Incidence and Prognostic Significance of Hormonal Receptors and HER2 Status Conversion in Recurrent Breast Cancer: A Retrospective Study in a Single Institute
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Patient Selection
2.3. Data Collection Tool
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Study Participants
3.2. Changing Patterns in Tumor Staging, Locoregional Progression, and Biomarker Profiles of Primary and Recurrent Breast Cancer
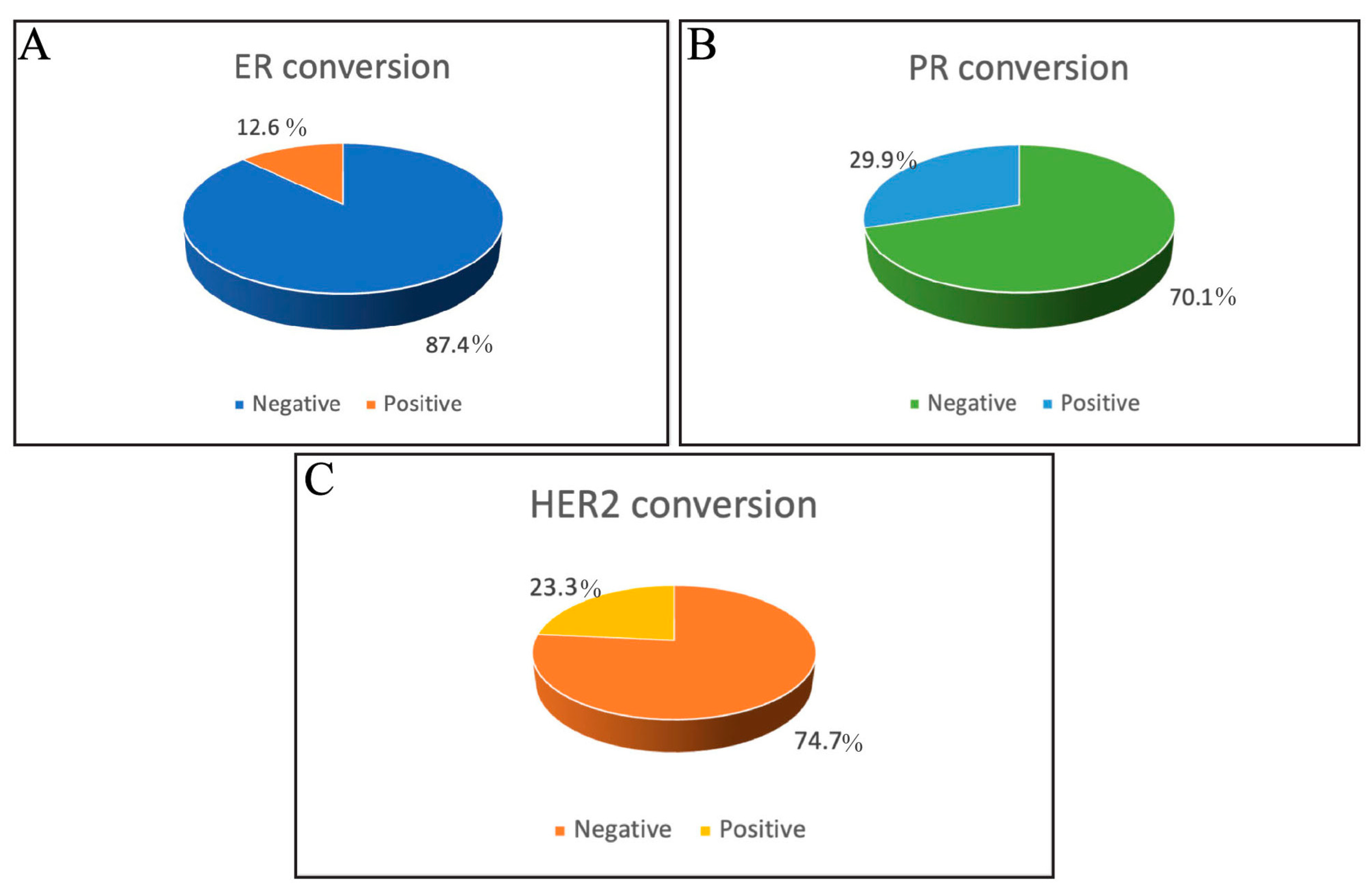
3.3. Incidence of ER, PR, and HER2 Receptor Conversion
3.4. Factors Influencing the Receptor Conversion
3.4.1. Univariate Analysis of Factors Affecting ER, PR, and HER2 Conversion
3.4.2. Multivariate Analysis of Factors Affecting ER, PR, and HER2 Conversion
3.5. Prognostic Effects of Receptor Conversion
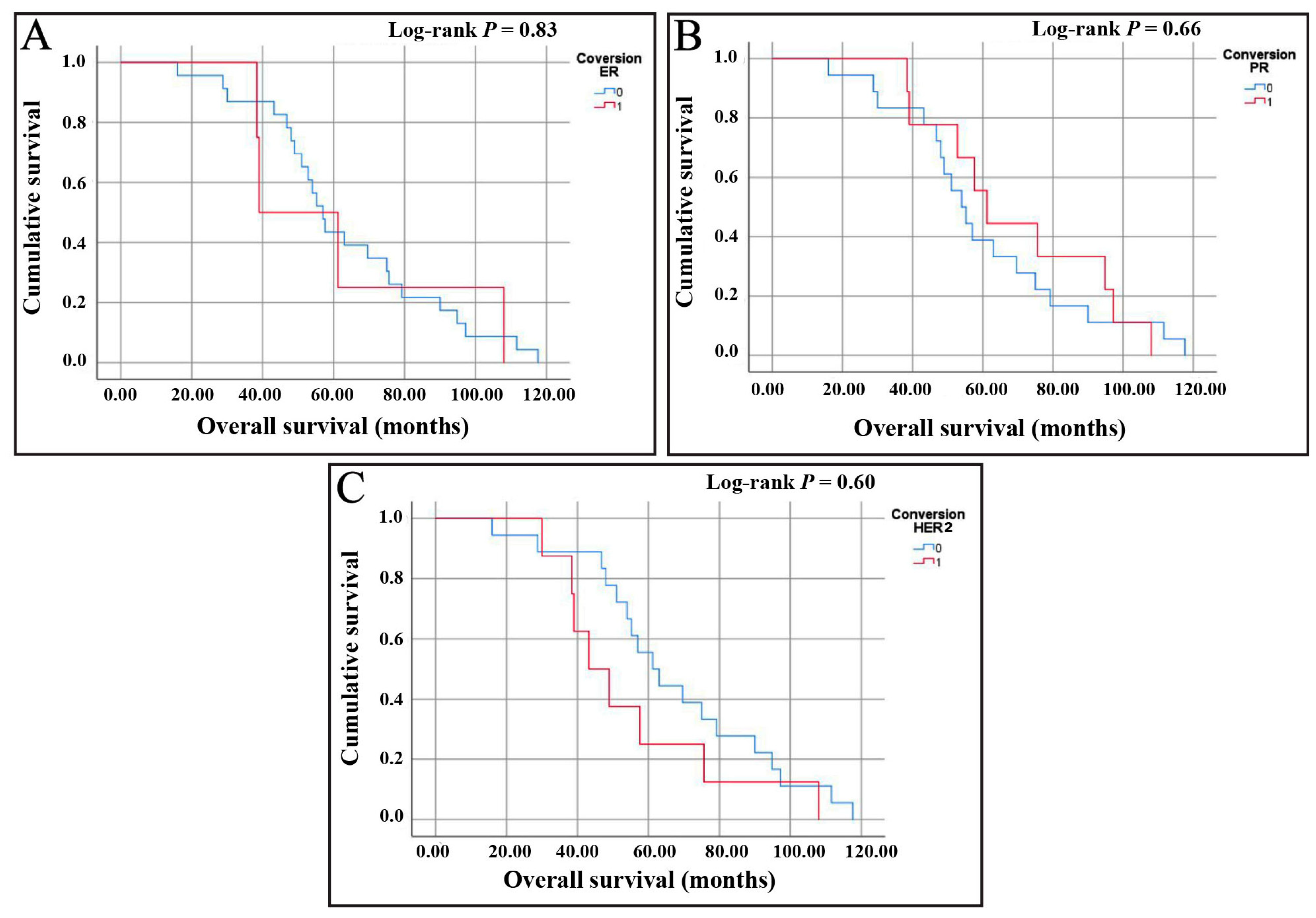
3.5.1. Assessment of Overall Survival (OS)
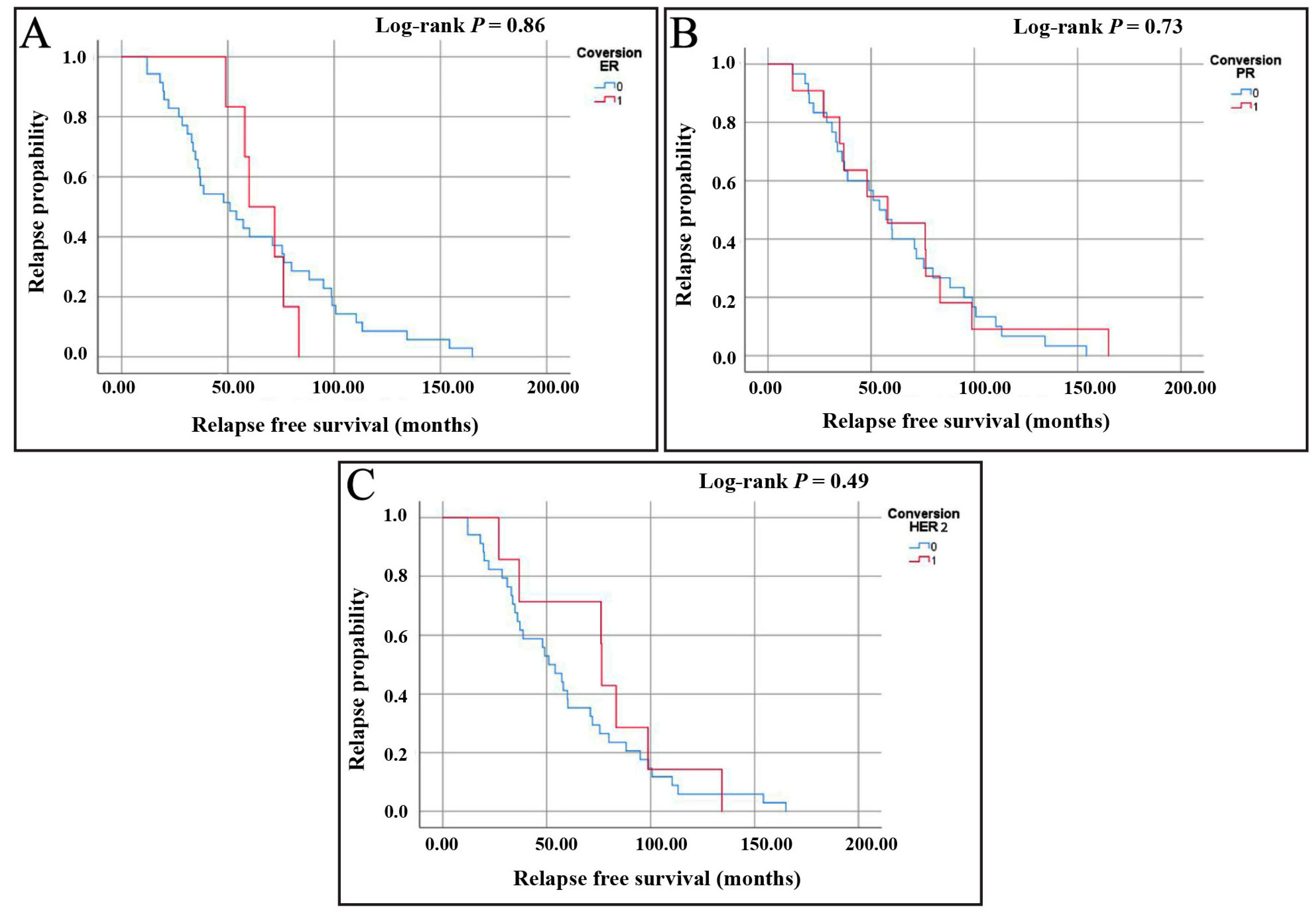
3.5.2. Assessment of Relapse-Free Survival (RFS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCO | American Society of Clinical Oncology |
| CAP | College of American Pathologists |
