Atherogenic Index of Plasma in Metabolic Syndrome—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Strategy
2.2. Inclusion Criteria
2.3. Risk of Bias Assessment in Individual Studies
2.4. Summary Measures and Synthesis of Results
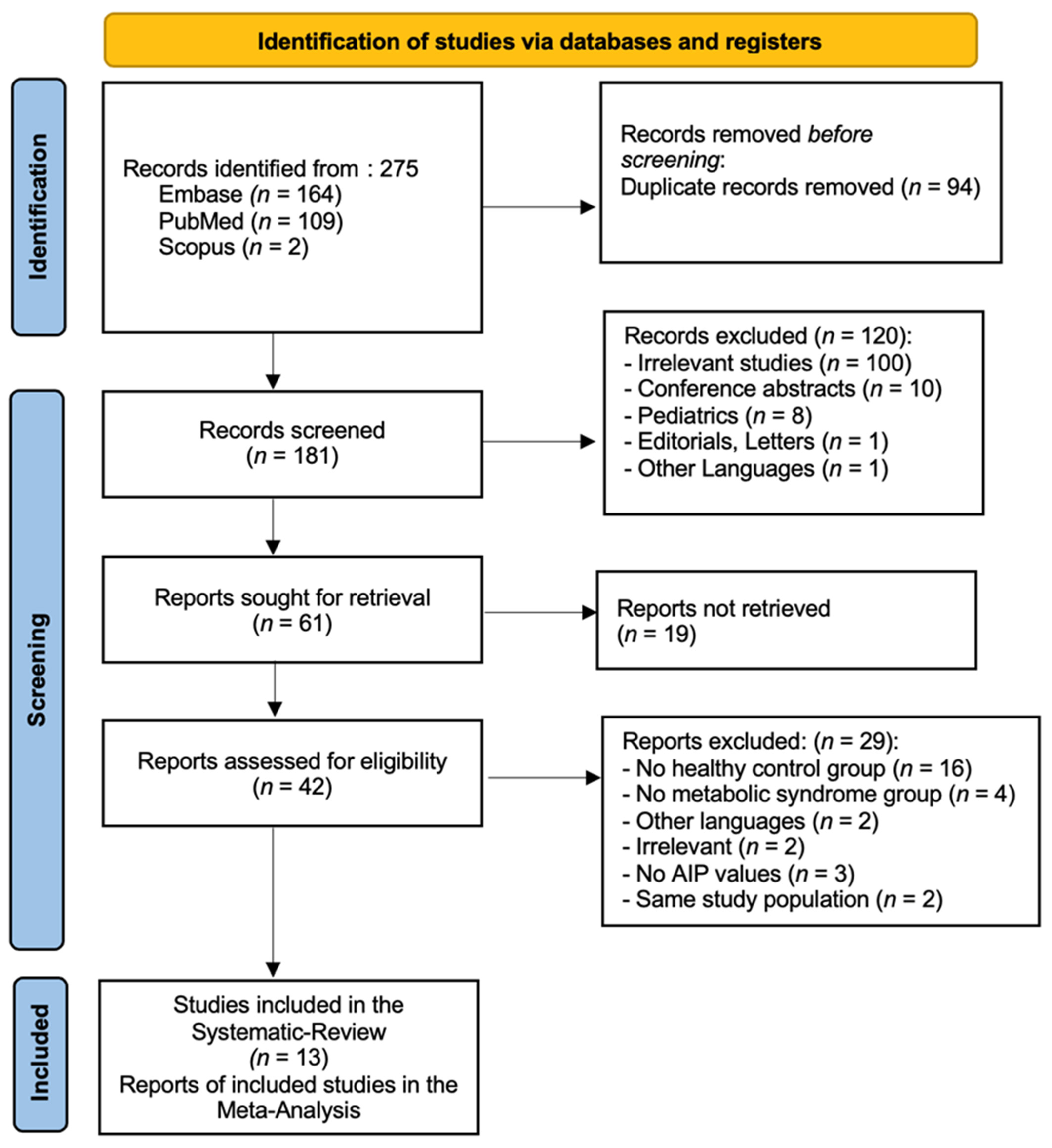
3. Results
3.1. General Results
3.2. Study Characteristics
3.3. Definition of MetS
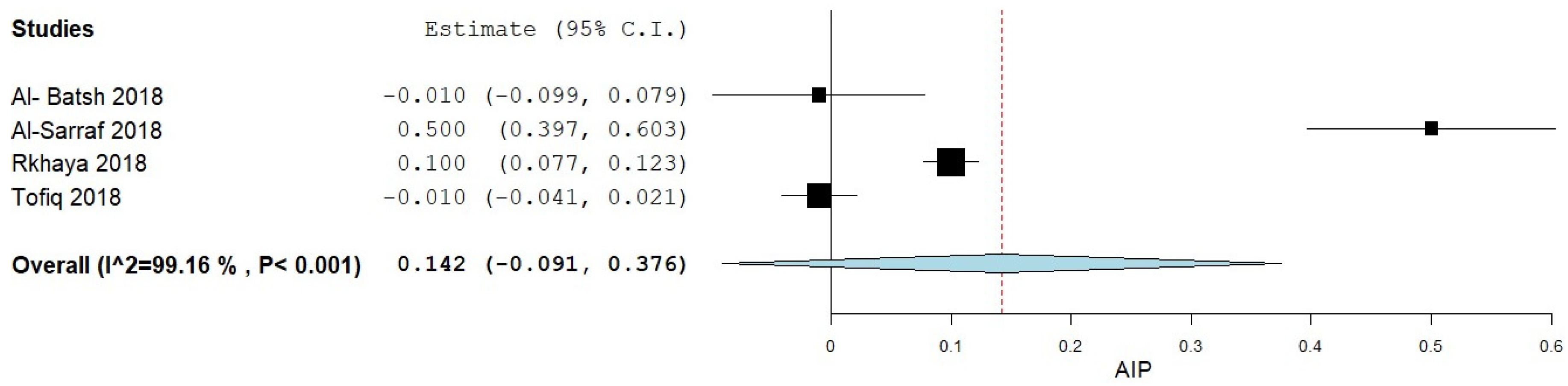
3.4. AIP Levels in MetS
3.4.1. AIP Levels in MetS Patients vs. Controls
3.4.2. Controls vs. Diabetic/Pre-Diabetic
3.5. AIP in Predicting MetS
3.6. Bias Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.-L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, C.; Ma, Y.; Yang, Y.; Liu, F.; Ma, X.; Li, X.; Xie, X.; Chen, B. Association of metabolic syndrome with various anthropometric and atherogenic parameters in the Kazakh population in China. Lipids Health Dis. 2016, 15, 166. [Google Scholar] [CrossRef]
- Dobiásová, M. AIP—Atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice. Vnitr. Lek. 2006, 52, 64–71. [Google Scholar]
- Wu, T.-T.; Gao, Y.; Zheng, Y.-Y.; Ma, Y.-T.; Xie, X. Atherogenic index of plasma (AIP): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018, 17, 197. [Google Scholar] [CrossRef]
- Wu, T.-T.; Zheng, Y.-Y.; Yang, Y.-N.; Li, X.-M.; Ma, Y.-T.; Xie, X. Age, Sex, and Cardiovascular Risk Attributable to Lipoprotein Cholesterol Among Chinese Individuals with Coronary Artery Disease: A Case–Control Study. Metab. Syndr. Relat. Disord. 2019, 17, 223–231. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 241–284. [Google Scholar]
- Bortolasci, C.C.; Vargas, H.O.; Nunes, S.O.V.; de Melo, L.G.P.; de Castro, M.R.P.; Moreira, E.G.; Dodd, S.; Barbosa, D.S.; Berk, M.; Maes, M. Factors influencing insulin resistance in relation to atherogenicity in mood disorders, the metabolic syndrome and tobacco use disorder. J. Affect. Disord. 2015, 179, 148–155. [Google Scholar] [CrossRef]
- Maia, D.G.; Augusto, K.L.; Bezerra, M.C.; Rodrigues, C.E.M. Metabolic syndrome in patients with ankylosing spondylitis receiving anti-TNF? therapy: Association with predictors of cardiovascular risk. Clin. Rheumatol. 2017, 2, 1129–2376. [Google Scholar] [CrossRef]
- Al-Batsh, M.M.W.; Albsoul-Younes, A.; Kasabri, V.; Suyagh, M.; Alalawi, S.; Yasin, N. Proportional correlates of cystatin-C with pentraxin-3, visceral adiposity index and atherogenicity index of plasma but not blood indices in metabolic syndrome patients with and without prediabetes. Horm. Mol. Biol. Clin. Investig. 2018, 36, 20180058. [Google Scholar] [CrossRef] [PubMed]
- Al-Sarraf, I.A.K.; Kasabri, V.; Akour, A.; Naffa, R. Melatonin and cryptochrome 2 in metabolic syndrome patients with or without diabetes: A cross-sectional study. Horm. Mol. Biol. Clin. Investig. 2018, 35, 20180016. [Google Scholar] [CrossRef]
