Serum Endocan Levels as a Risk Factor for Peripheral Artery Disease in Non-Dialysis Patients with Chronic Kidney Disease Stages 3–5
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Analysis
2.3. Biochemical Investigations
2.4. Blood Pressure and ABI Measurements
2.5. Statistical Analysis
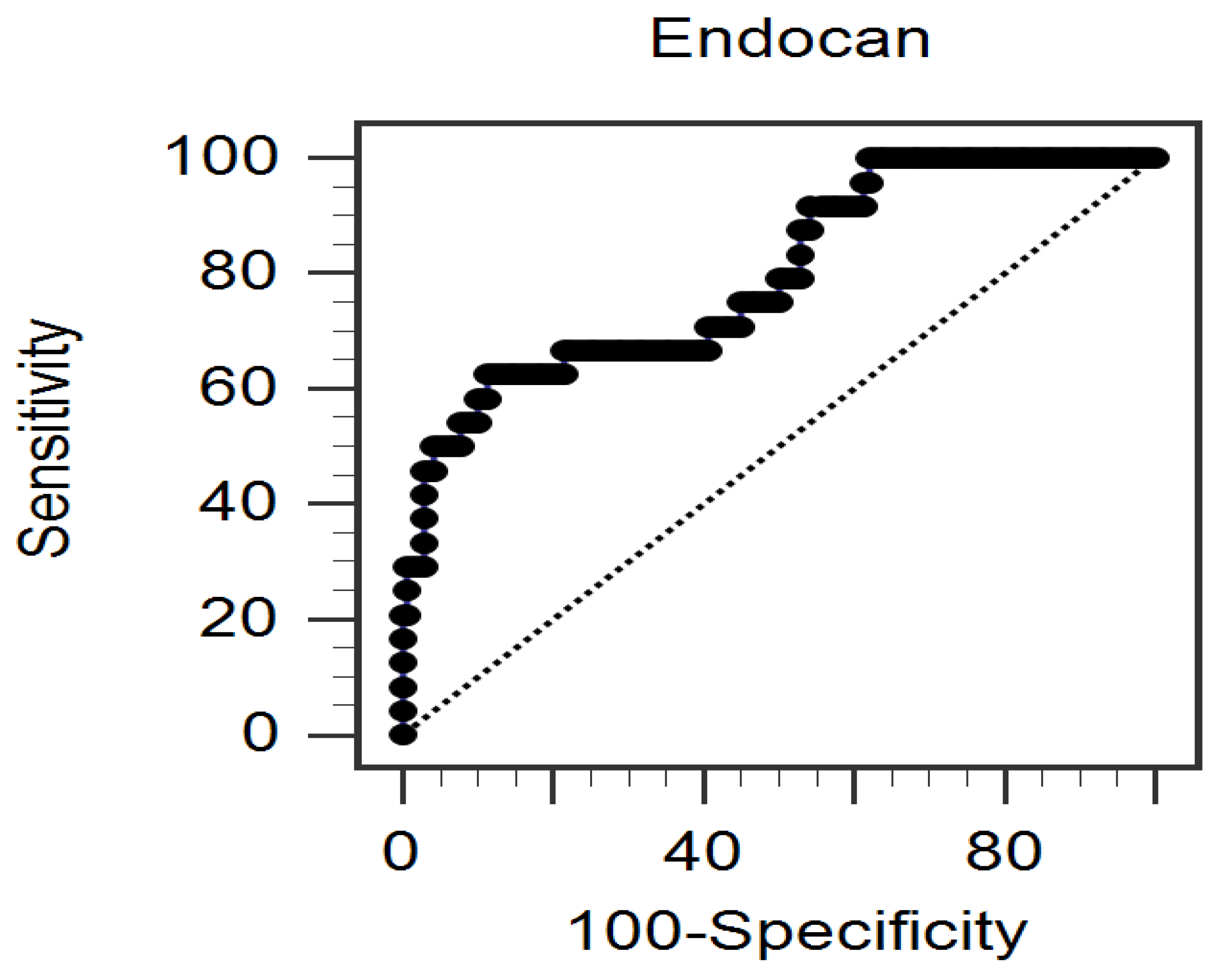
3. Results
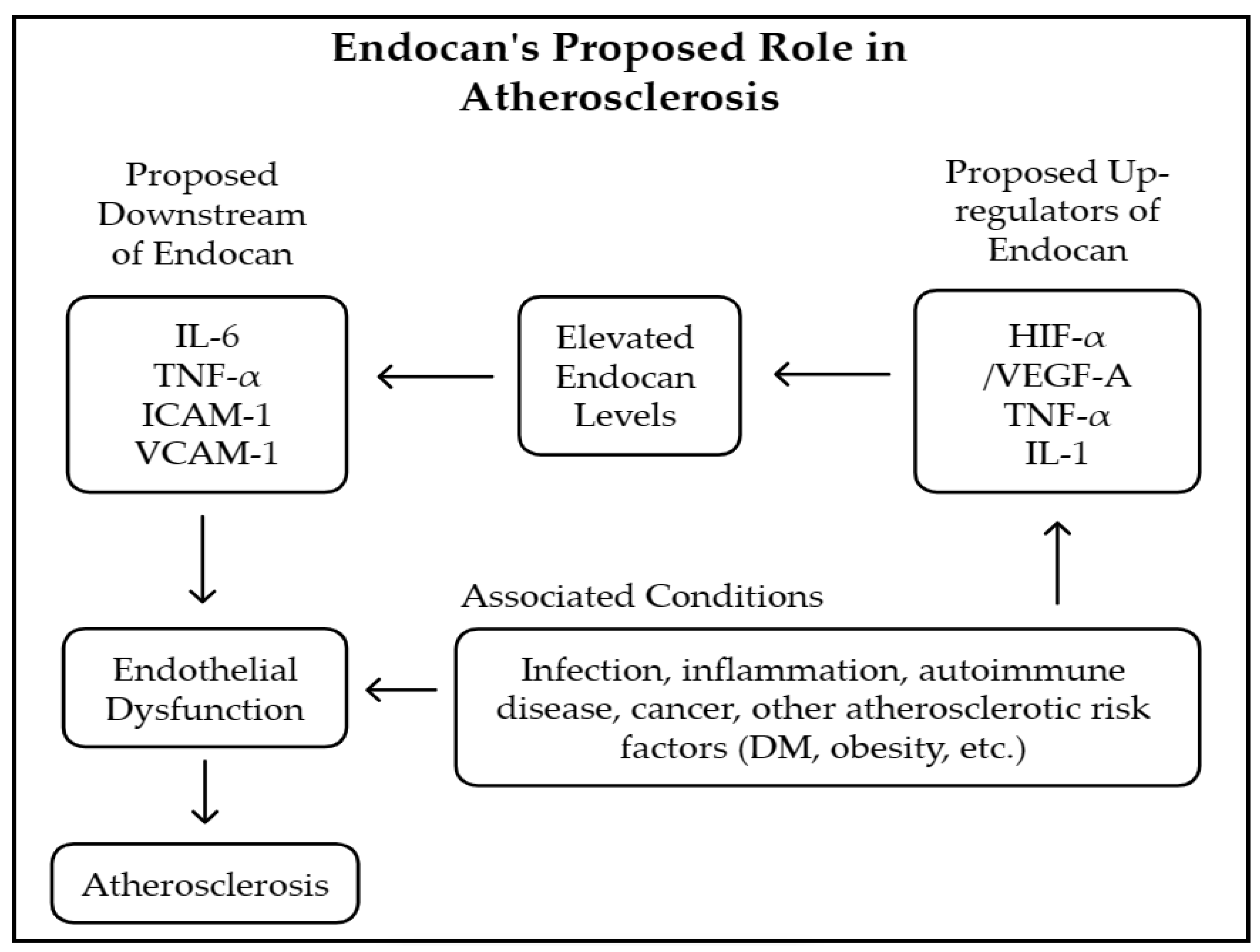
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adedokun, S.D.; Sarwar, M.; Hwang, K.; Hans, A.; Baskaran, J.; Anantha Narayanan, M. Outcomes of lower extremity peripheral arterial interventions in patients with and without chronic kidney disease or end-stage renal disease. J. Cardiovasc. Surg. (Torino) 2023, 64, 624–633. [Google Scholar] [CrossRef]
- O’Hare, A.M.; Vittinghoff, E.; Hsia, J.; Shlipak, M.G. Renal insufficiency and the risk of lower extremity peripheral arterial disease: Results from the heart and estrogen/progestin replacement study (HERS). J. Am. Soc. Nephrol. 2004, 15, 1046–1051. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wattanakit, K.; Folsom, A.R.; Selvin, E.; Coresh, J.; Hirsch, A.T.; Weatherley, B.D. Kidney function and risk of peripheral arterial disease: Results from the atherosclerosis risk in communities (ARIC) study. J. Am. Soc. Nephrol. 2007, 18, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Ballew, S.H.; Coresh, J. Influence of chronic kidney disease on cardiac structure and function. Curr. Hypertens. Rep. 2015, 17, 581. [Google Scholar] [CrossRef]
- Spertus, J.; Jones, P.; Poler, S.; Rocha-Singh, K. The peripheral artery questionnaire: A new disease-specific health status measure for patients with peripheral arterial disease. Am. Heart J. 2004, 147, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Eagle, K.A.; Ohman, E.M.; Hirsch, A.T.; Goto, S.; Mahoney, E.M.; Wilson, P.W.; Alberts, M.J.; D’Agostino, R.; Liau, C.S.; et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010, 04, 1350–1357. [Google Scholar] [CrossRef]
- Okamoto, S.; Iida, O.; Mano, T. Current perspective on hemodialysis patients with peripheral artery disease. Ann. Vasc. Dis. 2017, 10, 88–91. [Google Scholar] [CrossRef]