| CI | confidence intervals |
| ER | estrogen receptor |
| FFPE | formalin-fixed paraffin-embedded |
| FISH | fluorescence in situ hybridization |
| HER2 | human epidermal growth factor 2 |
| KM | Kaplan–Meier |
| OR | odds ratios |
| PR | progesterone receptor |
| RFS | relapse-free survival |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Breast Cancer: A Molecularly Heterogenous Disease Needing Subtype-Specific Treatments. Med. Sci. 2020, 8, 18. [Google Scholar] [CrossRef]
- Vemuru, S.; Huang, J.; Colborn, K.; Yoon, Y.; Huynh, V.; Leonard, L.; Ahrendt, G.; Christian, N.; Afghahi, A.; McLemore, L.; et al. Clinical implications of receptor conversions in breast cancer patients who have undergone neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2023, 200, 247–256. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, L.M.; Harhay, M.O.; Zhang, P.; Ugras, S. Impact of Neoadjuvant Chemotherapy on Breast Cancer Subtype: Does Subtype Change and, if so, How?: IHC Profile and Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2018, 25, 3535–3540. [Google Scholar] [CrossRef]
- Li, C.; Fan, H.; Xiang, Q.; Xu, L.; Zhang, Z.; Liu, Q.; Zhang, T.; Ling, J.; Zhou, Y.; Zhao, X.; et al. Prognostic value of receptor status conversion following neoadjuvant chemotherapy in breast cancer patients: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 178, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Yu, K.; Sun, X.; Xu, S.; Qiu, P.; Lv, Z.; Zhang, X.; Guo, A.; Xu, Y. Impact of hormone receptor, HER2, and Ki-67 status conversions on survival after neoadjuvant chemotherapy in breast cancer patients: A retrospective study. Ann. Transl. Med. 2022, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, K.; Salah, T.; Arafah, M.; Husain, S.; Al-Rikabi, A.; Abd El-Aziz, N. Prognostic significance of estrogen, progesterone and HER2 receptors’ status conversion following neoadjuvant chemotherapy in patients with locally advanced breast cancer: Results from a tertiary Cancer Center in Saudi Arabia. PLoS ONE 2021, 16, e0247802. [Google Scholar] [CrossRef]
- Lob, S.; Linsmeier, E.; Herbert, S.L.; Schlaiss, T.; Kiesel, M.; Wischhusen, J.; Salmen, J.; Kranke, P.; Quenzer, A.; Kurz, F.; et al. Prognostic effect of HER2 evolution from primary breast cancer to breast cancer metastases. J. Cancer Res. Clin. Oncol. 2023, 149, 5417–5428. [Google Scholar] [CrossRef]
