Improvement of Cardiometabolic Control with Dapagliflozin in Patients with Type 2 Diabetes in Primary Care: The AGORA-AP Study
Abstract
1. Introduction
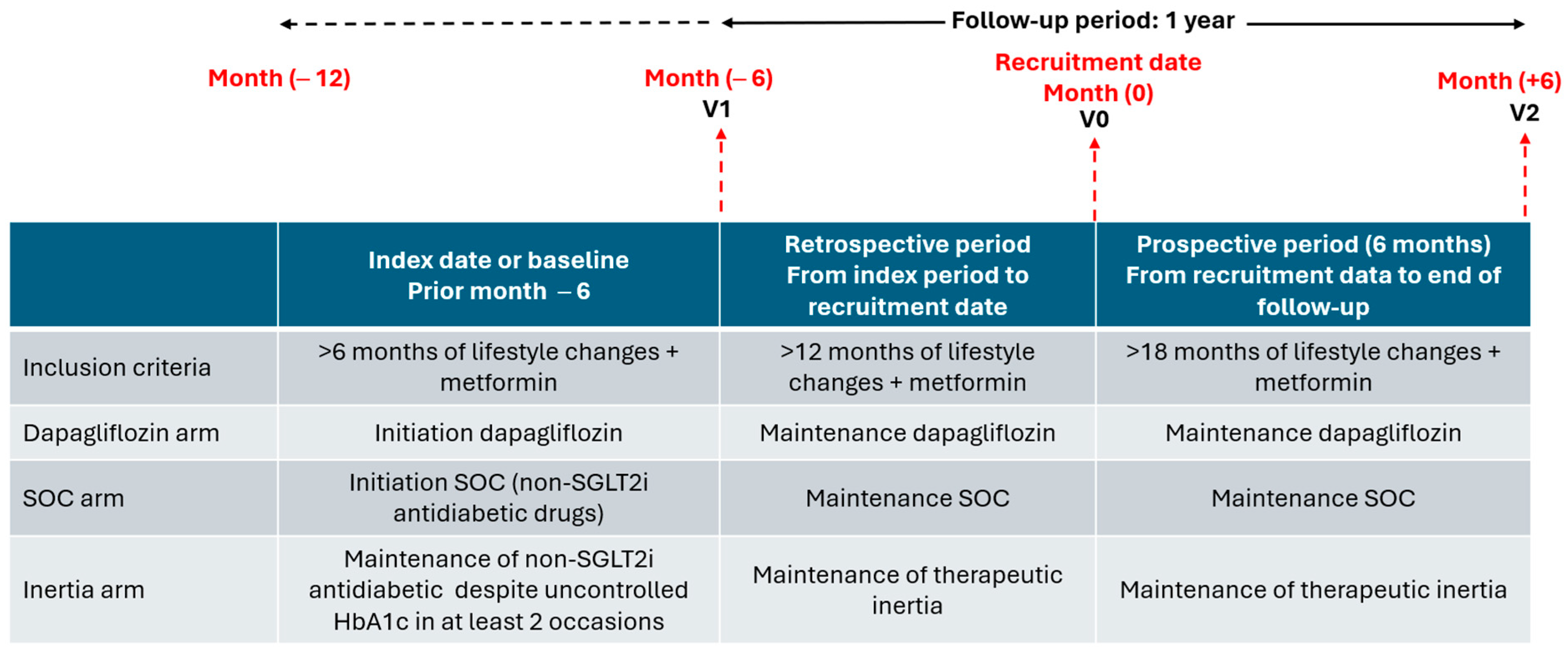
2. Methods
Statistical Analysis
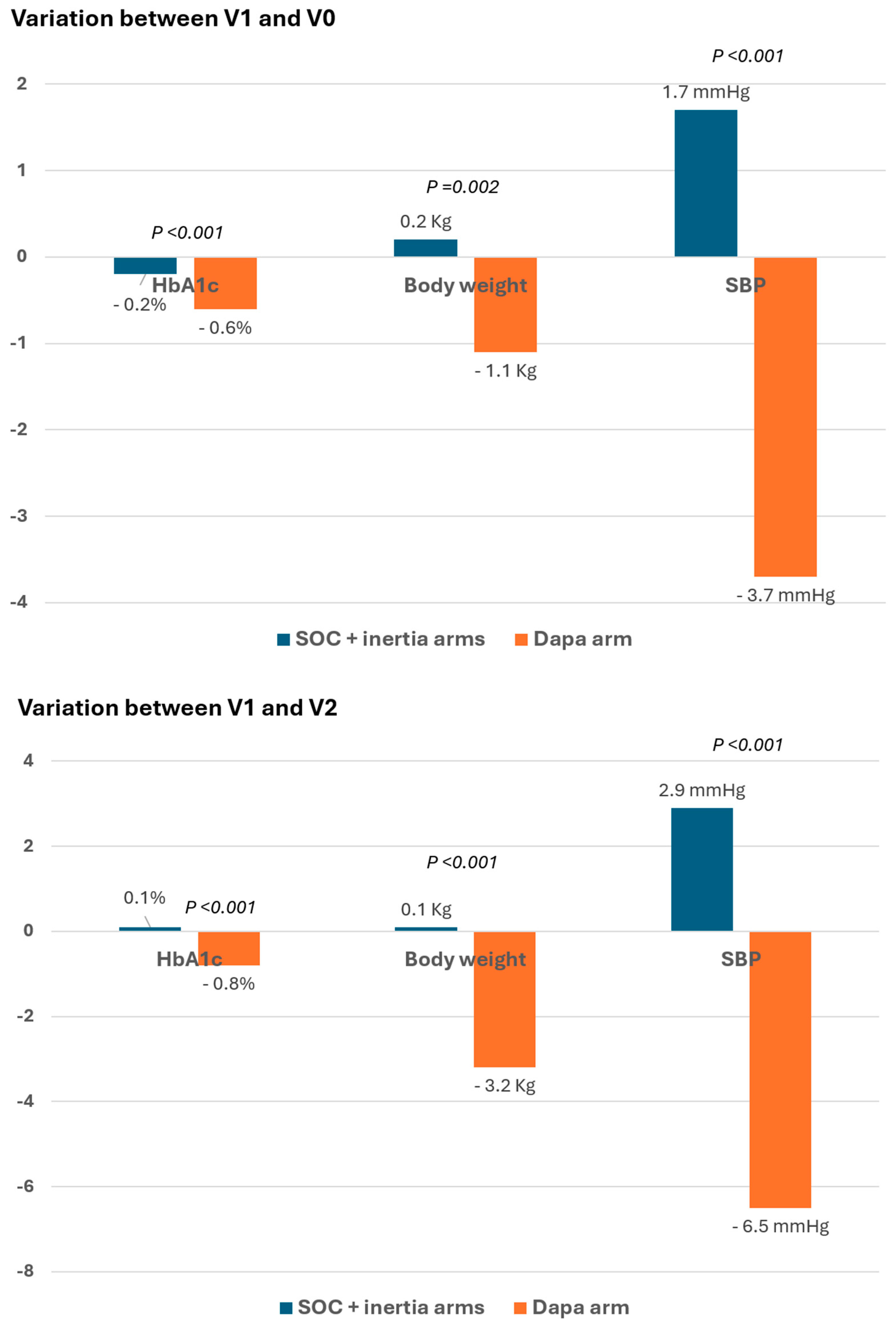
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Ruiz-García, A.; Arranz-Martínez, E.; García-Álvarez, J.C.; García-Fernández, M.E.; Palacios-Martínez, D.; Montero-Costa, A.; Ciria-de-Pablo, C.; López-Uriarte, B.; García-Pliego, R.A.; Chao-Escuer, P.; et al. Prevalence of diabetes mellitus in Spanish primary care setting and its association with cardiovascular risk factors and cardiovascular diseases. SIMETAP-DM study. Clin. Investig. Arterioscler. 2020, 32, 15–26. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study Group. VIII Study design, progress and performance. Diabetologia 1991, 34, 877–890. [Google Scholar] [CrossRef]
- Mann, C.; Braunwald, E.; Zelniker, T.A. Diabetic cardiomyopathy revisited: The interplay between diabetes and heart failure. Int. J. Cardiol. 2025, 438, 133554. [Google Scholar] [CrossRef]
- Dasgupta, I.; Zac-Varghese, S.; Chaudhry, K.; McCafferty, K.; Winocour, P.; Chowdhury, T.A.; Bellary, S.; Goldet, G.; Wahba, M.; De, P.; et al. Current management of chronic kidney disease in type-2 diabetes-A tiered approach: An overview of the joint Association of British Clinical Diabetologists and UK Kidney Association (ABCD-UKKA) guidelines. Diabet. Med. 2025, 42, e15450. [Google Scholar] [CrossRef] [PubMed]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- McLean, P.; Bennett, J.; “Trey” Woods, E.; Chandrasekhar, S.; Newman, N.; Mohammad, Y.; Khawaja, M.; Rizwan, A.; Siddiqui, R.; Birnbaum, Y.; et al. SGLT2 inhibitors across various patient populations in the era of precision medicine: The multidisciplinary team approach. Npj. Metab. Health Dis. 2025, 3, 29. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Fonseca, V.A.; et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2018 executive summary. Endocr. Pract. 2018, 24, 91–120. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycemia in type 2 diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes-2025. Diabetes. Care 2025, 48 (Suppl. S1), S128–S145. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S207–S238. [Google Scholar] [CrossRef]