- Mantha, Y.; Asif, A.; Fath, A.; Prasad, A. Implications of kidney disease in patients with peripheral arterial disease and vascular calcification. Interv. Cardiol. Clin. 2023, 12, 531–538. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2017, 135, e686–e725. [Google Scholar] [CrossRef]
- Cozzolino, M.; Ciceri, P.; Galassi, A.; Mangano, M.; Carugo, S.; Capelli, I.; Cianciolo, G. The key role of phosphate on vascular calcification. Toxins 2019, 11, 213. [Google Scholar] [CrossRef]
- Nelson, A.J.; Raggi, P.; Wolf, M.; Gold, A.M.; Chertow, G.M.; Roe, M.T. Targeting vascular calcification in chronic kidney disease. JACC Basic Transl. Sci. 2020, 5, 398–412. [Google Scholar] [CrossRef]
- Nalewajska, M.; Gurazda, K.; Marchelek-Myśliwiec, M.; Pawlik, A.; Dziedziejko, V. The role of endocan in selected kidney diseases. Int. J. Mol. Sci. 2020, 21, 6119. [Google Scholar] [CrossRef] [PubMed]
- Khalaji, A.; Behnoush, A.H.; Kia, Y.M.; Alehossein, P.; Bahiraie, P. High circulating endocan in chronic kidney disease? A systematic review and meta-analysis. PLoS ONE 2023, 18, e0289710. [Google Scholar] [CrossRef] [PubMed]
- Behnoush, A.H.; Khalaji, A.; Bahiraie, P.; Alehossein, P.; Shobeiri, P.; Peisepar, M.; Cannavo, A. Endocan as a marker of endothelial dysfunction in hypertension: A systematic review and meta-analysis. Hypertens. Res. 2023, 46, 2388–2399. [Google Scholar] [CrossRef]
- Khalaji, A.; Behnoush, A.H.; Saeedian, B.; Khanmohammadi, S.; Shokri Varniab, Z.; Peiman, S. Endocan in prediabetes, diabetes, and diabetes-related complications: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2023, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I.; Siriopol, D.; Saglam, M.; Kurt, Y.G.; Unal, H.U.; Eyileten, T.; Gok, M.; Cetinkaya, H.; Oguz, Y.; Sari, S.; et al. Plasma endocan levels associate with inflammation, vascular abnormalities, cardiovascular events, and survival in chronic kidney disease. Kidney Int. 2014, 86, 1213–1220. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Escobar, C.; Blanes, I.; Ruiz, A.; Vinuesa, D.; Montero, M.; Rodríguez, M.; Barbera, G.; Manzano, L. Prevalence and clinical profile and management of peripheral arterial disease in elderly patients with diabetes. Eur. J. Intern. Med. 2011, 22, 275–281. [Google Scholar] [CrossRef]