- Michel, A.; Oppong, M.D.; Rauschenbach, L.; Pierscianek, D.; Dinger, T.F.; Schmidt, T.; Hense, J.; Pottgen, C.; Kimmig, R.; Ahmadipour, Y.; et al. HER2 Receptor Conversion Is a strong Survival Predictor in Patients with Breast Cancer Brain Metastases. World Neurosurg. 2021, 152, e332–e343. [Google Scholar] [CrossRef]
- Woo, J.W.; Chung, Y.R.; Ahn, S.; Kang, E.; Kim, E.K.; Kim, S.H.; Kim, J.H.; Kim, I.A.; Park, S.Y. Changes in Biomarker Status in Metastatic Breast Cancer and Their Prognostic Value. J. Breast Cancer 2019, 22, 439–452. [Google Scholar] [CrossRef]
- Blancas, I.; Munoz-Serrano, A.J.; Legeren, M.; Ruiz-Avila, I.; Jurado, J.M.; Delgado, M.T.; Garrido, J.M.; Gonzalez, B.; Bayo, V.; Rodriguez-Serrano, F. Immunophenotypic Conversion between Primary and Relapse Breast Cancer and its Effects on Survival. Gynecol. Obstet. Investig. 2020, 85, 259–266. [Google Scholar] [CrossRef]
- Raosoft Sample Size Calculator. Available online: http://www.raosoft.com/samplesize.html (accessed on 20 November 2023).
