Scholar] [PubMed]
- van der Horst, M.P.; Garcia-Herrera, A.; Markiewicz, D.; Martin, B.; Calonje, E.; Brenn, T. Squamoid Eccrine Ductal Carcinoma: A Clinicopathologic Study of 30 Cases. Am. J. Surg. Pathol. 2016, 40, 755–760. [Google Scholar] [PubMed]
- Bayramoğlu, Z.; Ünal, B. Squamoid Eccrine Ductal Carcinoma: A Rare Case Report. Eur. Res. J. 2020, 6, 173–177. [Google Scholar] [CrossRef]
- Bellisario, J.A.; Lunardhi, A.; Huynh, K.; Iguh, C.; Stohl, H. Squamoid Eccrine Ductal Carcinoma: An Unusual Diagnosis in a Pregnant Patient. Cureus 2024, 16, e72123. [Google Scholar] [CrossRef]
- Frouin, E.; Vignon-Pennamen, M.D.; Balme, B.; Cavelier-Balloy, B.; Zimmermann, U.; Ortonne, N.; Carlotti, A.; Pinquier, L.; André, J.; Cribier, B. Anatomoclinical Study of 30 Cases of Sclerosing Sweat Duct Carcinomas (Microcystic Adnexal Carcinoma, Syringomatous Carcinoma and Squamoid Eccrine Ductal Carcinoma). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1978–1994. [Google Scholar] [CrossRef]
- Jung, Y.H.; Jo, H.J.; Kang, M.S. Squamoid Eccrine Ductal Carcinoma of the Scalp. Korean J. Pathol. 2012, 46, 278–281. [Google Scholar]
- Liang, C.A.; Busam, K.J.; Nehal, K.S. Microcystic Adnexal Carcinoma Associated with Multiple Benign Syringomatous Proliferations: A Report of Two Cases. Dermatol. Surg. 2011, 37, 1515–1518. [Google Scholar]
- Lobo-Jardim, M.M.; Souza, B.C.; Kakizaki, P.; Valente, N.Y.S. Dermoscopy of Squamoid Eccrine Ductal Carcinoma: An Aid for Early Diagnosis. An. Bras. Dermatol. 2018, 93, 893–895. [Google Scholar]
- Rovesti, M.; Satolli, F.; Ricci, R.; Manuguerra, R.; Zucchi, A.; Gandolfi, M.; Gianfaldoni, S.; Claudio, F.; Wollina, U.; Tchernev, G.; et al. Once in a Blue Moon … Rare Adnexal Tumor: From the Clinical and Videodermoscopic Aspects to the Mohs Surgery and the Histological Diagnosis. Open Access Maced. J. Med. Sci. 2018, 6, 146–148. [Google Scholar] [CrossRef]
- Lim, M.M.; Macdonald, J.A. Squamoid Eccrine Ductal Carcinoma: Treatment and Outcomes. Am. J. Dermatopathol. 2022, 44, 249–253. [Google Scholar] [PubMed]
- Saraiva, M.I.; Vieira, M.A.; Portocarrero, L.K.; Fraga, R.C.; Kakizaki, P.; Valente, N.Y. Squamoid Eccrine Ductal Carcinoma. An. Bras. Dermatol. 2016, 91, 799–802. [Google Scholar]
- Clark, S.; Young, A.; Piatigorsky, E.; Ravitskiy, L. Mohs Micrographic Surgery in the Setting of Squamoid Eccrine Ductal Carcinoma: Addressing a Diagnostic and Therapeutic Challenge. J. Clin. Aesthet. Dermatol. 2013, 6, 33–36. [Google Scholar]
- van der Horst, M.P.J.; Brenn, T. Update on Malignant Sweat Gland Tumors. Surg. Pathol. Clin. 2017, 10, 383–397. [Google Scholar]
- Elder, D.E.; Massi, D.; Scolyer, R.A.; Willemze, R. WHO Classification of Skin Tumors; WHO: Geneva, Switzerland, 2018; pp. 176–177. [Google Scholar]
