Silibinin Triggers Mitochondrial Apoptosis and Declines Clonogenic Potential in Detroit 562 Human Pharyngeal Carcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Lines and Cell Culture Conditions
2.3. Instruments
2.4. Cell Viability Evaluation Using MTT Colorimetric Assay
2.5. Cellular Morphology Assessment
2.6. Detection of Intracellular Reactive Oxygen Species (ROS)
2.7. Mitochondrial Membrane Potential (ΔΨm) Assay—JC-1 Staining
2.8. Mitochondria and Nuclei Immunofluorescence Staining
2.9. Caspase 3/7 and Caspase 9 Activation
2.10. Acridine Orange/Propidium Iodide (AO/PI) Assay
2.11. Colony Formation Assay
2.12. Statistical Analysis
3. Results
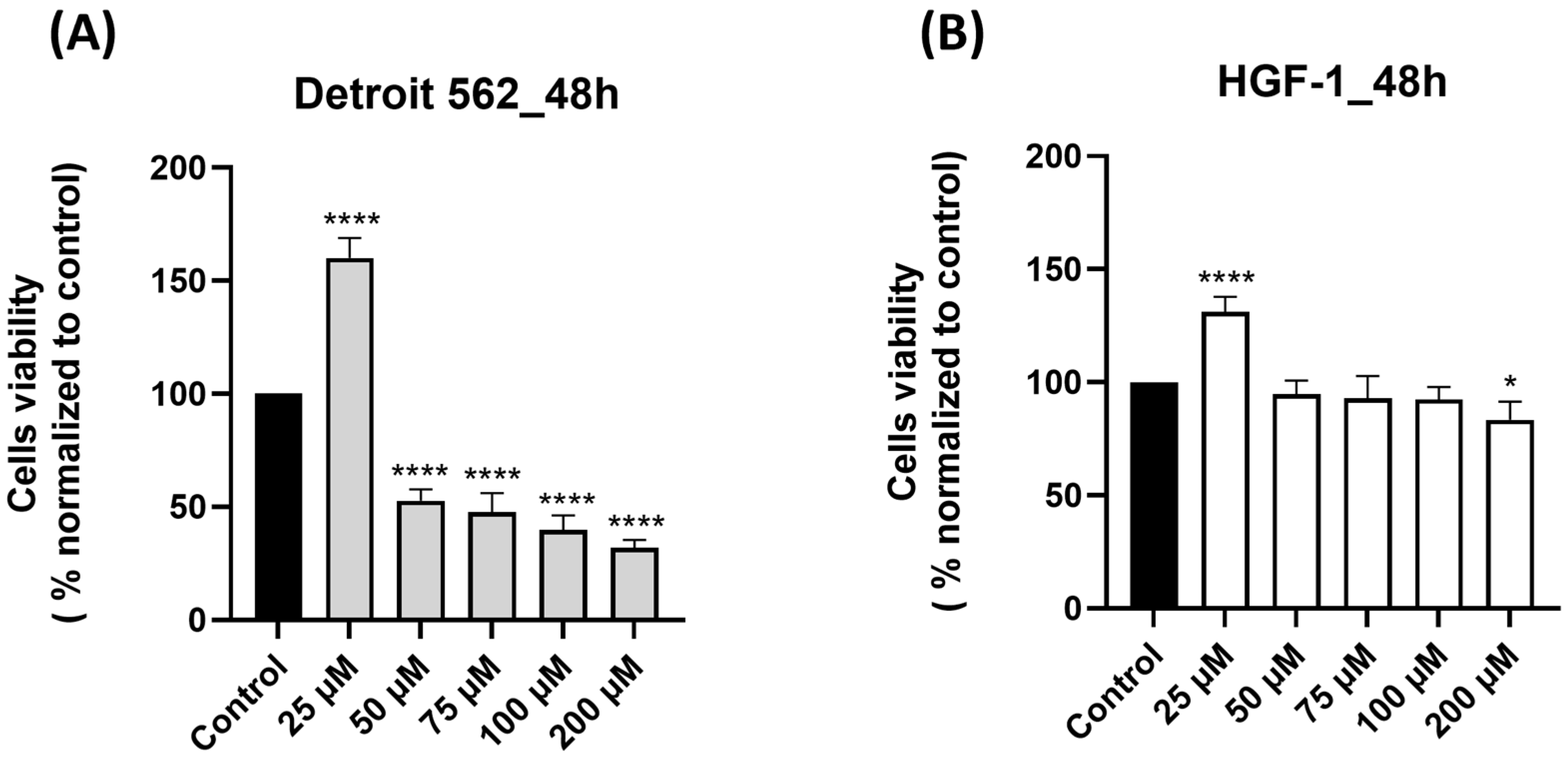
3.1. SIL Triggered a Cell-Type Dependent Cytotoxicity: Potent Effects in Detroit 562 Cancer Cells Versus Minimal Toxicity in HGF-1 Healthy Gingival Fibroblasts
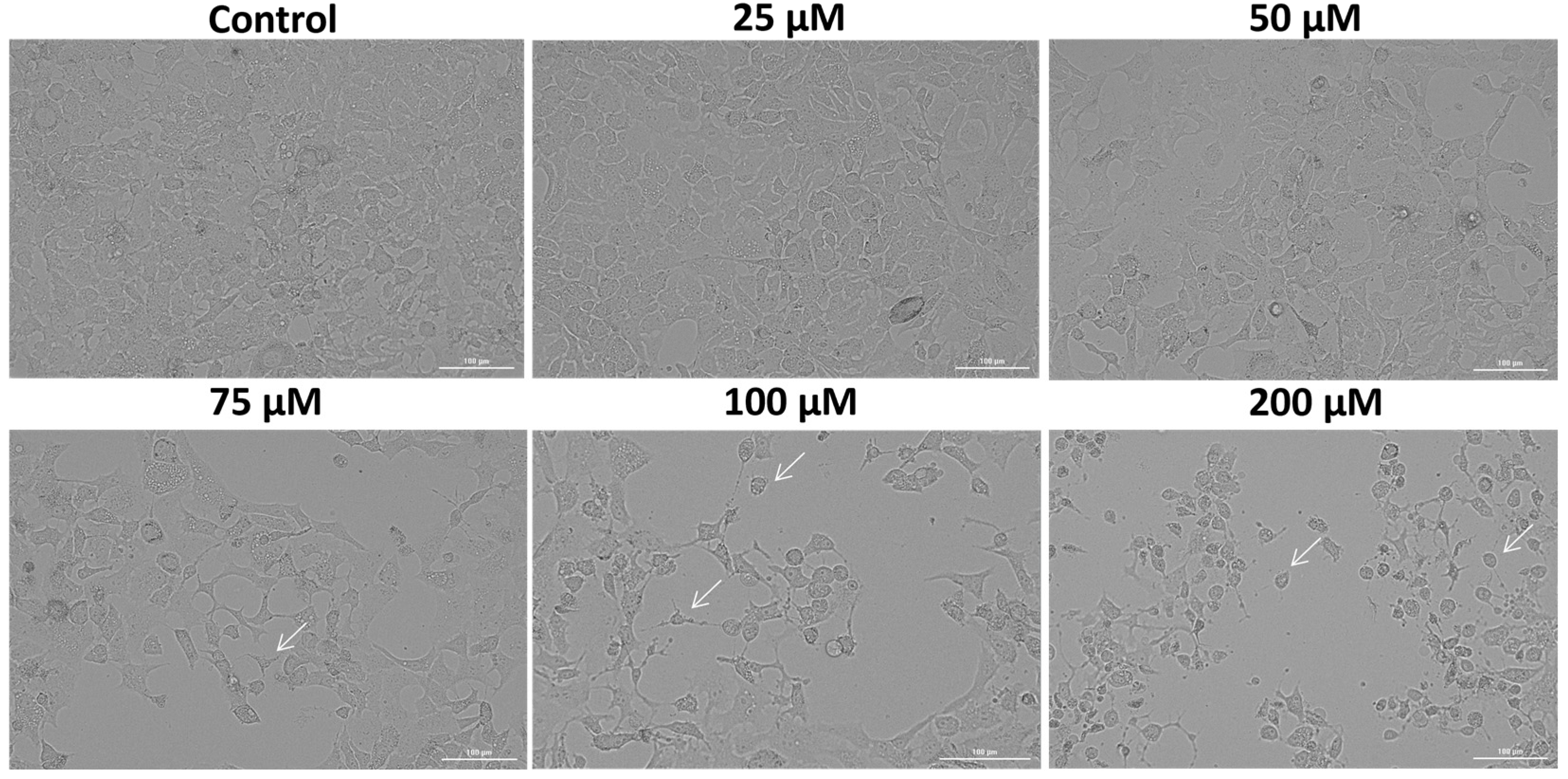
3.2. SIL Altered the Cellular Morphology of Detroit 562 Cancer Cells
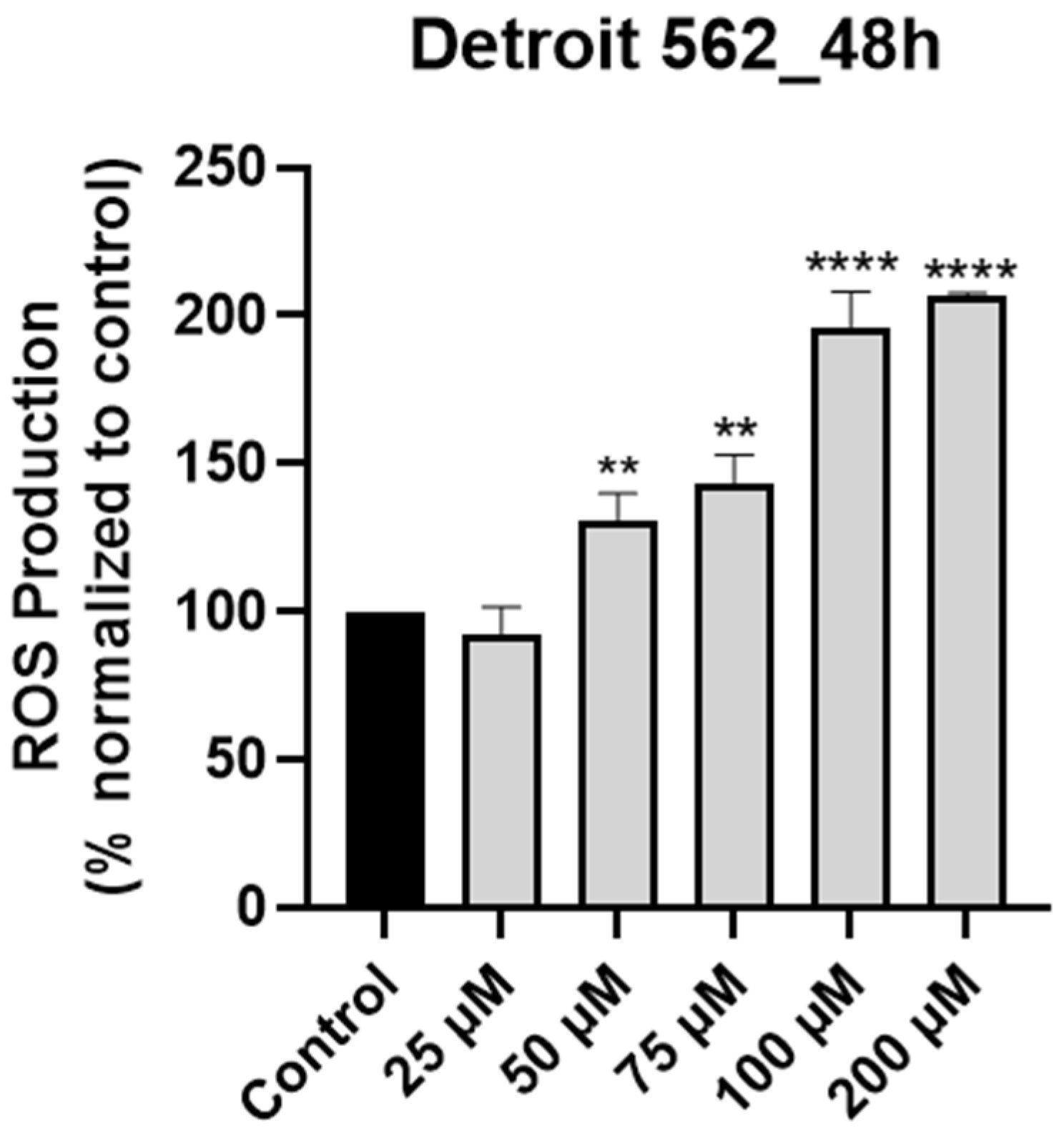
3.3. SIL Stimulates Intracellular ROS Production in Detroit 562 Cancer Cells in a Dose-Dependent Manner
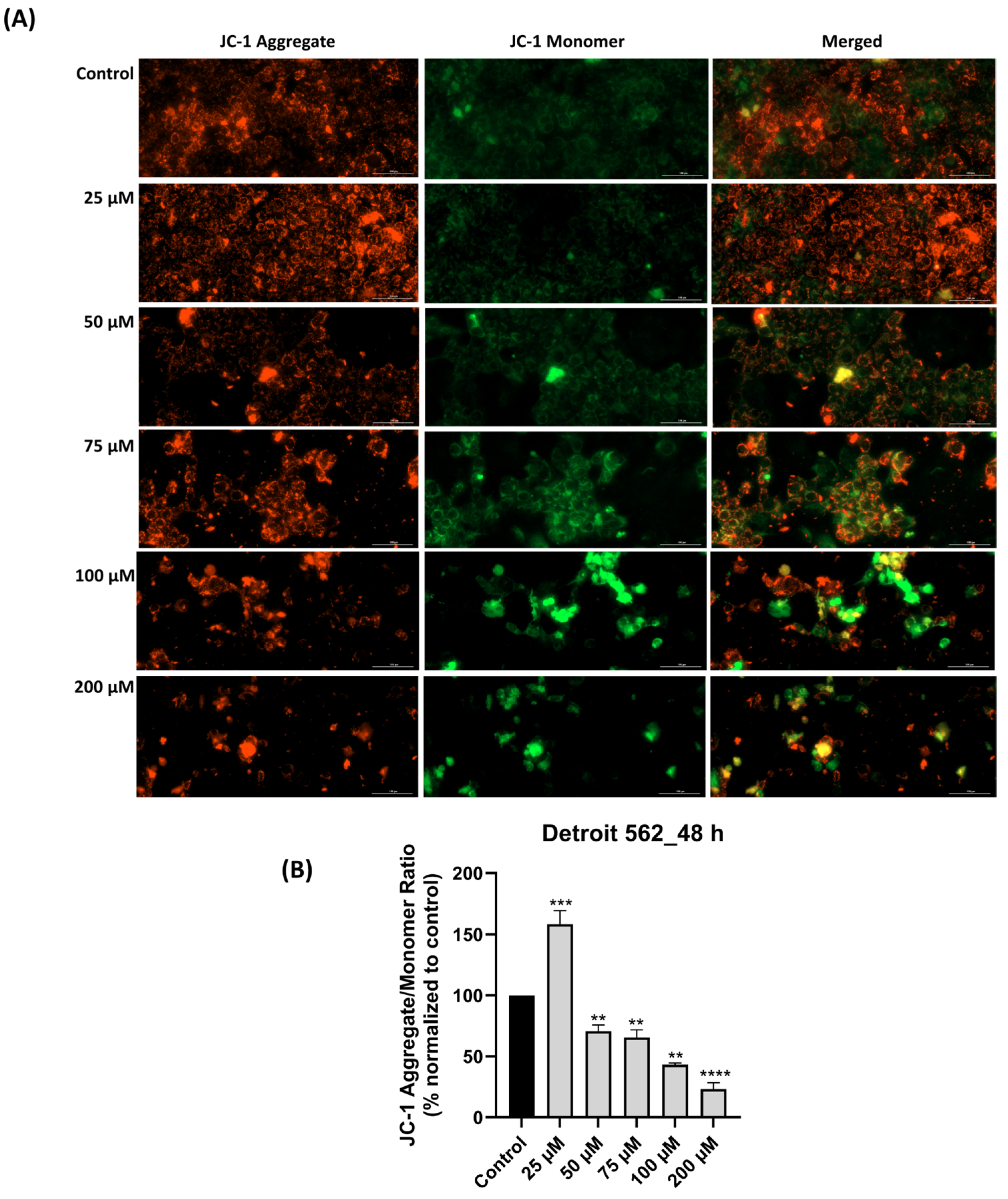
3.4. SIL-Elicited Mitochondrial Depolarization in Detroit 562 Cancer Cells
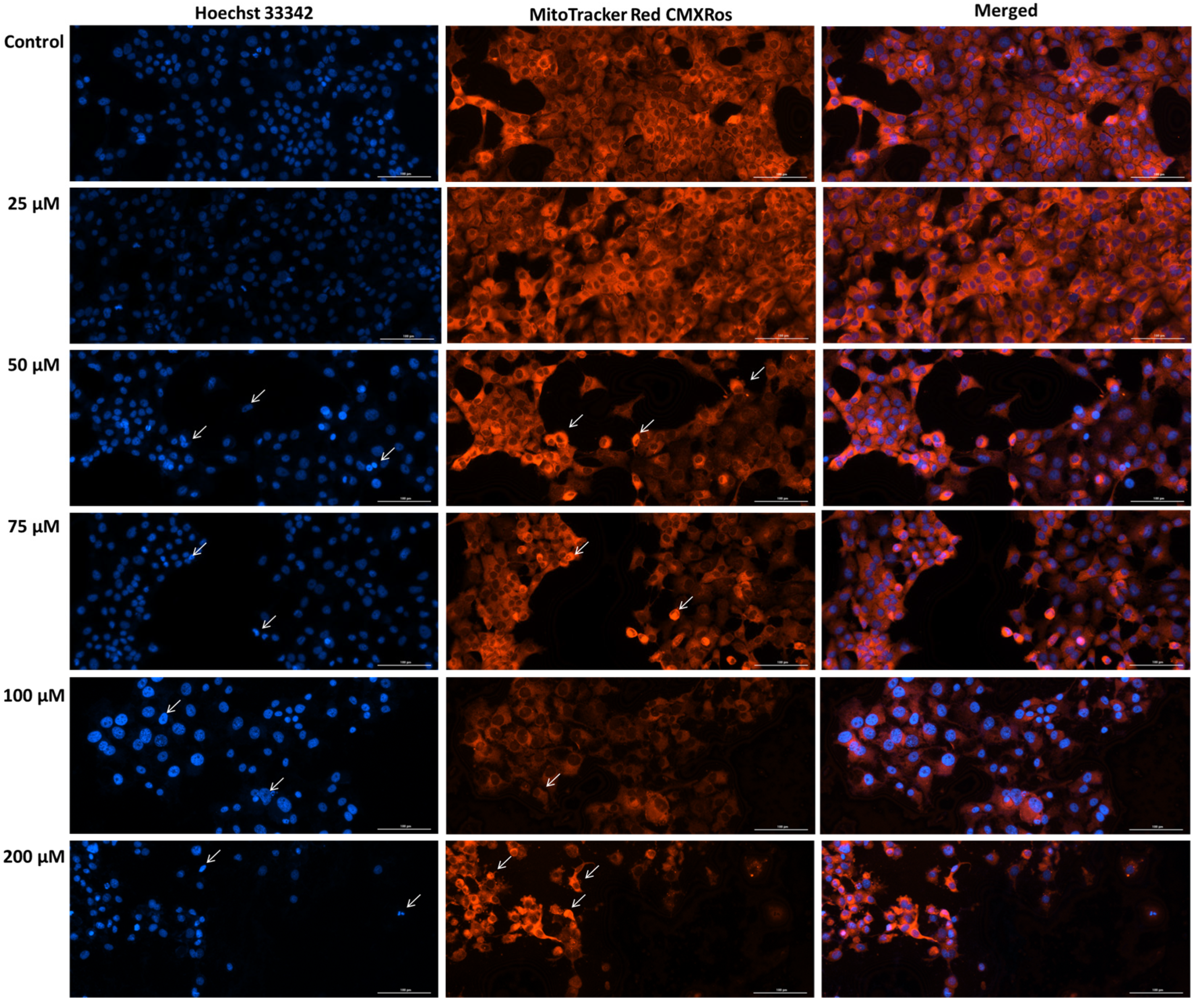
3.5. SIL Induces Mitochondrial Damage and Nuclear Morphology Alterations Dose-Dependently
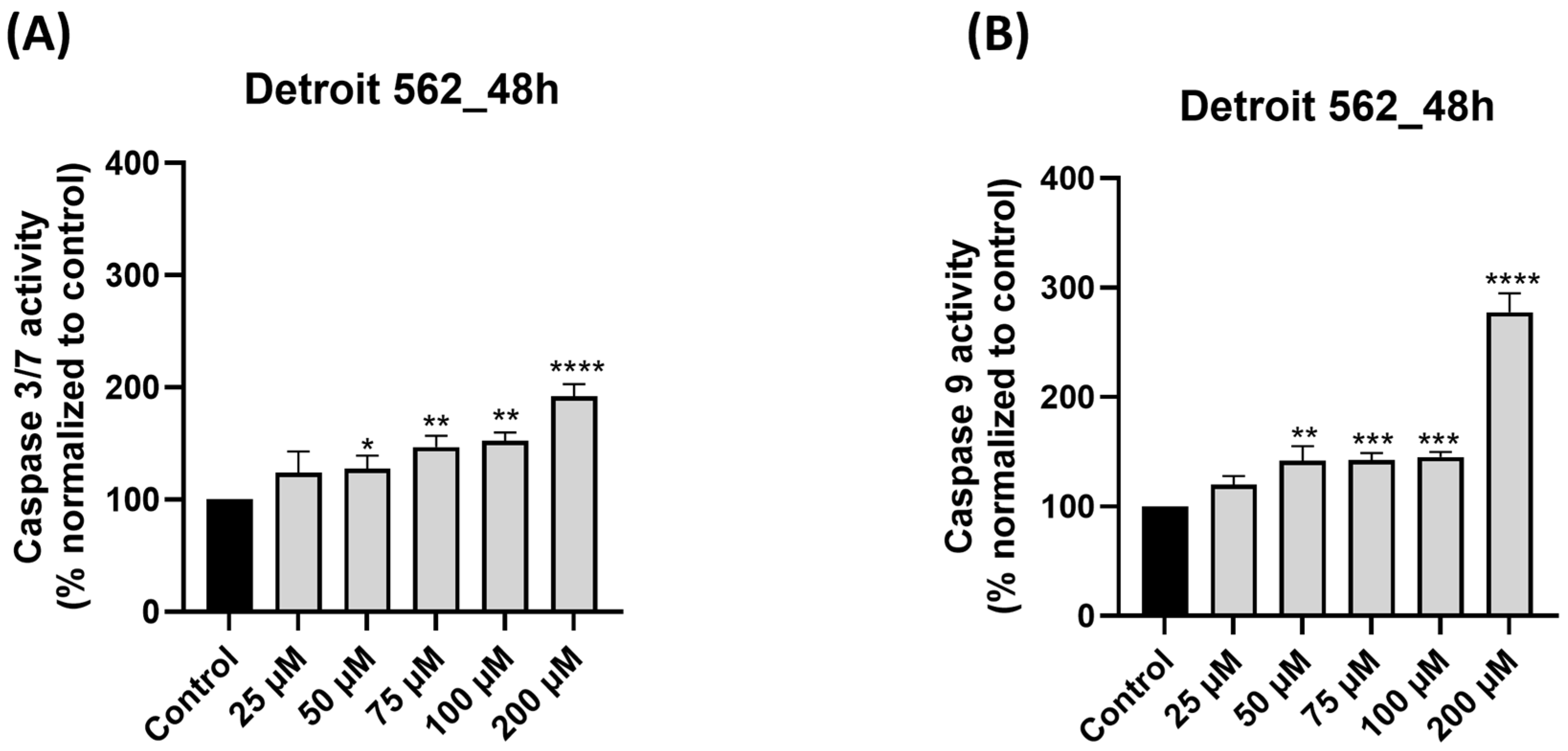
3.6. SIL Treatment Triggers Caspase 9 and Caspase 3/7 Activation
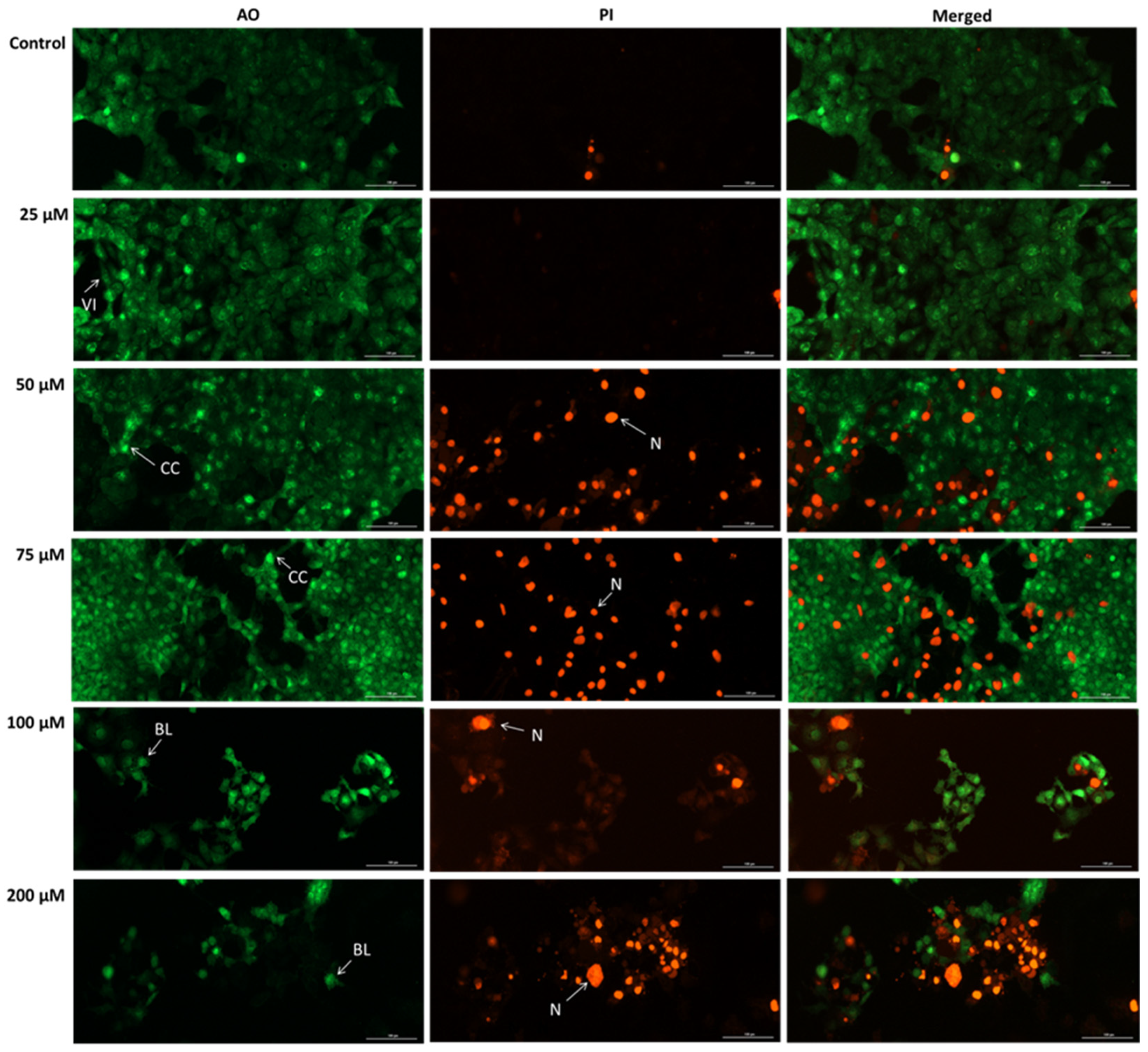
3.7. SIL Exhibits a Pro-Apoptotic Effect in Detroit 562 Cells
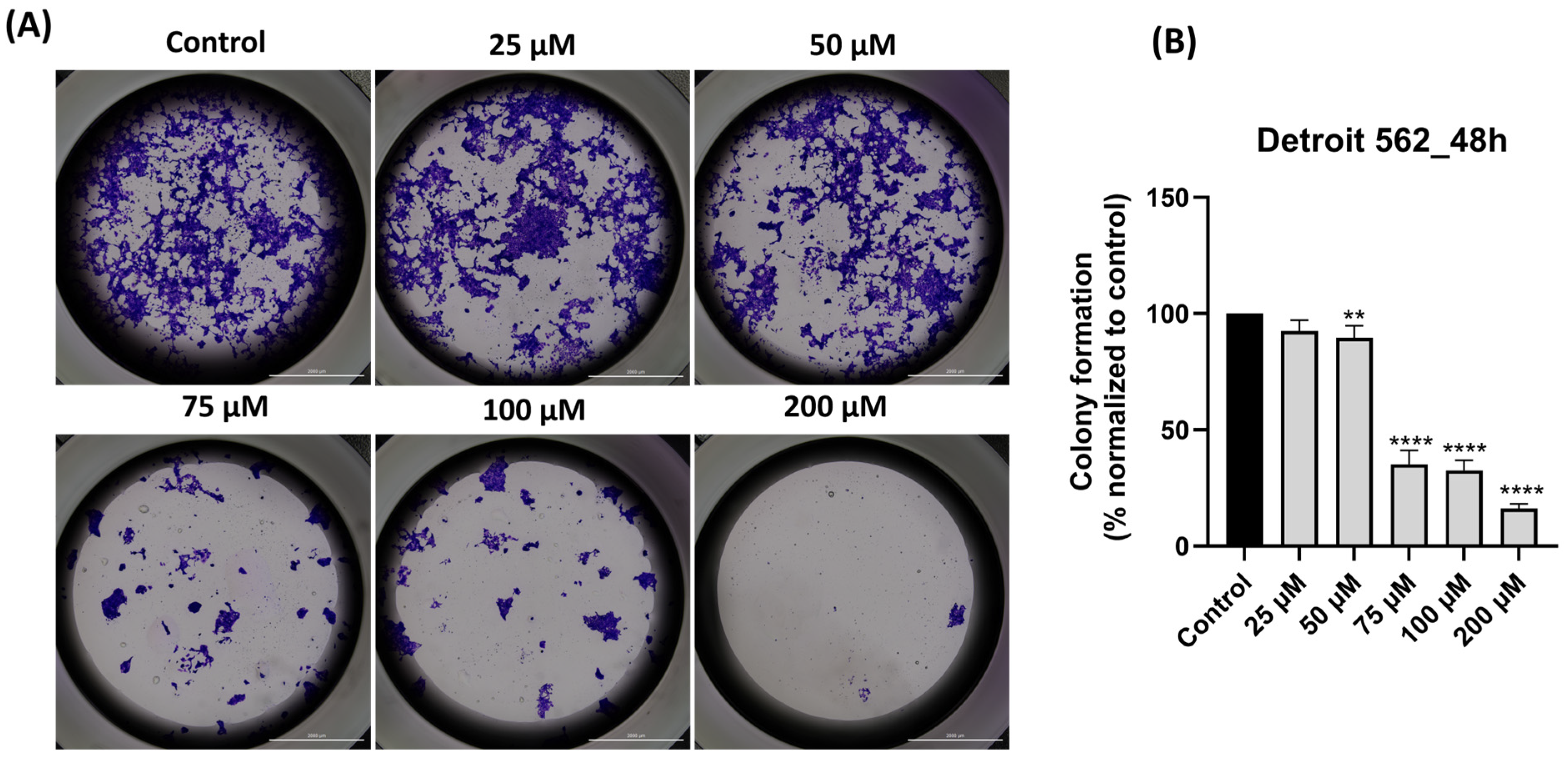