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thurlimann, B.; Senn, H.J.; Panel, m. Strategies for subtypes–dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, J.; Chen, H.; Zhou, Y.; Hong, L.; Ma, Y.; Chen, N.; Zhao, W.; Tong, Z. Clinical significance and prognostic value of receptor conversion after neoadjuvant chemotherapy in breast cancer patients. Front. Surg. 2022, 9, 1037215. [Google Scholar] [CrossRef]
- Hu, X.; Chen, W.; Li, F.; Ren, P.; Wu, H.; Zhang, C.; Gu, K. Expression changes of ER, PR, HER2, and Ki-67 in primary and metastatic breast cancer and its clinical significance. Front. Oncol. 2023, 13, 1053125. [Google Scholar] [CrossRef]
- Pegram, M.; Jackisch, C.; Johnston, S.R.D. Estrogen/HER2 receptor crosstalk in breast cancer: Combination therapies to improve outcomes for patients with hormone receptor-positive/HER2-positive breast cancer. NPJ Breast Cancer 2023, 9, 45. [Google Scholar] [CrossRef]
- Paakinaho, V.; Palvimo, J.J. Genome-wide crosstalk between steroid receptors in breast and prostate cancers. Endocr.-Relat. Cancer 2021, 28, R231–R250. [Google Scholar] [CrossRef]
- Mohammed, H.; Russell, I.A.; Stark, R.; Rueda, O.M.; Hickey, T.E.; Tarulli, G.A.; Serandour, A.A.; Birrell, S.N.; Bruna, A.; Saadi, A.; et al. Progesterone receptor modulates ERalpha action in breast cancer. Nature 2015, 523, 313–317. [Google Scholar] [CrossRef]
- Giuliano, M.; Trivedi, M.V.; Schiff, R. Bidirectional Crosstalk between the Estrogen Receptor and Human Epidermal Growth Factor Receptor 2 Signaling Pathways in Breast Cancer: Molecular Basis and Clinical Implications. Breast Care 2013, 8, 256–262. [Google Scholar] [CrossRef]
- Lee, A.V.; Cui, X.; Oesterreich, S. Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer. Clin. Cancer Res. 2001, 7, 4429s–4435s; discussion 4411s–4412s. [Google Scholar] [PubMed]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Sunden, M.; Norgren, S.; Lundqvist, R.; Andersson, A.; Sund, M.; Hemmingsson, O. Receptor conversion and survival in breast cancer liver metastases. Breast Cancer Res. 2023, 25, 105. [Google Scholar] [CrossRef] [PubMed]
- Talari, K.; Goyal, M. Retrospective studies—Utility and caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef]
| Variable | No (%) |
|---|---|
| Age of primary tumor * | 47.3 (10.1) |
| Age of recurrence * | 51.95 (10.6) |
| Difference between first diagnosis and relapse (y) * | 4.7 (3.4) |
| Family history of breast cancer | |
| Positive | 12 (13.8%) |
| Negative | 30 (34.5%) |
| Missing | 46 (51.7%) |
| Primary tumor size | |
| <2 cm | 16 (18.4%) |
| 2–5 cm | 17 (19.5%) |
| >5 cm | 13 (14.9%) |
| Unknown | 41 (47.1%) |
| Menopausal status | |
| Postmenopausal | 23 (26.4%) |
| Premenopausal | 38 (43.7%) |
| Missing | 26 (29.9%) |