- Saifi, B.; Maalouf, M.; Hasan, A.; Fadel, D.; Kassab, M.; Emmanuel, N. Squamoid Eccrine Ductal Carcinoma: A Systematic Review. Curr. Dermatol. Rep. 2025, 14, 4. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, A.R.; Yu, D.S. Mohs Micrographic Surgery for Squamoid Eccrine Ductal Carcinoma. Dermatol. Surg. 2005, 31, 1462–1464. [Google Scholar]
- Terushkin, E.; Leffell, D.J.; Futoryan, T.; Cowper, S.; Lazova, R. Squamoid Eccrine Ductal Carcinoma: A Case Report and Review of the Literature. Am. J. Dermatopathol. 2010, 32, 287–292. [Google Scholar] [CrossRef]
- Perkins, A.C.; Goldberg, D.; Maloney, M.E. Primary Eccrine Ductal Carcinoma Masquerading as Metastatic Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2011, 64, AB126. [Google Scholar] [CrossRef]
- Kim, J.W.; Jeon, M.K.; Kang, S.J.; Sun, H. Surgical Management of Recurrent Squamoid Eccrine Ductal Carcinoma of the Scalp. J. Craniofacial Surg. 2012, 23, 276–278. [Google Scholar]
- Ranasinghe, A.; Rytina, E.; Flanagan, N.; Jenkins, R.; Ha, T. Squamoid Eccrine Ductal Carcinoma: A Rare Adnexal Tumor. J. Am. Acad. Dermatol. 2013, 68, AB88. [Google Scholar] [CrossRef]
- Chan, H.; Howard, V.; Moir, D.; Dyall-Smith, D. Squamoid Eccrine Ductal Carcinoma of the Scalp. Australas. J. Dermatol. 2016, 57, e117–e119. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.; Krach, K. Squamoid Eccrine Ductal Carcinoma: A Unique Diagnostic and Therapeutic Challenge. J. Am. Acad. Dermatol. 2017, 76, AB240. [Google Scholar] [CrossRef]
- Sharma, B.; Malhotra, P.; Bhardwaj, M.; Bhartiya, R.; Kumari, N.; Agrawal, P.; Rajnish, K. Squamoid Eccrine Ductal Carcinoma: A Diagnostic Dilemma. Ann. Pathol. Lab. Med. 2018, 5, C119–C121. [Google Scholar] [CrossRef]
- Yim, S.; Lee, Y.H.; Chae, S.W.; Kim, W.S. Squamoid Eccrine Ductal Carcinoma of the Ear Helix. Clin. Case Rep. 2019, 7, 1409–1411. [Google Scholar] [CrossRef]
- Phan, K.; Kim, L.; Lim, P.; Cheung, K. A Case Report of Temple Squamoid Eccrine Ductal Carcinoma: A Diagnostic Challenge Beneath the Tip of the Iceberg. Dermatol. Ther. 2020, 33, e13213. [Google Scholar] [CrossRef]
- Rownose, C.S.; Mohamad Saupi, M.S.; Sharif, S.Z.; Lah, N.A.S.N. Aggressive Scalp and Sternal Lesion: A Presentation of Rare Case of Metastatic Eccrine Carcinoma. Ann. Med. Surg. 2021, 65, 102322. [Google Scholar] [CrossRef]
- Patel, N.; Alabiad, C.R.; Wick, M.R.; Elgart, G.W.; Tang, V.D.; Abou Khzam, R.A.; Dubovy, S.R. Squamoid Eccrine Ductal Carcinoma of the Eyelid: Clinicopathologic Correlation of a Case. Ophthalmic Plast. Reconstr. Surg. 2022, 38, e80–e82. [Google Scholar] [CrossRef]
- Kweon, H.T.; Lee, C.M.; Yeo, C.D.; Lee, E.J. A Case of Squamoid Eccrine Ductal Carcinoma of the Auricle Mimicking Perichondritis. Ear Nose Throat J. 2024. [Google Scholar] [CrossRef]
- Chan, C.X.; Sriharan, A.; LeBlanc, R.E.; Guill, M.A.; Glass, J.S.; Momtahen, S. Squamoid Eccrine Ductal Carcinoma: Clinical, Histological and Immunohistochemical Features. Dermatol. Online J. 2024, 30, 1–8. [Google Scholar] [CrossRef]