3.8. SIL Impairs Dose-Dependent the Clonogenic Potential of Detroit 562 Cancer Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jamal, Z.; Anjum, F. Oropharyngeal Squamous Cell Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563268/ (accessed on 15 November 2025).
- Sun, H.; Yu, M.; An, Z.; Liang, F.; Sun, B.; Liu, Y.; Zhang, S. Global burden of head and neck cancer: Epidemiological transitions, inequities, and projections to 2050. Front. Oncol. 2025, 15, 1665019. [Google Scholar] [CrossRef] [PubMed]
- Konings, H.; Stappers, S.; Geens, M.; De Winter, B.Y.; Lamote, K.; van Meerbeeck, J.P.; Specenier, P.; Vanderveken, O.M.; Ledeganck, K.J. A Literature Review of the Potential Diagnostic Biomarkers of Head and Neck Neoplasms. Front. Oncol. 2020, 10, 1020. [Google Scholar] [CrossRef] [PubMed]
- Racea, R.C.; Macasoi, I.G.; Dinu, S.; Pinzaru, I.; Marcovici, I.; Dehelean, C.; Rusu, L.C.; Chioran, D.; Rivis, M.; Buzatu, R. Eugenol: In Vitro and In Ovo Assessment to Explore Cytotoxic Effects on Osteosarcoma and Oropharyngeal Cancer Cells. Plants 2023, 12, 3549. [Google Scholar] [CrossRef]
- Cabral, L.G.d.S.; Martins, I.M.; Paulo, E.P.d.A.; Pomini, K.T.; Poyet, J.-L.; Maria, D.A. Molecular Mechanisms in the Carcinogenesis of Oral Squamous Cell Carcinoma: A Literature Review. Biomolecules 2025, 15, 621. [Google Scholar] [CrossRef]
- Lau, F.; Lisatchok, M.; Tamanini, J.B.; Gazmenga, F.P.; Texeira, D.N.A.; Couto, E.V.; Chone, C.T. Treatment outcome of oropharyngeal squamous cell carcinoma through propensity score analysis. Braz. J. Otorhinolaryngol. 2023, 89, 101335. [Google Scholar] [CrossRef]