| Histological subtype | |
| Ductal carcinoma in situ | 3 (3.4%) |
| Invasive duct carcinoma | 59 (67.8%) |
| Invasive adenosquamous carcinoma | 2 (2.3%) |
| invasive lobular carcinoma | 5 (5.7%) |
| Type of biopsy | |
| Core needle biopsy | 37 (42.5%) |
| Mastectomy | 18 (20.5%) |
| Missing | 32 (36.8%) |
| Treatment | |
| Neoadjuvant | 60 (69%) |
| Surgical | 82 (94.3%) |
| Adjuvant chemotherapy | 61 (70.1%) |
| Radiotherapy | 64 (73.6%) |
| Endocrinal therapy | 59 (67.8%) |
| Anti-HER2 | 39 (44.8%) |
| Variable | Primary No (%) | Recurrent No (%) |
|---|---|---|
| Stage of the tumor | ||
| I | 5 (5.7%) | 2 (2.3%) |
| II | 15 (17.2%) | 2 (2.3%) |
| III | 31 (35.6%) | 6 (6.8%) |
| IV | 14 (16.1%) | 64 (73.6%) |
| Missing | 22 (25.3%) | 12 (13.8%) |
| Locoregional involvement | ||
| Localized | 37 (42.5%) | 9 (10.3%) |
| Lymph node | 31 (35.6%) | 6 (6.9%) |
| Spread to one organ | 9 (10.3%) | 15 (17.2%) |
| Spread to more than one organ | 6 (6.9%) | 53 (60.9%) |
| Missing | 4 (4.6%) | 4 (4.6%) |
| Estrogen receptor | ||
| Positive | 66 (75.8%) | 65 (74.7%) |
| Negative | 21 (24.1%) | 22 (25.3%) |
| Progesterone receptor | ||
| Positive | 59 (67.8%) | 44 (50.6%) |
| Negative | 28 (32.2%) | 43 (49.4%) |
| HER2 | ||
| Positive | 28 (32.2%) | 32 (36.8%) |
| Negative | 56 (64.4%) | 50 (57.5%) |
| Equivalent | 3 (3.4%) | 5 (5.7%) |
| Ki-67 | ||
| >14% | 8 (9.2%) | 7 (8.05%) |
| <14% | 42 (48.3%) | 29 (33.3%) |
| Unknown | 37 (42.5%) | 51 (58.6%) |
| Variables | ER Conversion | PR Conversion | HER2 Conversion | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative No (%) | Positive No. (%) | p-Value | Negative No (%) | Positive No. (%) | p-Value | Negative No (%) | Positive No. (%) | p-Value | |
| Stage of recurrent tumor | |||||||||
| I | 2 (3%) | 0 | 0.71 | 2 (2.8%) | 1 (4.3%) | 0.37 | 1 (1.80%) | 2 (11.8%) | 0.15 |
| II | 1 (1.5%) | 1 (10%) | 2 (2.8%) | 0 | 1 (1.80%) | 2 (5.9%) | |||
| III | 6 (9.2%) | 0 | 3 (5.7%) | 3 (13%) | 4 (7.20%) | 2 (11.8%) | |||
| IV | 55 (84.6%) | 9 (90%) | 45 (86.5%) | 19 (82.6%) | 50 (89.3%) | 12 (70.6%) | |||
| Menopausal status | |||||||||
| Postmenopausal | 18 (33.3%) | 5 (71.4%) | 0.05 | 11 (25%) | 12 (70.6%) | 0.001 | 15 (31.3%) | 8 (66.7%) | 0.04 |
| Premenopausal | 36 (66.7%) | 2 (28.6%) | 33 (75%) | 5 (29.4%) | 33 (68.8%) | 4 (33.3%) | |||
| Histological subtype | |||||||||
| Ductal carcinoma in situ | 3 (4.8%) | 0 | 0.89 | 3 (6.1%) | 0 | 0.64 | 3 (5.70%) | 0 | 0.83 |
| Invasive duct carcinoma | 63 (85.5%) | 6 (85.7%) | 41 (83.7%) | 18 (90%) | 43 (82.7%) | 16 (94.1%) | |||
| Invasive adenosquamous carcinoma | 2 (3.2%) | 0 | 2 (4.1%) | 0 | 2 (3.80%) | 0 | |||
| Invasive lobular carcinoma | 4 (6.4%) | 1 (14.3%) | 3 (6.1%) | 2 (10%) | 4 (7.70%) | 1 (5.90%) | |||
| Locoregional involvement (primary) | |||||||||