- Anderson, T.J.; Grégoire, J.; Hegele, R.A.; Couture, P.; Mancini, G.B.; McPherson, R.; Francis, G.A.; Poirier, P.; Lau, D.C.; Grover, S.; et al. 2012 Update of the Canadian cardiovascular society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can. J. Cardiol. 2013, 29, 151–167. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Arinze, N.V.; Gregory, A.; Francis, J.M.; Farber, A.; Chitalia, V.C. Unique aspects of peripheral artery disease in patients with chronic kidney disease. Vasc. Med. 2019, 24, 251–260. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; et al. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index: US preventive services task force recommendation statement. JAMA 2018, 320, 177–183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- NKF. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am. J. Kidney Dis. 2005, 45 (Suppl 3), S1–S153. [Google Scholar]
- Mazzolai, L.; Teixido-Tura, G.; Lanzi, S.; Boc, V.; Bossone, E.; Brodmann, M.; Bura-Rivière, A.; De Backer, J.; Deglise, S.; Della Corte, A.; et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J. 2024, 45, 3538–3700. [Google Scholar] [CrossRef]
- Pan, C.F.; Chuang, S.M.; Lin, K.C.; Tsai, M.C.; Liao, W.T.; Zeng, Y.H.; Lee, C.C. Risk associated with estimated glomerular filtration rate and albuminuria for PAD among patients with type 2 diabetes. J. Investig. Med. 2021, 69, 1182–1188. [Google Scholar] [CrossRef]
- Sinjari, H.Y. Prevalence, clinical profile, and risk factors of peripheral artery disease in an Iraqi cohort of chronic kidney disease. Medicine 2023, 102, e35577. [Google Scholar] [CrossRef]
- Balta, S.; Mikhailidis, D.P.; Demirkol, S.; Ozturk, C.; Celik, T.; Iyisoy, A. Endocan: A novel inflammatory indicator in cardiovascular disease? Atherosclerosis 2015, 243, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Balta, S.; Balta, I.; Mikhailidis, D.P. Endocan: A new marker of endothelial function. Curr. Opin. Cardiol. 2021, 36, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.; Albino-Teixeira, A.; Reina-Couto, M.; Sousa, T. Endocan: A novel biomarker for risk stratification, prognosis and therapeutic monitoring in human cardiovascular and renal diseases. Clin. Chim. Acta 2020, 509, 310–335. [Google Scholar] [CrossRef]