- Ministry of Health. Spanish Agency for Medicines and Health Products. Available online: https://www.aemps.gob.es/industria-farmaceutica/comercializacion-de-medicamentos/ (accessed on 20 August 2025).
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; De Mets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Gregg, E.W.; Patorno, E.; Karter, A.J.; Mehta, R.; Huang, E.S.; White, M.; Patel, C.J.; McElvaine, A.T.; Cefalu, W.T.; Selby, J.; et al. Use of Real-World Data in Population Science to Improve the Prevention and Care of Diabetes-Related Outcomes. Diabetes Care 2023, 46, 1316–1326. [Google Scholar] [CrossRef]
- Toulis, K.A.; Willis, B.H.; Marshall, T.; Kumarendran, B.; Gokhale, K.; Ghosh, S.; Thomas, G.N.; Cheng, K.K.; Narendran, P.; Hanif, W.; et al. All-cause mortality in patients with diabetes under treatment with dapagliflozin: A population-based, open-cohort study in The Health Improvement Net-work database. J. Clin. Endocrin. Metab. 2017, 102, 1719–1725. [Google Scholar] [CrossRef]
- Pallarés-Carratalá, V.; Ruiz-García, A.; Serrano-Cumplido, A.; Segura-Fragoso, A.; Fernández-Pascual, V.; Sánchez-Sánchez, B.; Cervera-Pérez, M.I.; Alonso-Moreno, F.J.; Arranz-Martínez, E.; Barquilla-García, A.; et al. Comparison of baseline clinical characteristics among people with type 2 diabetes on second-line therapy previously added with dapagliflozin or another oral glucose-lowering drug: AGORA study. Clin. Investig. Arterioscler. 2025, 37, 100724. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint TaskForce of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Silva, C.; Catalán, V.; Rodríguez, A.; Galofré, J.C.; Escalada, J.; Valentí, V.; Rotellar, F.; Romero, S.; Ramírez, B.; et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care 2012, 35, 383–388. [Google Scholar] [CrossRef]
- Romero-Saldaña, M.; Fuentes-Jiménez, F.J.; Vaquero-Abellán, M.; Álvarez-Fernández, C.; Aguilera-López, M.D.; Molina-Recio, G. Predictive capacity and cutoff value of waist-to-height ratio in the incidence of metabolic syndrome. Clin. Nurs. Res. 2019, 28, 676–691. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC. Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105 (Suppl. S4), S117–S314. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S181–S206. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Morales, C.; Caballero, I.; González, B.; Tentolouris, N.; Consoli, A. Efficacy of Dapagliflozin in Southern Europe Across the Spectrum of Characteristics of Type 2 Diabetes: An International Real-World Analysis. Diabetes Metab. Syndr. Obes. 2022, 15, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Dapagliflozin: A Review in Type 2 Diabetes. Drugs 2019, 79, 1135–1146. [Google Scholar] [CrossRef]
- O’Donoghue, M.L.; Kato, E.T.; Mosenzon, O.; Murphy, S.A.; Cahn, A.; Herrera, M.; Tankova, T.; Šmahelová, A.; Merlini, P.; Gause-Nilsson, I.; et al. The efficacy and safety of dapagliflozin in women and men with type 2diabetes mellitus. Diabetologia 2021, 64, 1226–1234. [Google Scholar] [CrossRef]
- Scheerer, M.F.; Rist, R.; Proske, O.; Meng, A.; Kostev, K. Changes inHbA1c, body weight, and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy: A primary care database study. Diabetes Metab. Syndr. Obes. 2016, 9, 337–345. [Google Scholar] [CrossRef]
- Wilding, J.; Bailey, C.; Rigney, U.; Blak, B.; Beekman, W.; Emmas, C. Glycated hemoglobin, body weight and blood pressure in type 2 diabetes patients initiating dapagliflozin treatment in primary care: A retrospective study. Diabetes Ther. 2016, 7, 695–711. [Google Scholar] [CrossRef]
- Morieri, M.L.; Consoli, A.; Sesti, G.; Purrello, F.; Avogaro, A.; Fadini, G.P. Comparative effectiveness of dapagliflozin vs DPP-4inhibitors on a composite endpoint of HbA1c, body weight and blood pressure reduction in the real world. Diabetes Metab. Res. Rev. 2021, 37, e3353. [Google Scholar] [CrossRef] [PubMed]
- Deedwania, P. SGLT2 Inhibitors: The Dawn of a New Era in Cardio-Metabolic Therapeutics. Am. J. Cardiovasc. Drugs 2022, 22, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, N.; Aktaş, S.; Uyar, S.; Koca, N. Impact of SGLT2 Inhibitors on Cardiovascular Risk Scores, Metabolic Parameters, and Laboratory Profiles in Type 2 Diabetes. Life 2025, 15, 722. [Google Scholar] [CrossRef]