| Localized | 33 (45.2%) | 4 (40%) | 0.75 | 25 (43.1%) | 12 (48%) | 0.61 | 25 (41%) | 10 (50%) | 0.7 |
| Lymph node | 28 (38.4%) | 3 (30%) | 24 (41.4%) | 7 (28%) | 23 (37.7%) | 8 (40%) | |||
| Spread to one organ | 7 (9.6%) | 2 (20%) | 5 (8.6%) | 4(16%) | 8 (13.1%) | 1 (5%) | |||
| Spread to more than one organ | 5 (6.8%) | 1 (10%) | 4 (6.9%) | 2 (8%) | 5 (8.20%) | 1 (5%) | |||
| Locoregional involvement (recurrent) | |||||||||
| Localized | 9 (12.50%) | 0 | 0.24 | 7 (12.3%) | 2 (7.7%) | 0.31 | 5 (8.2%) | 4 (20%) | 0.39 |
| Lymph node | 4 (5.60%) | 2 (18.2%) | 4 (7%) | 2 (7.7%) | 4 (6.6%) | 2 (10%) | |||
| Spread to one organ | 14 (19.4%) | 1 (9.1%) | 13 (22.8%) | 2 (7.7%) | 12 (19.7%) | 2 (10%) | |||
| Spread to more than one organ | 45 (62.5%) | 8 (72.7%) | 33 (57.9%) | 20 (76.9%) | 40 (65.6%) | 12 (60%) | |||
| BRCA1 | |||||||||
| Negative | 15 (83.3%) | 3 (100%) | 0.45 | 13 (86.7%) | 5 (83.3%) | 0.84 | 15 (93.8%) | 3 (60%) | 0.23 |
| Positive | 3 (16.7%) | 0 | 2 (13.3%) | 1 (16.7%) | 1 (6.30%) | 2 (40%) | |||
| BRCA2 | |||||||||
| Negative | 16 (88.9%) | 3 (100%) | 0.54 | 14 (93.3%) | 5 (83.3%) | 0.48 | 15 (93.8%) | 4 (80%) | 0.36 |
| Positive | 2 (11.15) | 0 | 1 (6.7%) | 1 (16.7%) | 1 (6.3%) | 1 (20%) | |||
| Estrogen receptor (PR) conversion | |||||||||
| No | -- | -- | 58 (95.1%) | 18 (69.2%) | 0.001 | 59 (90.8%) | 17 (31.8%) | 0.1 | |
| Yes | -- | -- | 3 (4.9%) | 8 (30.8%) | 6 (9.2%) | 5 (22.7%) | |||
| Progesterone receptor (PR) conversion | |||||||||
| No | 58 (76.3%) | 3 (27.3%) | 0.001 | -- | -- | 54 (83.1%) | 7 (31.8%) | <0.001 | |
| Yes | 18 (23.7%) | 8 (72.7%) | -- | -- | 11 (16.9%) | 15 (68.2%) | |||
| HER2 conversion | |||||||||
| No | 59 (79.7%) | 6 (54.5%) | 0.06 | 54 (90%) | 11 (44%) | 0.001 | -- | -- | |
| Yes | 15 (20.3%) | 5 (45.5%) | 6 (10%) | 14 (56%) | -- | -- | |||
| ER Conversion | PR Conversion | HER2 Conversion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Negative No. (%) | Positive No. (%) | p-Value | Negative No. (%) | Positive No. (%) | p-Value | Negative No. (%) | Positive No. (%) | p-Value | |
| Neoadjuvant therapy | |||||||||
| Yes | 22 (28.9%) | 5 (45.5%) | 0.31 | 18 (29.5%) | 9 (34.6%) | 0.63 | 18 (27.7%) | 8 (40%) | 0.2 |
| No | 54 (71.10%) | 6 (54.5%) | 43 (70.5%) | 17 (65.4%) | 47 (72.3%) | 12 (60%) | |||
| Surgical treatment | |||||||||
| Yes | 4 (5.30%) | 1 (9.10%) | 0.61 | 4 (6.6%) | 1 (3.8%) | 0.62 | 3 (4.6%) | 2 (10%) | 0.58 |
| No | 72 (94.70%) | 10 (90.9%) | 57 (93.4%) | 25 (96.2%) | 62 (95.4%) | 18 (90%) | |||
| Chemotherapy | |||||||||
| Yes | 23 (30.3%) | 3 (27.3%) | 0.84 | 21 (34.4%) | 5 (19.2%) | 0.16 | 20 (30.8%) | 5 (25%) | 0.62 |
| No | 53 (69.7%) | 8 (72.7%) | 40 (65.6%) | 21 (80.8%) | 45 (69.2%) | 15 (75%) | |||
| Radiotherapy | |||||||||