- Eghtedari, M.; Lin, M.J.; Kim, L. Mohs Surgery for Squamoid Eccrine Ductal Carcinoma. J. Cutan. Aesthetic Surg. 2024, 17, 153–155. [Google Scholar] [CrossRef]
- Svoboda, S.A.; Rush, P.S.; Garofola, C.J.; Grider, D.J.; Prickett, K.A.; Phillips, M.A. Squamoid Eccrine Ductal Carcinoma. Cutis 2021, 107, E5–E9. [Google Scholar] [CrossRef]
- Honorato, C.M.A.; Carrascoza, G.G.; Abed, N.M.; Moya, F.G. Squamoid Eccrine Ductal Carcinoma: Series of Five Cases of a Rare Tumor. An. Bras. Dermatol. 2024, 99, 936–939. [Google Scholar] [CrossRef]
- Jacob, J.; Kugelman, L. Squamoid Eccrine Ductal Carcinoma. Cutis 2018, 101, 380–385. [Google Scholar]
- Ibrahim, Y.L.; Lambert, S.; Kazakov, D.V.; Kaya, G. An Unusual Morphological Presentation of Cutaneous Squamous Cell Carcinoma Mimicking Microcystic Adnexal Carcinoma: A Diagnostic Pitfall. Dermatopathology 2018, 5, 64–68. [Google Scholar]
- Macagno, N.; Sohier, P.; Kervarrec, T.; Pissaloux, D.; Jullie, M.-L.; Cribier, B.; Battistella, M. Recent Advances on Immunohistochemistry and Molecular Biology for the Diagnosis of Adnexal Sweat Gland Tumors. Cancers 2022, 14, 476. [Google Scholar] [CrossRef]
- Compton, L.A.; Murphy, G.F.; Lian, C.G. Diagnostic Immunohistochemistry in Cutaneous Neoplasia: An Update. Dermatopathology 2015, 2, 15–42. [Google Scholar] [CrossRef]
- Tuffaha, M.S.A.; Guski, H.; Kristiansen, G. Markers and Immunoprofile of Skin Tumors. In Immunohistochemistry in Tumor Diagnostics; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Harms, P.W.; Hovelson, D.H.; Cani, A.K.; Omata, K.; Haller, M.J.; Wang, M.L.; Arps, D.; Patel, R.M.; Fullen, D.R.; Wang, M.; et al. Porocarcinomas Harbor Recurrent HRAS-Activating Mutations and Tumor Suppressor Inactivating Mutations. Hum. Pathol. 2016, 51, 25–31. [Google Scholar] [CrossRef]
- López, V.; Rubio, M.; Santonja, N.; Jordá, E. Primary Cutaneous Mucoepidermoid Carcinoma. Am. J. Dermatopathol. 2010, 32, 618–620. [Google Scholar] [CrossRef]
- Sauerborn, D.; Vidaković, B.; Baranović, M. Metastatic Adenocarcinoma in the Head and Neck Region. In Adenocarcinoma: Pathogenesis, Treatment, and Prognosis; Longoria, M.A., Alcalá, J.I., Eds.; Nova Publishers: New York, NY, USA, 2012; pp. 135–156. [Google Scholar]
- Cardoso, J.C.; Calonje, E. Malignant Sweat Gland Tumors: An Update. Histopathology 2015, 67, 589–606. [Google Scholar] [CrossRef]
- McKissack, S.S.; Wohltmann, W.; Dalton, S.R.; Miletta, N.R. Squamoid Eccrine Ductal Carcinoma: An Aggressive Mimicker of Squamous Cell Carcinoma. Am. J. Dermatopathol. 2018, 41, 140–143. [Google Scholar] [CrossRef]
- Busam, K.J.; Jungbluth, A.A.; Rekthman, N.; Coit, D.; Pulitzer, M.; Bini, J.; Arora, R.; Hanson, N.C.; Tassello, J.A.; Frosina, D.; et al. Merkel Cell Polyomavirus Expression in Merkel Cell Carcinomas and Its Absence in Combined Tumors and Pulmonary Neuroendocrine Carcinomas. Am. J. Surg. Pathol. 2009, 33, 1378–1385. [Google Scholar] [CrossRef]