- Matteucci, A.; Pandozi, C.; Bonanni, M.; Mariani, M.V.; Sgarra, L.; Nesti, L.; Pierucci, N.; La Fazia, V.M.; Lavalle, C.; Nardi, F.; et al. Impact of empagliflozin and dapagliflozin on sudden cardiac death: A systematic review and meta-analysis of adjudicated randomized evidence. Heart Rhythm 2025. Sep 17 Epub ahead of print: S1547-5271(25)02890-5. [Google Scholar] [CrossRef] [PubMed]
| Dapa Arm (n = 226) | SOC Arm (n = 197) | Inertia Arm (n = 112) | Total (n = 535) | p | |
|---|---|---|---|---|---|
| Socio-demographic data | |||||
| Age, years | 62.3 (7.9) | 64.9 (8.2) | 64.6 (8.2) | 63.8 (8.2) | 0.002 |
| Sex (women), n (%) | 90 (39.8) | 72 (36.5) | 47 (42.0) | 209 (39.1) | 0.614 |
| Residence setting, n (%) | 0.003 | ||||
| Urban (>20,000 inhabitants) | 178 (78.8) | 153 (77.7) | 82 (73.2) | 413 (77.2) | |
| Semi-urban (5000 to 20,000 inhabitants) | 24 (10.6) | 12 (6.1) | 3 (2.7) | 39 (7.3) | |
| Rural (<5000 inhabitants) | 24 (10.6) | 32 (16.2) | 27 (24.1) | 83 (15.5) | |
| Race/ethnicity, n (%) | 0.29 | ||||
| White | 218 (96.5) | 190 (96.4) | 106 (94.6) | 514 (96.1) | |
| Black | 4 (1.8) | 0 (0) | 1 (0.9) | 5 (0.9) | |
| Latin American | 2 (0.9) | 4 (2.0) | 1 (0.9) | 7 (1.3) | |
| Asian | 0 (0) | 1 (0.5) | 2 (1.8) | 3 (0.6) | |
| Maghrebi/Arab | 2 (0.9) | 2 (1.0) | 2 (1.8) | 6 (1.1) | |
| Education level, n (%) | <0.001 | ||||
| Without studies | 11 (4.9) | 13 (6.6) | 6 (5.4) | 30 (5.6) | |
| Primary studies | 121 (53.5) | 108 (54.8) | 80 (71.4) | 309 (57.8) | |
| Higher studies | 77 (34.1) | 66 (33.5) | 18 (16.1) | 161 (30.1) | |
| University studies | 17 (7.5) | 10 (5.1) | 4 (3.6) | 31 (5.8) | |
| Housewife | 0 (0) | 0 (0) | 4 (3.6) | 4 (0.7) | |
| Employment status, n (%) | 0.017 | ||||
| Employed | 94 (41.6) | 66 (33.5) | 32 (28.6) | 192 (35.9) | |
| Unemployed | 7 (3.1) | 2 (1.0) | 5 (4.5) | 14 (2.6) | |
| Retired/pensioner | 96 (42.5) | 111 (56.3) | 56 (50.0) | 263 (49.2) | |
| Homework | 29 (12.8) | 18 (9.1) | 19 (17.0) | 66 (12.3) | |
| Alcohol consumption, n (%) | 28 (12.4) | 25 (12.7) | 14 (12.5) | 67 (12.5) | 0.996 |
| Physical examination | |||||
| BMI, Kg/m2 | 31.0 (5.1) | 29.8 (4.6) | 30.1 (4.6) | 30.4 (4.9) | 0.040 |
| Body weight, Kg | 84.9 (16.2) | 81.7 (15.6) | 83.5 (16.4) | 83.4 (16.0) | 0.114 |
| Waist-to-height ratio | 0.63 (0.06) | 0.63 (0.07) | 0.64 (0.08) | 0.63 (0.07) | 0.601 |
| Waist circumference, cm | 104.3 (10.2) | 103.3 (10.6) | 104.0 (12.7) | 103.9 (10.8) | 0.717 |