| Yes | 19 (25%) | 4 (36.4%) | 0.4 | 13 (21.3%) | 10 (38.5%) | 0.097 | 16 (24.6%) | 7 (35%) | 0.39 |
| No | 57 (75%) | 7 (63.6%) | 48 (78.7%) | 16 (61.5%) | 49 (75.4%) | 13 (65%) | |||
| Hormonal therapy | |||||||||
| Yes | 25 (32.9%) | 3 (27.3%) | 0.71 | 22 (36.10%) | 6 (23.1%) | 0.23 | 24 (36.9%) | 4 (20%) | 0.16 |
| No | 51 (67.10%) | 8 (72.7%) | 39 (63.90%) | 20 (76.9%) | 41 (63.1%) | 16 (80%) | |||
| Receptor | Predictors | B (Coefficient) | p-Value | OR (95% CI) |
|---|---|---|---|---|
| ER | PR Conversion (Reference: No conversion) | 2.293 | 0.02 | 9.89 (1.33–73.50) |
| HER2 Conversion (Reference: No conversion) | 1.703 | 0.1 | 5.49 (0.68–44.087) | |
| Menopausal status (Reference: Postmenopausal) | −0.309 | 0.15 | 0.734 (1.12–0.150) | |
| PR | ER Conversion (Reference: No conversion) | 2.074 | 0.076 | 7.958 (0.81–78.6) |
| Menopausal status (Reference: Postmenopausal) | −1.877 | 0.019 | 0.153 (0.03–0.74) | |
| HER2 Conversion (Reference: No conversion) | 2.763 | 0.003 | 15.84 (2.6–95.7) | |
| HER2 | ER Conversion (Reference: No conversion) | 0.207 | 0.7 | 1.23 (0.39–3.84) |
| PR Conversion (Reference: No conversion) | 0.93 | 0.02 | 2.52 (1.12–5.69) | |
| Menopausal status (Reference: Postmenopausal) | −2.16 | 0.001 | 0.125 (0.76–0.17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousef, E.M.; Alswilem, A.M.; Alfaraj, Z.S.; Alhamood, D.J.; Ghashi, G.K.; Alruwaily, H.S.; Al Yahya, S.S.; Alsaeed, E. Incidence and Prognostic Significance of Hormonal Receptors and HER2 Status Conversion in Recurrent Breast Cancer: A Retrospective Study in a Single Institute. Medicina 2025, 61, 563. https://doi.org/10.3390/medicina61040563
Yousef EM, Alswilem AM, Alfaraj ZS, Alhamood DJ, Ghashi GK, Alruwaily HS, Al Yahya SS, Alsaeed E. Incidence and Prognostic Significance of Hormonal Receptors and HER2 Status Conversion in Recurrent Breast Cancer: A Retrospective Study in a Single Institute. Medicina. 2025; 61(4):563. https://doi.org/10.3390/medicina61040563
Chicago/Turabian StyleYousef, Einas M., Abdullah Mansour Alswilem, Zahrah S. Alfaraj, Danya J. Alhamood, Ghfran K. Ghashi, Hanan S. Alruwaily, Shouq S. Al Yahya, and Eyad Alsaeed. 2025. "Incidence and Prognostic Significance of Hormonal Receptors and HER2 Status Conversion in Recurrent Breast Cancer: A Retrospective Study in a Single Institute" Medicina 61, no. 4: 563. https://doi.org/10.3390/medicina61040563
APA StyleYousef, E. M., Alswilem, A. M., Alfaraj, Z. S., Alhamood, D. J., Ghashi, G. K., Alruwaily, H. S., Al Yahya, S. S., & Alsaeed, E. (2025). Incidence and Prognostic Significance of Hormonal Receptors and HER2 Status Conversion in Recurrent Breast Cancer: A Retrospective Study in a Single Institute. Medicina, 61(4), 563. https://doi.org/10.3390/medicina61040563