- Mordzińska-Rak, A.; Telejko, I.; Adamczuk, G.; Trombik, T.; Stepulak, A.; Błaszczak, E. Advancing Head and Neck Cancer Therapies: From Conventional Treatments to Emerging Strategies. Biomedicines 2025, 13, 1046. [Google Scholar] [CrossRef] [PubMed]
- Katabi, N.; Lewis, J.S. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: What Is New in the 2017 WHO Blue Book for Tumors and Tumor-Like Lesions of the Neck and Lymph Nodes. Head Neck Pathol. 2017, 11, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Maria de França, G.; Andrade, A.C.M.; Felix, F.A.; da Silva, W.R.; Almeida, D.R.M.F.; Leite, R.B.; Galvão, H.C.; Miguel, M.C.D.C. Survival-related epithelial-mesenchymal transition proteins in oropharyngeal squamous cell carcinoma: A systematic review and meta-analysis. Arch. Oral Biol. 2021, 131, 105267. [Google Scholar] [CrossRef]
- Panda, S.; Gurusamy, K.S.; Thakar, A.; Mitra, S.; Dwivedi, R.; Chiumenti, F.A. Treatment outcomes for human papillomavirus negative oropharyngeal cancer: A meta-analysis. Eur. J. Surg. Oncol. 2025, 51, 110005. [Google Scholar] [CrossRef] [PubMed]
- Silbermann, M.; Pitsillides, B.; Al-Alfi, N.; Omran, S.; Al-Jabri, K.; Elshamy, K.; Ghrayeb, I.; Livneh, J.; Daher, M.; Charalambous, H.; et al. Multidisciplinary care team for cancer patients and its implementation in several Middle Eastern countries. Ann. Oncol. 2013, 24, vii41–vii47. [Google Scholar] [CrossRef]
- Choi, S.E.; Choudhary, A.; Sonis, S.; Villa, A. Benefits of the Involvement of Dentists in Managing Oral Complications Among Patients With Oral Cavity and Oropharyngeal Cancer: An Analysis of Claims Data. JCO Oncol. Pract. 2021, 17, e1668–e1677. [Google Scholar] [CrossRef]