- Lin, L.Y.; Chang, T.T.; Leu, H.B.; Huang, C.C.; Wu, T.C.; Chou, R.H.; Huang, P.H.; Yin, W.H.; Tseng, W.K.; Wu, Y.W.; et al. Novel prognostic impact and cell specific role of endocan in patients with coronary artery disease. Clin. Res. Cardiol. 2025, in press. [CrossRef]
- Pawlak, K.; Mysliwiec, M.; Pawlak, D. Endocan—the new endothelial activation marker independently associated with soluble endothelial adhesion molecules in uraemic patients with cardiovascular disease. Clin. Biochem. 2015, 48, 425–430. [Google Scholar] [CrossRef]
- Kim, J.S.; Ko, G.J.; Kim, Y.G.; Lee, S.Y.; Lee, D.Y.; Jeong, K.H.; Lee, S.H. Plasma endocan as a predictor of cardiovascular event in patients with end-stage renal disease on hemodialysis. J. Clin. Med. 2020, 9, 4086. [Google Scholar] [CrossRef] [PubMed]
- El-Senosy, F.M.; Abd El Aziz, R.E.M.; Kasim, S.A.; Gad, L.A.; Hassan, D.A.; Sabry, S.; El Mancy, I.M.; Shawky, T.A.; Mohamed, I.G.R.; Elmonier, R.; et al. Serum endocan levels and subclinical atherosclerosis in patients with chronic kidney and end-stage renal diseases. Int. J. Clin. Pract. 2022, 2022, 4524637. [Google Scholar] [CrossRef] [PubMed]
- Yazman, S.; Depboylu, B.C.; Saruhan, E.; Cenikli, U.; Harmandar, B.; Arslan, K.; İlhan, G.; İştar, H. New biomarkers (endocan, interleukin-17, and thrombospondin-4) for the diagnosis, assessment of severity, and follow-up of peripheral arterial disease. Angiology 2023, 74, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Scuruchi, M.; D’Ascola, A.; Avenoso, A.; Mandraffino, G.; Campo, S.; Campo, G.M. Endocan, a Novel Inflammatory Marker, Is Upregulated in Human Chondrocytes Stimulated with IL-1 Beta. Mol. Cell. Biochem. 2021, 476, 1589–1597. [Google Scholar] [CrossRef]
- Voiosu, T.; Bălănescu, P.; Bengus, A.; Voiosu, A.; Baicus, C.; Barbu, M.A.; Lădaru, A.; Nitipir, C.; Mateescu, B.; Diculescu, M.; et al. Serum Endocan Levels Are Increased in Patients with Inflammatory Bowel Disease. Clin. Lab. 2014, 60, 505–510. [Google Scholar] [CrossRef]
- El-Kamshoushi, A.A.M.; Hassan, E.M.; El Abd, A.M.; Hassan, S.Z.; Maher, A.A. Serum Endocan as a Predictive Biomarker of Cardiovascular Risk in Erectile Dysfunction Patients. Andrologia 2018, 50, e13113. [Google Scholar] [CrossRef]