- Abu Rkhaya, S.; Bulatova, N.; Kasabri, V.; Naffa, R.; Alquoqa, R. Increased malondialdehyde vs. reduced sirtuin 1 in relation with adiposity, atherogenicity and hematological indices in metabolic syndrome patients with and without prediabetes. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Tofiq, K.O.; Bulatova, N.; Kasabri, V.; Suyagh, M.; Halaseh, L.; Alalawi, S. Increased lipocalin-2 vs reduced oxytocin in relation with adiposity, atherogenicity and hematological indices in metabolic syndrome patients with and without prediabetes. Bratisl. Med. J. 2019, 119, 762–769. [Google Scholar] [CrossRef]
- Štěpánek, L.; Horáková, L.; Cibičková, Ľ.; Karásek, D.; Vaverková, H.; Nakládalová, M.; Juríčková, Ľ.; Kollárová, H. Associations Between Homeostasis Model Assessment (HOMA) and Routinely Examined Parameters in Individuals with Metabolic Syndrome. Physiol. Res. 2019, 68, 921–930. [Google Scholar] [CrossRef]
- Amirkhizi, F.; Khademi, Z.; Hamedi−Shahraki, S.; Rahimlou, M. Vitamin D insufficiency and its association with adipokines and atherogenic indices in patients with metabolic syndrome: A case-control study. Front. Endocrinol. 2023, 14, 1080138. [Google Scholar] [CrossRef]
- Mohammedsaeed, W.; Ahmed, A.; Alharbi, N.; Aljohani, A.; Alruwaithi, R.; Alharbi, R.; Alahmadi, S. Evaluation of Adiponectin and ANGPTL8 in Women With Metabolic Syndrome in the Madinah Region of Saudi Arabia. Cureus 2023, 15, e44219. [Google Scholar] [CrossRef] [PubMed]
- Tien, Y.-T.; Wang, L.-J.; Lee, Y.; Lin, P.-Y.; Hung, C.-F.; Chong, M.-Y.; Huang, Y.-C. Comparative predictive efficacy of atherogenic indices on metabolic syndrome in patients with schizophrenia. Schizophr. Res. 2023, 262, 95–101. [Google Scholar] [CrossRef]
- Rattanatham, R.; Tangpong, J.; Chatatikun, M.; Sun, D.; Kawakami, F.; Imai, M.; Klangbud, W.K. Assessment of eight insulin resistance surrogate indexes for predicting metabolic syndrome and hypertension in Thai law enforcement officers. PeerJ 2023, 11, e15463. [Google Scholar] [CrossRef]
- Abolnezhadian, F.; Hosseini, S.A.; Alipour, M.; Zakerkish, M.; Cheraghian, B.; Ghandil, P.; Cheraghpour, M. Association Metabolic Obesity Phenotypes with Cardiometabolic Index, Atherogenic Index of Plasma and Novel Anthropometric Indices: A Link of FTO-rs9939609 Polymorphism. Vasc. Health Risk Manag. 2020, 16, 249–256. [Google Scholar] [CrossRef]
- Li, Y.-W.; Kao, T.-W.; Chang, P.-K.; Chen, W.-L.; Wu, L.-W. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: A 9-year longitudinal study. Sci. Rep. 2021, 11, 9900. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Pucci, G.; Alcidi, R.; Tap, L.; Battista, F.; Mattace-Raso, F.; Schillaci, G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol. Res. 2017, 120, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Swarup, S.; Ahmed, I.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, K.C.; Tzima, G.I.; Tentolouris, K.N.; Vasileiadis, A.I. Common Pathogenetic Pathways of Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2023, 19, 96–114. [Google Scholar] [CrossRef]