- Swiecicki, P.L.; Malloy, K.M.; Worden, F.P. Advanced oropharyngeal squamous cell carcinoma: Pathogenesis, treatment, and novel therapeutic approaches. World J. Clin. Oncol. 2016, 7, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-Y.; Tseng, Y.-H. The Potential of Phytochemicals in Oral Cancer Prevention and Therapy: A Review of the Evidence. Biomolecules 2020, 10, 1150. [Google Scholar] [CrossRef]
- Ray, P.P.; Islam, M.A.; Islam, M.S.; Han, A.; Geng, P.; Aziz, M.A.; Mamun, A.A. A comprehensive evaluation of the therapeutic potential of silibinin: A ray of hope in cancer treatment. Front. Pharmacol. 2024, 15, 1349745. [Google Scholar] [CrossRef]
- Mishra, S.D.; Mendonca, P.; Kaur, S.; Soliman, K.F.A. Silibinin Anticancer Effects Through the Modulation of the Tumor Immune Microenvironment in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 6265. [Google Scholar] [CrossRef]
- Chen, P.N.; Hsieh, Y.S.; Chiang, C.L.; Chiou, H.L.; Yang, S.F.; Chu, S.C. Silibinin inhibits invasion of oral cancer cells by suppressing the MAPK pathway. J. Dent. Res. 2006, 85, 220–225. [Google Scholar] [CrossRef]
- Murali Iyangar, R.; Devaraj, E. Silibinin Triggers the Mitochondrial Pathway of Apoptosis in Human Oral Squamous Carcinoma Cells. Asian Pac. J. Cancer Prev. 2020, 21, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Jalil, A.T.; Riyad Muedii, Z.A.; Aminov, Z.; Alsaikhan, F.; Ramírez-Coronel, A.A.; Ramaiah, P.; Farhood, B. The Radiosensitizing Potentials of Silymarin/Silibinin in Cancer: A Systematic Review. Curr. Med. Chem. 2024, 31, 6992–7014. [Google Scholar] [CrossRef] [PubMed]