- Louveau, B.; Nakouri, I.; Jouenne, F.; Baroudjian, B.; Sadoux, A.; Da Meda, L.; Osio, A.; Reinhart, F.; Robert, J.; Herms, F.; et al. Genomic Profiling of a Skin Adnexal Carcinomas Cohort Using a Comprehensive High-Throughput Sequencing Approach. Br. J. Dermatol. 2024, 191, 639–641. [Google Scholar] [CrossRef]
- Harms, P.W.; Runge, M.; Chan, M.P.; Liu, C.J.; Qin, Z.; Worden, F.; Robinson, D.R.; Chinnaiyan, A.M.; Mclean, S.A.; Harms, K.L.; et al. Squamoid Eccrine Ductal Carcinoma Displays Ultraviolet Mutations and Intermediate Gene Expression Relative to Squamous Cell Carcinoma, Microcystic Adnexal Carcinoma, and Porocarcinoma. Mod. Pathol. 2024, 37, 100592. [Google Scholar] [CrossRef]
- Wang, B.; Jarell, A.D.; Bingham, J.L.; Bonavia, G.H. PET/CT Imaging of Squamoid Eccrine Ductal Carcinoma. Clin. Nucl. Med. 2015, 40, 322–324. [Google Scholar] [CrossRef]
- Hutchinson, M.K.N.D.; Mierzwa, M.; D’Silva, N.J. Radiation Resistance in Head and Neck Squamous Cell Carcinoma: Dire Need for an Appropriate Sensitizer. Oncogene 2020, 39, 3638–3649. [Google Scholar] [CrossRef]
- Stevenson, M.L.; Criscito, M.C.; Wilken, R.; Doudican, N.A.; Bain, E.E., III; Parashar, B.; Carucci, J.A. Use of Adjuvant Radiotherapy in the Treatment of High-Risk Cutaneous Squamous Cell Carcinoma with Perineural Invasion. JAMA Dermatol. 2020, 156, 918–921. [Google Scholar] [CrossRef]
- De Iuliis, F.; Amoroso, L.; Taglieri, L.; Vendittozzi, S.; Blasi, L.; Salerno, G.; Lanza, R.; Scarpa, S. Chemotherapy of Rare Skin Adnexal Tumors: A Review of Literature. Anticancer Res. 2014, 34, 5263–5268. [Google Scholar]
- Płachta, I.; Kleibert, M.; Czarnecka, A.M.; Spałek, M.; Szumera-Ciećkiewicz, A.; Rutkowski, P. Current Diagnosis and Treatment Options for Cutaneous Adnexal Neoplasms with Apocrine and Eccrine Differentiation. Int. J. Mol. Sci. 2021, 22, 5077. [Google Scholar] [CrossRef]
- Fadhil, M.; Lochhead, A.; Trinh, H.; Brungs, D. Metastatic Ductal Eccrine Adenocarcinoma with Excellent Response to Immunotherapy. Case Rep. Oncol. 2023, 16, 1415–1424. [Google Scholar] [CrossRef]
- Wee, S.; Jagani, Z.; Xiang, K.X.; Loo, A.; Dorsch, M.; Yao, Y.M.; Sellers, W.R.; Lengauer, C.; Stegmeier, F. PI3K Pathway Activation Mediates Resistance to MEK Inhibitors in KRAS Mutant Cancers. Cancer Res. 2009, 69, 4286–4293. [Google Scholar] [CrossRef]
- Janku, F.; Wheler, J.J.; Westin, S.N.; Moulder, S.L.; Naing, A.; Tsimberidou, A.M.; Fu, S.; Falchook, G.S.; Hong, D.S.; Garrido-Laguna, I.; et al. PI3K/AKT/mTOR Inhibitors in Patients with Breast and Gynecologic Malignancies Harboring PIK3CA Mutations. J. Clin. Oncol. 2012, 30, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Pirotte, B.D.; Benson, S. Refusal of Care. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560886/ (accessed on 20 March 2025).