| Percentage of body fat CUNBAE (%), mean (SD) | 38.0 (7.9) | 36.0 (7.3) | 38.0 (8.1) | 37.3 (7.8) | 0.035 |
| SBP, mmHg | 129.3 (12.7) | 133.2 (13.4) | 133.5 (15.5) | 131.6 (13.7) | 0.004 |
| DBP, mmHg | 77.7 (8.8) | 78.5 (9.2) | 77.2 (8.8) | 77.9 (8.9) | 0.391 |
| Pulse pressure, mmHg | 51.6 (10.2) | 54.8 (10.6) | 55.4 (13.3) | 53.5 (11.2) | 0.002 |
| Heart rate, bpm | 71.6 (10.1) | 73.4 (10.3) | 73.7 (10.7) | 72.7 (10.3) | 0.113 |
| CVR factors | |||||
| Dyslipidemia, n (%) | 192 (85.0) | 157 (79.7) | 82 (73.2) | 431 (80.6) | 0.034 |
| Important dyslipidemia, n (%) | 7 (3.1) | 2 (1.0) | 2 (1.8) | 11 (2.1) | 0.314 |
| Hypertension, n (%) | 160 (70.8) | 137 (69.5) | 80 (71.4) | 377 (70.5) | 0.931 |
| Overweight (BMI 25 to 29.99 Kg/m2), n (%) | 88 (39.1) | 89 (45.4) | 41 (36.6) | 218 (40.9) | 0.237 |
| Obesity (BMI ≥ 30 Kg/m2), n (%) | 118 (52.4) | 87 (44.4) | 55 (49.1) | 260 (48.8) | |
| Sedentarism, n (%) | 96 (42.5) | 92 (46.7) | 57 (50.9) | 245 (45.8) | 0.326 |
| Smoking, n (%) | 0.081 | ||||
| Current | 48 (21.2) | 37 (18.8) | 12 (10.7) | 97 (18.1) | |
| Former | 72 (31.9) | 61 (31.0) | 31 (27.7) | 164 (30.7) | |
| Never | 106 (46.9) | 99 (50.3) | 69 (61.6) | 274 (51.2) | |
| Target organ damage | |||||
| Eye fundus examination performed, n (%) | 163 (72.1) | 132 (67.0) | 62 (55.4) | 357 (66.7) | 0.009 |
| LVH, n (%) | 9 (4.0) | 10 (5.1) | 9 (8.0) | 28 (5.2) | 0.287 |
| ABI < 0.9, n (%) | 13 (5.8) | 6 (3.0) | 7 (6.3) | 26 (4.9) | 0.323 |
| Carotid atherosclerotic plaques, n (%) | 6 (2.7) | 6 (3.0) | 7 (6.3) | 19 (3.6) | 0.217 |
| Increased carotid Intima-media thickness, n (%) | 6 (2.7) | 5 (2.5) | 6 (5.4) | 17 (3.2) | 0.334 |
| CV disease | |||||
| Coronary artery disease, n (%) | 19 (18.8) | 12 (11.7) | 11 (20.0) | 42 (16.2) | 0.264 |
| Stroke, n (%) | 9 (8.9) | 16 (15.4) | 6 (10.9) | 31 (11.9) | 0.348 |
| Albuminuria (30–299 mg/g), n (%) | 23 (10.2) | 21 (10.7) | 15 (13.4) | 59 (11.0) | 0.660 |
| Peripheral artery disease, n (%) | 11 (11.1) | 11 (10.7) | 3 (6.0) | 25 (9.9) | 0.582 |
| CKD (eGFR < 60 mL/min), n (%) | 7 (3.1) | 1 (0.5) | 4 (3.6) | 12 (2.2) | 0.113 |
| Proteinuria (≤300 mg/g), n (%) | 4 (1.8) | 1 (0.5) | 3 (2.7) | 8 (1.5) | 0.289 |
| Heart failure, n (%) | 5 (2.2) | 3 (1.5) | 0 (0) | 8 (1.5) | 0.288 |