- Chioreanu, A.; Balica, N.C.; Mot, C.I.; Bugari, R.; Morar, R.; Baderca, F.; Marti, T.D.; Boru, C.; Avram, C.R.; Dema, S.; et al. A Retrospective Analysis from Western Romania Comparing the Treatment and Survivability of p16-Positive versus p16-Negative Oropharyngeal Cancer. Cancers 2024, 16, 945. [Google Scholar] [CrossRef]
- Ursu, R.G.; Danciu, M.; Spiridon, I.A.; Ridder, R.; Rehm, S.; Maffini, F.; McKay-Chopin, S.; Carreira, C.; Lucas, E.; Costan, V.V.; et al. Role of mucosal high-risk human papillomavirus types in head and neck cancers in Romania. PLoS ONE 2018, 13, e0199663. [Google Scholar] [CrossRef]
- Iliescu, D.; Marcovici, I.; Susan, M.; Susan, R.; Pinzaru, I.; Dumache, R.; Chiriac, S. In vitro evaluation of alphatocopherol as potential agent in combating the hepatotoxicity induced by ethinylestradiol. Farmacia 2024, 71, 533–540. [Google Scholar] [CrossRef]
- Yonbawi, A.R.; Abdallah, H.M.; Alkhilaiwi, F.A.; Koshak, A.E.; Heard, C.M. Anti-Proliferative, Cytotoxic and Antioxidant Properties of the Methanolic Extracts of Five Saudi Arabian Flora with Folkloric Medicinal Use: Aizoon canariense, Citrullus colocynthis, Maerua crassifolia, Rhazya stricta and Tribulus macropterus. Plants 2021, 10, 2073. [Google Scholar] [CrossRef]
- Chou, S.H.; Lan, J.; Esposito, E.; Ning, M.; Balaj, L.; Ji, X.; Lo, E.H.; Hayakawa, K. Extracellular Mitochondria in Cerebrospinal Fluid and Neurological Recovery After Subarachnoid Hemorrhage. Stroke 2017, 48, 2231–2237. [Google Scholar] [CrossRef]