- Sokoya, M.; Cohn, J.E.; Kohlert, S.; Lee, T.; Kadakia, S.; Ducic, Y. Considerations in Orbital Exenteration. Semin. Plast. Surg. 2019, 33, 103–105. [Google Scholar] [CrossRef] [PubMed]
- McLean, L.S.; Lim, A.M.; Webb, A.; Cavanagh, K.; Thai, A.; Magarey, M.; Fox, C.; Kleid, S.; Rischin, D. Immunotherapy to Avoid Orbital Exenteration in Patients with Cutaneous Squamous Cell Carcinoma. Front. Oncol. 2022, 11, 796197. [Google Scholar] [CrossRef]
- Borg, T.M.; Eisold, J.; Miyanjo, Y.; Pappa, E. The Effect of COVID-19 on Surgical Management of Skin Cancers of the Head, Face and Neck in Elderly Patients. Skin Health Dis. 2023, 3, e175. [Google Scholar] [CrossRef]
- Sengar, M.; Chinnaswamy, G.; Ranganathan, P.; Ashok, A.; Bhosale, S.; Biswas, S.; Chaturvedi, P.; Dhamne, C.; Divatia, J.; D’Sa, K.; et al. TMH COVID-19 Action Group. Outcomes of COVID-19 and Risk Factors in Patients with Cancer. Nat. Cancer 2022, 3, 547–551. [Google Scholar] [CrossRef]
| Author, Year [Reference Number] | Gender/Age | Past Medical History | Site of the Tumor | Size (cm) | Presence of PNI and LVI | Management, Follow-Up, and Outcome |
|---|---|---|---|---|---|---|
| Wong et al., 1996 [2] | M/81 | NA | Ear | ≤2.5 | PNI | Excision with 3 recurrences within 36 mo |
| Kim et al., 2005 [18] | F/30 | Not remarkable | Neck | 2.5 | NA | Negative CT, Mohs, no recurrence at 5 mo |
| Terushkin et al., 2010 [19] | M/63 | Chronic lymphocytic leukemia | Cheek | 2.7 | No | Mohs, no recurrence at 10 mo |
| Perkins et al., 2011 [20] | M/72 | Multiple SCCs and esophageal adenocarcinoma | Ear | NA | LVI | Mohs, follow-up NA |
| Jung et al., 2012 [8] | M/53 | Not remarkable | Scalp | 2.6 | NA | CT, excision, fine needle aspiration with metastasis to lymph node at 5 mo, excision and lymph node dissection |
| Kim et al., 2012 [21] | M/53 | NA | Scalp | 2.6 | NA | CT, excision, fine needle aspiration with metastasis to lymph node at 5 mo, excision, and lymph node dissection |
| Ranasinghe et al., 2013 [22] | M/65 | NA | Nose | 0.7 | PNI | Mohs, follow-up NA |
| Chan et al., 2016 [23] | M/85 | Multiple nonmelanoma skin cancers and superficial spreading melanoma, myasthenia gravis | Scalp | NA | No | Excision, follow-up NA |
| Saraiva et al., 2016 [13] | F/72 | Not remarkable | Nose | NA | NA | Excision 2× and RT for recurrence at 5 mo, no recurrence at 23 mo |
| Graham et al., 2017 [24] | M/80 | NA | Forehead | NA | NA | Mohs, no recurrence at 24 mo |
| Sharma et al., 2018 [25] | M/50 | NA | Scalp | 1 | NA | No recurrence at 5 mo |
| Rovesti et al., 2018 [11] | M/75 | Colon cancer | Temple | 2 | PNI | Mohs, no recurrence at 12 mo |
| Lobo-Jardim et al., 2018 [10] | F/76 | Bowen disease | Nose | NA | NA | Excision, follow-up NA |
| Yim et al., 2019 [26] | M/80 | Not remarkable | Ear | 1.5 | NA | CT scan, excision, no recurrence at 9 mo |
| Bayramoğlu and Ünal, 2020 [5] | M/79 | Not remarkable | Scalp | 1.2 | No | Local excision, follow-up NA |
| Phan et al., 2020 [27] | M/92 | Thrombocytopenia, infiltrative BCC of the nose | Temple | 2 × 2.2 | Few foci of PNI | Local excision and watch-and-wait approach, no recurrence at 6 mo |
| Rownose et al., 2021 [28] | F/41 | Not remarkable | Scalp | 4 | PNI and LVI | Wedge excision biopsy, suggested RT and ChT refused; died 3 mo after |
| Patel et al., 2022 [29] | M/76 | SCC of the right naris, right nasal ala and scalp | Eyelid | 2.5 × 1.5 | No | Mohs stage 2, no recurrence at 27 mo |
| Kweon et al., 2024 [30] | M/84 | Not remarkable | Ear auricle | NA | LVI | Parotidectomy, selective neck dissection, no recurrence at 12 mo |
| Chan et al., 2024 [31] | F/88 | NA | Temple | 2 | NA | Mohs, follow-up NA |