- Borzan, V.; Lerchbaum, E.; Missbrenner, C.; Heijboer, A.C.; Goschnik, M.; Trummer, C.; Theiler-Schwetz, V.; Haudum, C.; Gumpold, R.; Schweighofer, N.; et al. Risk of Insulin Resistance and Metabolic Syndrome in Women with Hyperandrogenemia: A Comparison between PCOS Phenotypes and Beyond. J. Clin. Med. 2021, 10, 829. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Bittner, V.A. The New 2019 AHA/ACC Guideline on the Primary Prevention of Cardiovascular Disease. Circulation 2020, 142, 2402–2404. [Google Scholar] [CrossRef]
- Liu, H.; Liu, K.; Pei, L.; Li, S.; Zhao, J.; Zhang, K.; Zong, C.; Zhao, L.; Fang, H.; Wu, J.; et al. Atherogenic Index of Plasma Predicts Outcomes in Acute Ischemic Stroke. Front. Neurol. 2021, 12, 741754. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, Q.; Wei, Z.; Wei, J.; Cui, M. Atherogenic Index of Plasma and Coronary Artery Disease in the Adult Population: A Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 817441. [Google Scholar] [CrossRef]
- Xue, J.; He, L.; Xie, H.; Xie, X.; Wang, H. An Inverse Correlation between the Atherogenic Index of Plasma and Heart Failure: An Analysis of the National Health and Nutrition Examination Survey 2017–March 2020 Pre-Pandemic Data. J. Cardiovasc. Dev. Dis. 2022, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yu, L.; Zhou, H.; Ma, Q.; Zhou, X.; Lei, T.; Hu, J.; Xu, W.; Yi, N.; Lei, S. Atherogenic index of plasma is a novel and better biomarker associated with obesity: A population-based cross-sectional study in China. Lipids Health Dis. 2018, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, Z.; Heshka, S.; Heo, M.; Faith, M.S.; Heymsfield, S.B. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: Clinical action thresholds. Am. J. Clin. Nutr. 2002, 76, 743–749. [Google Scholar] [CrossRef]
- Hanson, R.L.; Imperatore, G.; Bennett, P.H.; Knowler, W.C. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes 2002, 51, 3120–3127. [Google Scholar]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef]
- Laaksonen, D.E.; Lakka, H.-M.; Niskanen, L.K.; Kaplan, G.A.; Salonen, J.T.; Lakka, T.A. Metabolic Syndrome and Development of Diabetes Mellitus: Application and Validation of Recently Suggested Definitions of the Metabolic Syndrome in a Prospective Cohort Study. Am. J. Epidemiol. 2002, 156, 1070–1077. [Google Scholar] [CrossRef]
- Ford, E.S.; Li, C.; Sattar, N. Metabolic syndrome and incident diabetes: Current state of the evidence. Diabetes Care 2008, 31, 1898–1904. [Google Scholar] [CrossRef]