- Wen, Y.; Gorsic, L.K.; Wheeler, H.E.; Ziliak, D.M.; Huang, R.S.; Dolan, M.E. Chemotherapeutic-induced apoptosis: A phenotype for pharmacogenomics studies. Pharmacogenet. Genom. 2011, 21, 476–488. [Google Scholar] [CrossRef]
- Dahma, R.; Motoc, M.; Dumitrel, Ș.-I.; Hajali, D.; Morariu-Briciu, D.-M.; Bonțe, D. Digoxin Exhibits Anticancer Activity in A375 and RPMI7951 Cutaneous Melanoma Cells: A Drug Repurposing Approach. Farmacia 2025, 73, 1306–1316. [Google Scholar] [CrossRef]
- Marcovici, I.; Chioibas, R.; Zupko, I.; Pinzaru, I.; Moaca, A.; Ledeti, A.; Barbu-Tudoran, L.; Geamantan, A.; Predescu, I.; Dehelean, C.A. Preclinical pharmaco-toxicological screening of biomimetic melanin-like nanoparticles as a potential therapeutic strategy for cutaneous melanoma. Front. Pharmacol. 2025, 16, 1487854. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yuan, B.; Shimada, R.; Hayashi, H.; Si, N.; Zhao, H.Y.; Bian, B.; Takagi, N. Cytocidal effects of arenobufagin and hellebrigenin, two active bufadienolide compounds, against human glioblastoma cell line U-87. Int. J. Oncol. 2018, 53, 2488–2502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, X.; Shen, Q.; Xing, D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat. Commun. 2019, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Monreal, A.V. Squamous Cell Carcinoma: A Silent Killer. Oral Pathology and Radiology, 8 December 2023. Available online: https://ostrowonline.usc.edu/squamous-cell-carcinoma-unveiling-the-faces-of-a-silent-killer-2/ (accessed on 15 November 2025).