| Eghtedari, Lin and Kim, 2024 [32] | F/82 | Not remarkable | Cheek | NA | No | Mohs, no recurrence at 12 mo |
| Špiljak et al. [this case] | F/80 | Not remarkable | Left nasal region | 1 × 1.5 | PNI | Mohs, adjuvant RT; recurrence after 20 mo; declined further surgery; died of COVID-19 39 mo after disease progression |
| Author, Year [Reference Number] | Number of Cases | Gender/Mean Age | Past Medical History | Site of the Tumor | Mean Size (cm) | Presence of PNI and LVI | Management, Follow-Up, and Outcome |
|---|---|---|---|---|---|---|---|
| Frouin et al., 2015 [7] | 7 | 6 F and 1 M/81.3 | BCC (2×), SCC (2×), Bowen disease (2×), SSM (1×), breast cancer—radiation surgery (1×), kidney transplantat recipient (1×), liver transplantat recipient (1×) | 3× cheek, 2× nose, 1× forehead, 1× canthus | 3.45 (3× NA) | 4× PNI and 4× LVI | Excision (3×), 2× excision (2×), 5× excision with enucleation, amputation and RT (1×), Mohs (1×), no recurrence at 44–156 mo (5×); recurred at 32 mo (1×), died from SEDC at 42 mo (1×) |
| Van der Horst, 2016 [4] | 23 | 7 F and 16 M/68.4 | NA | Cheek (5×), forehead (4×), ear (4×), scalp (3×), nose (3×), neck (2×), temple (1×), lip (1×) | 1.045 | NA | No recurrence at 6–51 mo (12×), recurrence at 7–20 mo and no recurrence at 13–50 mo (2×), recurrence at 24 mo (1×), lymph node metastasis and no recurrence at 99 mo—(1×), lymph node metastasis at 8 mo and recurrence at 14 mo—died at 32 mo (2×), died at 7–32 mo (3×), follow-up NA (2×) |
| Svoboda et al., 2021 [33] | 5 | 1 F and 4 M/80.8 | Active hepatitis B infection (1×), Crohn disease treated with azathioprine and history of melanoma (1×), chronic lymphocitic leukemia (1×), HIV and polymiositis treated with tacrolimus and intravenous immunoglobulin (1×) | Scalp (3×), eyebrow (1×), forehead (1×), | 2.5 × 2.2 | PNI (4×) | Excision (5×), adjuvant RT (2×), no recurrence at 6.5–18 mo (4×), newly identified case (1×) |
| Honorato et al., 2024 [34] | 5 | 5 M/68 | Kidney transplant recipients (3×) | Eyebrow (1×), temporal region (1×), forehead (1×), cervical region (1×), scalp (1×) | NA | PNI (3×) and LVI (1×) | Surgical excision (4×), NA (1×), no recurrence at 21–35 mo (2×), recurrence at 36 mo (1×); recurrence, lymph node and lung metastasis at 22 mo resulting in death (1×) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Špiljak, B.; Sauerborn, D.; Tomas, M.; Gregorić Butina, B.; Mahovne, I.; Erić, S.; Vidaković, B.; Lešić, S. Aggressive Squamoid Eccrine Ductal Carcinoma of the Face: A Rare and Challenging Diagnosis—Case Report and Literature Review. Medicina 2025, 61, 612. https://doi.org/10.3390/medicina61040612
Špiljak B, Sauerborn D, Tomas M, Gregorić Butina B, Mahovne I, Erić S, Vidaković B, Lešić S. Aggressive Squamoid Eccrine Ductal Carcinoma of the Face: A Rare and Challenging Diagnosis—Case Report and Literature Review. Medicina. 2025; 61(4):612. https://doi.org/10.3390/medicina61040612
Chicago/Turabian StyleŠpiljak, Bruno, Damir Sauerborn, Matej Tomas, Brankica Gregorić Butina, Ivana Mahovne, Suzana Erić, Bruno Vidaković, and Stjepanka Lešić. 2025. "Aggressive Squamoid Eccrine Ductal Carcinoma of the Face: A Rare and Challenging Diagnosis—Case Report and Literature Review" Medicina 61, no. 4: 612. https://doi.org/10.3390/medicina61040612
APA StyleŠpiljak, B., Sauerborn, D., Tomas, M., Gregorić Butina, B., Mahovne, I., Erić, S., Vidaković, B., & Lešić, S. (2025). Aggressive Squamoid Eccrine Ductal Carcinoma of the Face: A Rare and Challenging Diagnosis—Case Report and Literature Review. Medicina, 61(4), 612. https://doi.org/10.3390/medicina61040612