- Wu, X.; Luo, J.; Huang, W.; Jia, B.; Luo, T. Role of Ascitic Endocan Levels in the Diagnosis of Spontaneous Bacterial Peritonitis in Decompensated Cirrhosis. Biomarkers 2020, 25, 360–366. [Google Scholar] [CrossRef]
- Kumar, S.K.; Mani, K.P. Proinflammatory Signaling Mechanism of Endocan in Macrophages: Involvement of TLR2 Mediated MAPK-NFkB Pathways. Cytokine 2024, 175, 156482. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, L.; Yu, X.-H.; Hu, M.; Zhang, Y.; Liu, X.; He, P.; Ouyang, X.-P. Endocan: A Key Player of Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 8, 798699. [Google Scholar] [CrossRef]
- Chen, J.M.; He, H.; Starcke, C.C.; Guo, Y.; Geng, S.; Chen, C.-S.; Mahone, E.B.; Batuman, V.; Hamm, L.L.; He, J. Accuracy of Ankle-Brachial Index, Toe-Brachial Index, and Risk Classification Score in Discriminating Peripheral Artery Disease in Patients With Chronic Kidney Disease. Am. J. Cardiol. 2021, 160, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-G.; Seo, J.; Kim, G.S.; Jin, M.N.; Lee, H.; Byun, Y.S.; Kim, B.-K. Elevated C-Reactive Protein/Albumin Ratio Is Associated With Lesion Complexity, Multilevel Involvement, and Adverse Outcomes in Patients With Peripheral Artery Disease. Angiology 2022, 73, 843–851. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| CKD stages 3 to 5 | Dialysis |
| Adults (not less than 18 years old) | Acute infection |
| Malignancy | |
| Stroke | |
| Heart failure | |
| Under cilostazol or pentoxifylline | |
| ABI > 1.3 |
| Characteristics | All Patients (n = 164) | Control Group (n = 140) | Low-ABI Group (n = 24) | p Value |
|---|---|---|---|---|
| Age (years) | 68.48 ± 13.37 | 66.06 ± 12.55 | 80.54 ± 11.33 | <0.001 * |
| Body mass index (kg/m2) | 26.35 ± 4.80 | 26.58 ± 4.58 | 24.98 ± 5.85 | 0.132 |
| Left-ankle–brachial index | 1.07 (0.99–1.13) | 1.08 (1.03–1.14) | 0.77 (0.66–0.85) | <0.001 * |
| Right-ankle–brachial index | 1.09 (1.02–1.14) | 1.10 (1.05–1.14) | 0.78 (0.67–0.87) | <0.001 * |
| Hemoglobin (g/dL) | 11.67 ± 2.48 | 11.78 ± 2.57 | 11.04 ± 1.77 | 0.179 |
| Total cholesterol (mg/dL) | 159.11 ± 33.53 | 158.71 ± 33.71 | 161.46 ± 30.03 | 0.712 |