- Budrukkar, A.; Kashid, S.R.; Swain, M.; Ghosh Laskar, S.; Mittal, N.; Mahimkar, M.; Sasidharan, A.; Patil, A.; Patel, U.; Murthy, V.; et al. Long-term outcomes of consecutive patients of oropharyngeal cancer treated with radical radiotherapy. BJC Rep. 2025, 3, 54. [Google Scholar] [CrossRef]
- Ramsridhar, S.; Rajkumar, C.; Veeraraghavan, V.P.; Francis, A.P.; Balasubramaniam, M.; Bharkavi, I. From cell lines to animal models: “Plant-derived chemotherapeutics unlocking new frontiers against oral squamous cell carcinoma”—A comprehensive systematic review. Discov. Oncol. 2025, 16, 340. [Google Scholar] [CrossRef] [PubMed]
- Crooker, K.; Aliani, R.; Ananth, M.; Arnold, L.; Anant, S.; Thomas, S.M. A Review of Promising Natural Chemopreventive Agents for Head and Neck Cancer. Cancer Prev. Res. 2018, 11, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.T.; Fecchi, K.; Pierdominici, M.; Ortona, E.; Peruzzu, D. Human Monocyte-Derived Dendritic Cells Are the Pharmacological Target of the Immunosuppressant Flavonoid Silibinin. Int. J. Mol. Sci. 2022, 23, 10417. [Google Scholar] [CrossRef]
- Borji, S.; Shahriarinour, M.; Shariati, S.; Ranji, N.; Nikpassand, M. Enhanced therapeutic efficacy of silibinin loaded silica coated magnetic nanocomposites against Pseudomonas aeruginosa in Combination with Ciprofloxacin and HepG2 cancer cells. Sci. Rep. 2025, 15, 21498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Hong, M.; Sun, X.Y.; Huang, D.W.; He, D.H.; Chen, Y.F.; Yuan, Y.; Liu, Y.Q. Silybin has therapeutic efficacy against non-small cell lung cancer through targeting of Skp2. Acta Mater. Medica 2022, 1, 302–313. [Google Scholar] [CrossRef]
- Nuth, M.; Benakanakere, M.R.; Ricciardi, R.P. Discovery of a potent cytotoxic agent that promotes G2/M phase cell cycle arrest and apoptosis in a malignant human pharyngeal squamous carcinoma cell line. Int. J. Oncol. 2022, 60, 41. [Google Scholar] [CrossRef]
- Hyodo, T.; Kuribayashi, N.; Fukumoto, C.; Komiyama, Y.; Shiraishi, R.; Kamimura, R.; Sawatani, Y.; Yaguchi, E.; Hasegawa, T.; Izumi, S.; et al. The mutational spectrum in whole exon of p53 in oral squamous cell carcinoma and its clinical implications. Sci. Rep. 2022, 12, 21695. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, A.; Kubina, R.; Wojtyczka, R.D.; Tanasiewicz, M.; Varoni, E.M.; Iriti, M. Flavonoids Induce Migration Arrest and Apoptosis in Detroit 562 Oropharynx Squamous Cell Carcinoma Cells. Processes 2021, 9, 426. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Dobryakova, N.V.; Ezhov, A.A.; Kudryashova, E.V. Achievement of the Selectivity of Cytotoxic Agents against Cancer Cells by Creation of Combined Formulation with Terpenoid Adjuvants as Prospects to Overcome Multidrug Resistance. Int. J. Mol. Sci. 2023, 24, 8023. [Google Scholar] [CrossRef] [PubMed]
- Ringer, J.; Morrison, B.; Kingsley, K. Evaluation of Hyaluronic Acid to Modulate Oral Squamous Cell Carcinoma Growth In Vitro. J. Funct. Biomater. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Gag, O.; Macasoi, I.; Pinzaru, I.; Dinu, S.; Popovici, R.; Cosoroaba, M.-R.; Buzatu, R.; Cabuta, M.; Chiriac, S.D. In Vitro Assessment of the Impact of Ultraviolet B Radiation on Oral Healthy and Tumor Cells. Photonics 2023, 10, 464. [Google Scholar] [CrossRef]
- Brizuela, M.; Winters, R. Histology, Oral Mucosa. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572115/ (accessed on 4 December 2025).