| CVR score, n (%) | 0.001 | ||||
| High | 83 (36.7) | 82 (41.6) | 66 (58.9) | 231 (43.2) | |
| Very high | 119 (52.7) | 87 (44.2) | 38 (33.9) | 244 (45.6) | |
| Extreme | 24 (10.6) | 28 (14.2) | 8 (7.1) | 60 (11.2) | |
| Biochemical parameters | |||||
| Hemoglobin (g/dL), mean (SD) | 15.2 (1.5) | 14.4 (1.2) | 14.7 (1.3) | 14.8 (1.4) | <0.001 |
| FPG (mg/dL), mean (SD) | 138.6 (33.8) | 133.5 (32.7) | 134.3 (29.3) | 135.8 (32.5) | 0.242 |
| HbA1c (%), mean (SD) | 7.1 (0.9) | 6.7 (0.8) | 7.0 (0.9) | 6.93 (0.9) | <0.001 |
| TC (mg/dL), mean (SD) | 168.3 (35.8) | 169.0 (36.0) | 171.5 (33.3) | 169.2 (35.4) | 0.73 |
| HDL-c (mg/dL), mean (SD) | 47.7 (11.4) | 48.7 (11.1) | 47.5 (12.1) | 48.0 (11.4) | 0.587 |
| LDL-c (mg/dL), mean (SD) | 90.1 (31.7) | 90.5 (31.3) | 93.1 (29.2) | 90.9 (31.0) | 0.7 |
| TG (mg/dL), mean (SD) | 156.6 (66.3) | 145.7 (81.2) | 149.7 (68.8) | 151.1 (72.6) | 0.299 |
| Sodium (mEq/L), mean (SD) | 140.8 (2.2) | 140.4 (2.8) | 140.8 (2.7) | 140.6 (2.5) | 0.314 |
| Potassium (mEq/L), mean (SD) | 4.6 (0.4) | 4.6 (0.4) | 4.6 (0.4) | 4.6 (0.4) | 0.338 |
| SUA (mg/dL), mean (SD) | 4.9 (1.2) | 5.5 (1.2) | 5.5 (1.4) | 5.2 (1.3) | <0.001 |
| AST (U/L), mean (SD) | 26.4 (13.0) | 25.0 (14.0) | 26.5 (13.0) | 25.9 (13.4) | 0.702 |
| ALT (U/L), mean (SD) | 28.2 (17.6) | 24.7 (13.1) | 27.0 (14.8) | 26.7 (15.6) | 0.087 |
| GGT (U/L), mean (SD) | 30.9 (19.5) | 34.7 (24.7) | 31.3 (20.1) | 32.3 (21.6) | 0.247 |
| FLI, mean (SD) | 69.8 (21.3) | 66.7 (23.1) | 66.9 (26.1) | 68.2 (22.9) | 0.46 |
| Creatinine (mg/dL), mean (SD) | 0.8 (0.2) | 0.9 (0.2) | 0.9 (0.2) | 0.8 (0.2) | 0.106 |
| eGFR (mL/min/1.73 m2), mean (SD) | 99.2 (16.3) | 96.7 (15.9) | 96.6 (18.9) | 97.7 (16.7) | 0.23 |
| uACR (mg/g), mean (SD) | 13.3 (12.8) | 15.3 (15.2) | 19.6 (21.0) | 15.2 (15.6) | 0.012 |
| Reduction HbA1c ≥ 0.5% + reduction body weight ≥ 2 kg + reduction SBP ≥ 2 mmHg between V1 and V0 | Adjusted for age, residence setting, education level, employment status | ||
| OR (95% CI) | p | ||
| SOC + Inertia arms, n (%) | 6 (1.9) | 1 Reference | <0.001 |
| DAPA arm, n (%) | 49 (21.8) | 12.7 (5.3–30.6) | |
| Reduction HbA1c ≥ 0.5% + reduction body weight ≥ 2 kg + reduction SBP ≥ 2 mmHg between V1 and V2 | Adjusted for age, residence setting, education level, employment status | ||