| Triglyceride (mg/dL) | 119.00 (87.25–164.00) | 119.00 (86.25–163.50) | 117.50 (91.75–166.25) | 0.867 |
| LDL-C (mg/dL) | 91.45 ± 29.46 | 90.67 ± 28.35 | 96.00 ± 33.82 | 0.410 |
| Fasting glucose (mg/dL) | 104.50 (96.00–133.00) | 101.50 (96.00–130.75) | 109.50 (95.00–142.50) | 0.310 |
| Albumin (g/dL) | 4.04 ± 0.33 | 4.06 ± 0.34 | 3.95 ± 0.26 | 0.145 |
| Blood urea nitrogen (mg/dL) | 35.50 (26.00–48.00) | 38.00 (26.00–49.00) | 32.00 (26.00–38.75) | 0.153 |
| Creatinine (mg/dL) | 2.00 (1.50–2.78) | 2.10 (1.50–2.98) | 1.80 (1.40–2.28) | 0.117 |
| eGFR (mL/min) | 30.42 ± 14.51 | 30.26 ± 15.07 | 31.38 ± 10.82 | 0.729 |
| UPCR (mg/g) | 182.00 (71.55–453.00) | 131.00 (61.95–475.75) | 266.80 (187.50–344.75) | 0.031 * |
| CRP (mg/dL) | 0.07 (0.05–0.64) | 0.05 (0.05–0.47) | 0.56 (0.05–2.64) | 0.027 * |
| Endocan (ng/mL) | 15.75 (9.30–24.12) | 14.74 (8.73–21.71) | 29.42 (15.08–46.07) | <0.001 * |
| Female, n (%) | 67 (40.9) | 57 (40.7) | 10 (41.7) | 0.930 |
| Diabetes mellitus, n (%) | 51 (31.1) | 39 (27.9) | 12 (50.0) | 0.030 * |
| Hypertension, n (%) | 134 (81.7) | 114 (81.4) | 20 (83.3) | 0.824 |
| Glomerulonephritis, n (%) | 25 (15.2) | 21 (15.0) | 4 (16.7) | 0.834 |
| Current smoking, n (%) | 22 (13.4) | 18 (12.9) | 4 (16.7) | 0.613 |
| CKD stage 3, n (%) | 76 (46.3) | 63 (45.0) | 13 (54.2) | 0.322 |
| CKD stage 4, n (%) | 56 (34.1) | 47 (33.6) | 9 (37.5) | |
| CKD stage 5, n (%) | 32 (19.5) | 30 (21.4) | 2 (8.3) |
| Variables | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Endocan, 1 ng/mL | 1.098 | 1.042–1.157 | 0.001 * |
| Age, 1 year | 1.097 | 1.038–1.159 | 0.001 * |
| Diabetes mellitus, present | 3.437 | 1.053–11.225 | 0.041 * |
| C-reactive protein, 1 mg/dL | 1.119 | 0.816–1.535 | 0.486 |
| UPCR, 1 mg/g | 1.000 | 0.999–1.001 | 0.795 |
| Variables | Left Log-ABI | Right Log-ABI | Log-Endocan (ng/mL) | |||
|---|---|---|---|---|---|---|
| Spearman Coefficient of Correlation | p Value | Spearman Coefficient of Correlation | p Value | Spearman Coefficient of Correlation | p Value | |
| Age (years) | –0.401 | <0.001 * | –0.367 | <0.001 * | 0.129 | 0.099 |
| Body mass index (kg/m2) | 0.127 | 0.105 | 0.142 | 0.070 | 0.077 | 0.329 |
| Left log-ABI | — | — | 0.905 | <0.001 * | –0.347 | <0.001 * |
| Right log-ABI | 0.905 | <0.001 * | — | — | –0.320 | <0.001 * |