- Li, X.; González-Maroto, C.; Tavassoli, M. Crosstalk between CAFs and Tumour Cells in Head and Neck Cancer. Cell Death Discov. 2024, 10, 303. [Google Scholar] [CrossRef]
- Smirani, R.; Rémy, M.; Devillard, R.; Naveau, A. Use of Human Gingival Fibroblasts for Pre-Vascularization Strategies in Oral Tissue Engineering. Tissue Eng. Regen. Med. 2022, 19, 525–535. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhu, W.W.; Wang, M.Y.; Zhai, R.D.; Wang, Q.; Shen, W.L.; Liu, L.K. Cancer-Associated Fibroblasts Promote Oral Squamous Cell Carcinoma Progression through LOX-Mediated Matrix Stiffness. J. Transl. Med. 2021, 19, 513. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, H.; Kim, S.Y.; Hai, H.; Kim, E.; Ma, L.; Kim, D.; Kim, C.Y.; Park, K.; Park, S.; et al. Silibinin induces oral cancer cell apoptosis and reactive oxygen species generation by activating the JNK/c-Jun pathway. J. Cancer 2023, 14, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ali, M.; Biswal, P.; Jaiswal, A.; Mishra, D.; Agarwal, R.; Zaidi, R.; Singh, R.P. Inhibition of Growth and Survival of Head and Neck Cancer Cells by Silibinin Involves the Down-Regulation of Erk1/2, AKT and STAT3 Signaling. Mol. Carcinog. 2025, 64, 1735–1750. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, C.L.; Lu, D.W.; Liang, C.M.; Tai, M.C.; Chen, J.T. Silibinin Inhibits Platelet-Derived Growth Factor-Driven Cell Proliferation via Downregulation of N-Glycosylation in Human Tenon’s Fibroblasts in a Proteasome-Dependent Manner. PLoS ONE 2016, 11, e0168765. [Google Scholar] [CrossRef]
- Venugopal, D.C.; Viswanathan, P.; Ravindran, S.; Punnoose, A.M.; Yasasve, M.; Dicky John, D.G.; Prabhakar, L.; Ramanathan, G.; Sankarapandian, S.; Ramshankar, V. Antifibrotic effect of silymarin on arecoline-induced fibrosis in primary human buccal fibroblasts: An in silico and in vitro analysis. Mol. Biol. Rep. 2024, 51, 303. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Hormesis Defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jodynis-Liebert, J.; Kujawska, M. Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence. J. Clin. Med. 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Roman, A.; Boru, C.; Iftode, A.; Smeu, A.; Boța, M.; Vlaia, L.; Sașco, D.; Nicolov, M.; Iliescu, D. Genistein−quercetin combinatorial treatment yields enhanced anticancer activity in A-431 and A-375 human skin cancer cells. Farmacia 2025, 73, 576–590. [Google Scholar] [CrossRef]
- Raina, K.; Agarwal, C.; Wadhwa, R.; Serkova, N.J.; Agarwal, R. Energy deprivation by silibinin in colorectal cancer cells: A double-edged sword targeting both apoptotic and autophagic machineries. Autophagy 2013, 9, 697–713. [Google Scholar] [CrossRef]
- Ionescu, C.; Kamal, F.Z.; Ciobica, A.; Halitchi, G.; Burlui, V.; Petroaie, A.D. Oxidative Stress in the Pathogenesis of Oral Cancer. Biomedicines 2024, 12, 1150. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-Protocol 2019, 9, e3128. [Google Scholar] [CrossRef]
- Gottlieb, E.; Armour, S.M.; Harris, M.H.; Thompson, C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef]
- Wang, C.; He, C.; Lu, S.; Wang, X.; Wang, L.; Liang, S.; Wang, X.; Piao, M.; Cui, J.; Chi, G.; et al. Autophagy activated by silibinin contributes to glioma cell death via induction of oxidative stress-mediated BNIP3-dependent nuclear translocation of AIF. Cell Death Dis. 2020, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Esselun, C.; Bruns, B.; Hagl, S.; Grewal, R.; Eckert, G.P. Differential Effects of Silibinin A on Mitochondrial Function in Neuronal PC12 and HepG2 Liver Cells. Oxid. Med. Cell. Longev. 2019, 2019, 1652609. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ge, S.; Liu, J.; Sun, D.; Xi, Y.; Chen, P. Nuclear Structure, Size Regulation, and Role in Cell Migration. Cells 2024, 13, 2130. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhang, Y.; Chen, Y.; Li, Q.; Chen, J.; Dong, Y.; Shi, W. Silibinin Causes Apoptosis and Cell Cycle Arrest in Some Human Pancreatic Cancer Cells. Int. J. Mol. Sci. 2011, 12, 4861–4871. [Google Scholar] [CrossRef] [PubMed]
- Greier, M.C.; Runge, A.; Dudas, J.; Pider, V.; Skvortsova, I.I.; Savic, D.; Riechelmann, H. Mitochondrial dysfunction and epithelial to mesenchymal transition in head neck cancer cell lines. Sci. Rep. 2022, 12, 13255. [Google Scholar] [CrossRef]
- Dho, S.H.; Cho, M.; Woo, W.; Jeong, S.; Kim, L.K. Caspases as master regulators of programmed cell death: Apoptosis, pyroptosis and beyond. Exp. Mol. Med. 2025, 57, 1121–1132. [Google Scholar] [CrossRef]
- Hussain, H.; Ahmad, S.; Abd. Razak, M.F.; Wan Mohamud, W.N.; Bakar, J.; Ghazali, H.M. Determination of cell viability using acridine orange/propidium iodide dual-spectrofluorometry assay. Cogent Food Agric. 2019, 5, 1582398. [Google Scholar] [CrossRef]
- Vakili Zahir, N.; Nakhjavani, M.; Hajian, P.; Shirazi, F.H.; Mirzaei, H. Evaluation of Silibinin Effects on the Viability of HepG2 and HUVEC Cell Lines. Iran J. Pharm. Res. 2018, 17, 261–267. [Google Scholar] [PubMed]
- Tang, H.L.; Tang, H.M.; Hardwick, J.M.; Fung, M.C. Strategies for Tracking Anastasis, a Cell Survival Phenomenon That Reverses Apoptosis. J. Vis. Exp. 2015, 96, 51964. [Google Scholar] [CrossRef]
- Tang, H.M.; Talbot, C.C., Jr.; Fung, M.C.; Tang, H.L. Molecular Signature of Anastasis for Reversal of Apoptosis. F1000Research 2017, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Guzman, E.; Balasanyan, V.; Conner, C.M.; Wong, K.; Zhou, H.R.; Kosik, K.S.; Montell, D.J. A Molecular Signature for Anastasis, Recovery from the Brink of Apoptotic Cell Death. J. Cell Biol. 2017, 216, 3355–3368. [Google Scholar] [CrossRef] [PubMed]
- Deep, G.; Kumar, R.; Nambiar, D.K.; Jain, A.K.; Ramteke, A.M.; Serkova, N.J.; Agarwal, C.; Agarwal, R. Silibinin inhibits hypoxia-induced HIF-1α-mediated signaling, angiogenesis and lipogenesis in prostate cancer cells. Mol. Carcinog. 2017, 56, 833–848. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talpos, S.; Chioran, D.; Alexandru, G.C.; Dinu, Ș.; Coricovac, E.-D.; Smeu, A.; Ali, D.H.; Szuhanek, C.; Popa, M. Silibinin Triggers Mitochondrial Apoptosis and Declines Clonogenic Potential in Detroit 562 Human Pharyngeal Carcinoma Cells. Medicina 2025, 61, 2197. https://doi.org/10.3390/medicina61122197
Talpos S, Chioran D, Alexandru GC, Dinu Ș, Coricovac E-D, Smeu A, Ali DH, Szuhanek C, Popa M. Silibinin Triggers Mitochondrial Apoptosis and Declines Clonogenic Potential in Detroit 562 Human Pharyngeal Carcinoma Cells. Medicina. 2025; 61(12):2197. https://doi.org/10.3390/medicina61122197
Chicago/Turabian StyleTalpos, Serban, Doina Chioran, George Cătălin Alexandru, Ștefania Dinu, Elena-Dorina Coricovac, Andreea Smeu, Diana Haj Ali, Camelia Szuhanek, and Malina Popa. 2025. "Silibinin Triggers Mitochondrial Apoptosis and Declines Clonogenic Potential in Detroit 562 Human Pharyngeal Carcinoma Cells" Medicina 61, no. 12: 2197. https://doi.org/10.3390/medicina61122197
APA StyleTalpos, S., Chioran, D., Alexandru, G. C., Dinu, Ș., Coricovac, E.-D., Smeu, A., Ali, D. H., Szuhanek, C., & Popa, M. (2025). Silibinin Triggers Mitochondrial Apoptosis and Declines Clonogenic Potential in Detroit 562 Human Pharyngeal Carcinoma Cells. Medicina, 61(12), 2197. https://doi.org/10.3390/medicina61122197