| OR (95% CI) | p | ||
| SOC + Inertia arms, n (%) | 12 (4.0) | 1 Reference | <0.001 |
| DAPA arm, n (%) | 82 (37.3) | 14.4 (7.5–27.7) | |
| Reduction HbA1c ≥ 0.5% + reduction body weight ≥ 2 kg + reduction SBP ≥ 2 mmHg between V1 and V0 | Adjusted for age, residence setting, education level, employment status | ||
| OR (95% CI) | p | ||
| Inertia arm, n (%) | 2 (1.8) | 1 Reference | 0.001 |
| DAPA arm, n (%) | 49 (21.8) | 13.3 (3.1–57.5) | |
| Reduction HbA1c ≥ 0.5% + reduction body weight ≥ 2 kg + reduction SBP ≥ 2 mmHg between V1 and V2 | Adjusted for age, residence setting, education level, employment status | ||
| OR (95% CI) | p | ||
| Inertia arm, n (%) | 5 (4.5) | 1 Reference | <0.001 |
| DAPA arm, n (%) | 82 (37.3) | 12.0 (4.6–31.5) | |
| Reduction HbA1c ≥ 0.5% + reduction body weight ≥ 2 kg + reduction SBP ≥ 2 mmHg between V1 and V0 | Adjusted for age, residence setting, education level, employment status | ||
| OR (95% CI) | p | ||
| Inertia arm, n (%) | 2 (1.8) | 1 Reference | 0.983 |
| SOC arm, n (%) | 4 (2.0) | 1.0 (0.2–6.2) | |
| Reduction HbA1c ≥ 0.5% + reduction body weight ≥ 2 kg + reduction SBP ≥ 2 mmHg between V1 and V2 | Adjusted for age, residence setting, education level, employment status | ||
| OR (95% CI) | p | ||
| Inertia arm, n (%) | 5 (4.5) | 1 Reference | 0.523 |
| SOC arm, n (%) | 7 (3.6) | 0.7 (0.2–2.3) | |
| SOC + Inertia Arms | Dapa Arm | Adjusted for Age, Residence Setting, Education Level, Employment Status | ||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Incidence Density Per 1000 Patient-Years | n (%) | Incidence Density Per 1000 Patient-Years | OR (95% CI) | p | Rate Ratio (95% CI) | p | |
| Incidence of dyslipidemia | 12 (4.3) | 7 (3.3) | 0.6 (0.2–1.6) | 0.286 | ||||
| Incidence of hypertension | 15 (5.7) | 11 (6.0) | 0.9 (0.4–2.1) | 0.791 | ||||
| Incidence of atrial fibrillation | 8 (2.6) | 4.8 | 7 (3.1) | 6.5 | 1.2 (0.4–3.5) | 0.769 | 1.4 (0.4–4.3) | 0.122 |
| Incidence of heart failure | 1 (0.3) | 0.6 | 2 (0.9) | 1.8 | 3.5 (0.2–49.9) | 0.363 | 3.1 (0.2–180.6) | 0.448 |
| Incidence of heart failure hospitalization | 0 (0.0) | 0 (0.0) | NC | |||||
| Incidence of myocardial infarction | 4 (1.3) | 2.4 | 2 (0.9) | 1.8 | 0.7 (0.1–4.2) | 0.705 | 0.8 (0.07–5.3) | 0.098 |