| Log-endocan (ng/mL) | –0.347 | <0.001 * | –0.320 | <0.001 * | — | — |
| SBP (mmHg) | –0.117 | 0.137 | –0.146 | 0.063 | –0.055 | 0.483 |
| DBP (mmHg) | 0.122 | 0.120 | 0.124 | 0.113 | –0.152 | 0.053 |
| Hemoglobin (g/dL) | 0.080 | 0.308 | 0.048 | 0.544 | –0.013 | 0.871 |
| Total cholesterol (mg/dL) | –0.055 | 0.484 | –0.074 | 0.349 | 0.116 | 0.138 |
| Log-triglyceride (mg/dL) | –0.017 | 0.832 | –0.035 | 0.659 | 0.157 | 0.045 * |
| LDL-C (mg/dL) | –0.052 | 0.510 | –0.083 | 0.289 | 0.136 | 0.082 |
| Log-glucose (mg/dL) | –0.042 | 0.592 | –0.091 | 0.245 | 0.116 | 0.140 |
| Albumin (g/dL) | 0.125 | 0.112 | 0.106 | 0.177 | 0.024 | 0.763 |
| Log-BUN (mg/dL) | 0.062 | 0.432 | 0.063 | 0.420 | –0.108 | 0.167 |
| Log-creatinine (mg/dL) | 0.142 | 0.070 | 0.136 | 0.082 | –0.096 | 0.222 |
| eGFR (mL/min) | 0.006 | 0.943 | –0.013 | 0.865 | 0.080 | 0.309 |
| Log-UPCR (mg/g) | –0.117 | 0.135 | –0.115 | 0.144 | –0.016 | 0.844 |
| Log-CRP (mg/L) | –0.295 | <0.001 * | –0.312 | <0.001 * | 0.195 | 0.012 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, K.-J.; Chang, H.-T.; Chern, Y.-B.; Wu, C.-F.; Tsai, J.-P.; Hsu, B.-G. Serum Endocan Levels as a Risk Factor for Peripheral Artery Disease in Non-Dialysis Patients with Chronic Kidney Disease Stages 3–5. Medicina 2025, 61, 577. https://doi.org/10.3390/medicina61040577
Cheng K-J, Chang H-T, Chern Y-B, Wu C-F, Tsai J-P, Hsu B-G. Serum Endocan Levels as a Risk Factor for Peripheral Artery Disease in Non-Dialysis Patients with Chronic Kidney Disease Stages 3–5. Medicina. 2025; 61(4):577. https://doi.org/10.3390/medicina61040577
Chicago/Turabian StyleCheng, Kai-Jen, Hsiao-Teng Chang, Yahn-Bor Chern, Chun-Feng Wu, Jen-Pi Tsai, and Bang-Gee Hsu. 2025. "Serum Endocan Levels as a Risk Factor for Peripheral Artery Disease in Non-Dialysis Patients with Chronic Kidney Disease Stages 3–5" Medicina 61, no. 4: 577. https://doi.org/10.3390/medicina61040577
APA StyleCheng, K.-J., Chang, H.-T., Chern, Y.-B., Wu, C.-F., Tsai, J.-P., & Hsu, B.-G. (2025). Serum Endocan Levels as a Risk Factor for Peripheral Artery Disease in Non-Dialysis Patients with Chronic Kidney Disease Stages 3–5. Medicina, 61(4), 577. https://doi.org/10.3390/medicina61040577