- Lioy, B.; Webb, R.J.; Amirabdollahian, F. The Association between the Atherogenic Index of Plasma and Cardiometabolic Risk Factors: A Review. Healthcare 2023, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Macías, J.C.; Ochoa-Martínez, A.C.; Varela-Silva, J.A.; Pérez-Maldonado, I.N. Atherogenic Index of Plasma: Novel Predictive Biomarker for Cardiovascular Illnesses. Arch. Med. Res. 2019, 50, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-W.; Deng, F.-Y.; Lei, S.-F. Meta-analysis of Atherogenic Index of Plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim. Care Diabetes 2015, 9, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Wu, Z.; Xia, Y.; Xiao, S.; Chen, L.; Li, Y. Non-linear association of atherogenic index of plasma with insulin resistance and type 2 diabetes: A cross-sectional study. Cardiovasc. Diabetol. 2023, 22, 157. [Google Scholar] [CrossRef]
- Edwards, C.M.; Cusi, K. Prediabetes: A Worldwide Epidemic. Endocrinol. Metab. Clin. N. Am. 2016, 45, 751–764. [Google Scholar] [CrossRef]
- Kim, J.J.; Yoon, J.; Lee, Y.-J.; Park, B.; Jung, D.-H. Predictive Value of the Atherogenic Index of Plasma (AIP) for the Risk of Incident Ischemic Heart Disease among Non-Diabetic Koreans. Nutrients 2021, 13, 3231. [Google Scholar] [CrossRef]
- Hu, Y.-M.; Tian, H.-M.; Liu, R.; Chen, X. Atherogenic index of plasma is associated with carotid intima-media thickness in patients with type 2 diabetes mellitus. Sichuan Da Xue Xue Bao Yi Xue Ban 2004, 35, 696–698, 707. [Google Scholar]
- Manohar, S.M.; Vaikasuvu, S.R.; Deepthi, K.; Sachan, A.; Narasimha, S.R. An association of hyperglycemia with plasma malondialdehyde and atherogenic lipid risk factors in newly diagnosed Type 2 diabetic patients. J. Res. Med. Sci. 2013, 18, 89–93. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andraschko, L.M.; Gazi, G.; Leucuta, D.-C.; Popa, S.-L.; Chis, B.A.; Ismaiel, A. Atherogenic Index of Plasma in Metabolic Syndrome—A Systematic Review and Meta-Analysis. Medicina 2025, 61, 611. https://doi.org/10.3390/medicina61040611
Andraschko LM, Gazi G, Leucuta D-C, Popa S-L, Chis BA, Ismaiel A. Atherogenic Index of Plasma in Metabolic Syndrome—A Systematic Review and Meta-Analysis. Medicina. 2025; 61(4):611. https://doi.org/10.3390/medicina61040611
Chicago/Turabian StyleAndraschko, Leia Mossane, Gabi Gazi, Daniel-Corneliu Leucuta, Stefan-Lucian Popa, Bogdan Augustin Chis, and Abdulrahman Ismaiel. 2025. "Atherogenic Index of Plasma in Metabolic Syndrome—A Systematic Review and Meta-Analysis" Medicina 61, no. 4: 611. https://doi.org/10.3390/medicina61040611
APA StyleAndraschko, L. M., Gazi, G., Leucuta, D.-C., Popa, S.-L., Chis, B. A., & Ismaiel, A. (2025). Atherogenic Index of Plasma in Metabolic Syndrome—A Systematic Review and Meta-Analysis. Medicina, 61(4), 611. https://doi.org/10.3390/medicina61040611