| Incidence of stroke | 8 (2.6) | 4.9 | 1 (0.4) | 0.9 | 0.2 (0.03–1.9) | 0.178 | 0.2 (0.004–1.4) | 0.32 |
| Incidence of peripheral artery disease | 3 (1.0) | 1.8 | 3 (1.3) | 2.7 | 1.2 (0.2–6.5) | 0.803 | 1.5 (0.2–11.5) | 0.17 |
| Death (V2) | 2 (0.6) | 1.2 | 3 (1.3) | 2.7 | 2.7 (0.4–18.4) | 0.3 | 2.3 (0.3–27.5) | 0.34 |
| Cardiovascular death (V2) | 0 (0.0) | 0 (0.0) | NC | |||||
| Incidence of MACE | 10 (3.2) | 6.0 | 3 (1.3) | 2.7 | 0.5 (0.1–1.8) | 0.275 | 0.5 (0.08–1.8) | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallarés-Carratalá, V.; Ruiz-García, A.; Fragoso, A.S.; Escobar-Cervantes, C.; Cervera-Pérez, M.I.; Alonso-Moreno, F.J.; Arranz-Martínez, E.; Barquilla-García, A.; Rey-Aldana, D.; Polo-García, J.; et al. Improvement of Cardiometabolic Control with Dapagliflozin in Patients with Type 2 Diabetes in Primary Care: The AGORA-AP Study. Medicina 2025, 61, 2087. https://doi.org/10.3390/medicina61122087
Pallarés-Carratalá V, Ruiz-García A, Fragoso AS, Escobar-Cervantes C, Cervera-Pérez MI, Alonso-Moreno FJ, Arranz-Martínez E, Barquilla-García A, Rey-Aldana D, Polo-García J, et al. Improvement of Cardiometabolic Control with Dapagliflozin in Patients with Type 2 Diabetes in Primary Care: The AGORA-AP Study. Medicina. 2025; 61(12):2087. https://doi.org/10.3390/medicina61122087
Chicago/Turabian StylePallarés-Carratalá, Vicente, Antonio Ruiz-García, Antonio Segura Fragoso, Carlos Escobar-Cervantes, María Inmaculada Cervera-Pérez, Francisco Javier Alonso-Moreno, Ezequiel Arranz-Martínez, Alfonso Barquilla-García, Daniel Rey-Aldana, José Polo-García, and et al. 2025. "Improvement of Cardiometabolic Control with Dapagliflozin in Patients with Type 2 Diabetes in Primary Care: The AGORA-AP Study" Medicina 61, no. 12: 2087. https://doi.org/10.3390/medicina61122087
APA StylePallarés-Carratalá, V., Ruiz-García, A., Fragoso, A. S., Escobar-Cervantes, C., Cervera-Pérez, M. I., Alonso-Moreno, F. J., Arranz-Martínez, E., Barquilla-García, A., Rey-Aldana, D., Polo-García, J., & Cinza-Sanjurjo, S. (2025). Improvement of Cardiometabolic Control with Dapagliflozin in Patients with Type 2 Diabetes in Primary Care: The AGORA-AP Study. Medicina, 61(12), 2087. https://doi.org/10.3390/medicina61122087







