A Clinical Prediction Model for Atypical Tuberculosis Manifestations Among Older Adults
Abstract
1. Introduction
2. Materials and Methods
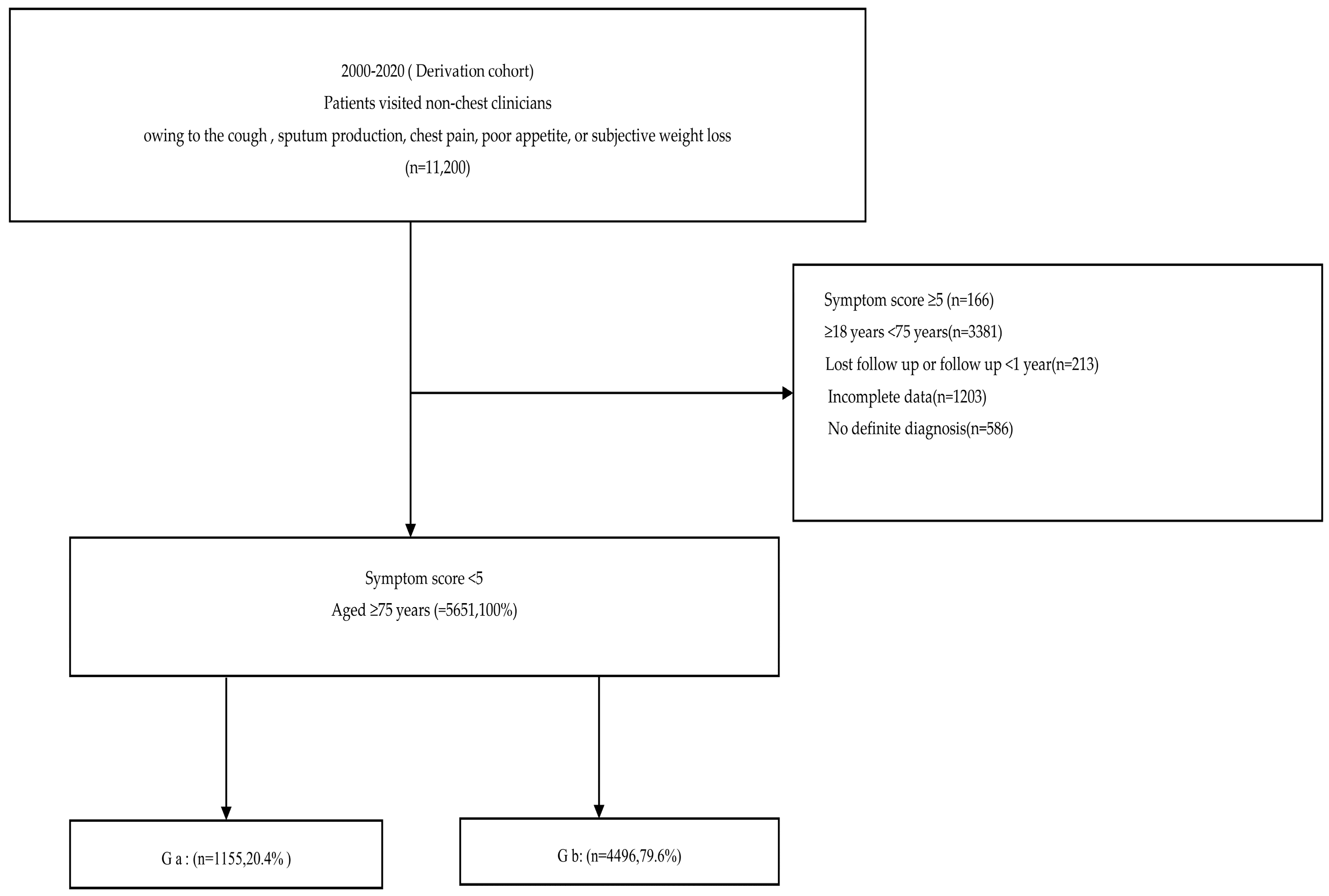
2.1. Derivation Cohort
2.1.1. Patient Selection and Grouping
2.1.2. Data Collection
2.1.3. Imaging Assessment
2.1.4. Statistical Analysis
2.2. Methods
2.2.1. Validation Cohort
2.2.2. Subgroup Analyses
2.2.3. Ethical Approval
3. Results
3.1. Baseline Characteristics
3.2. Screening Potential Risk Factors
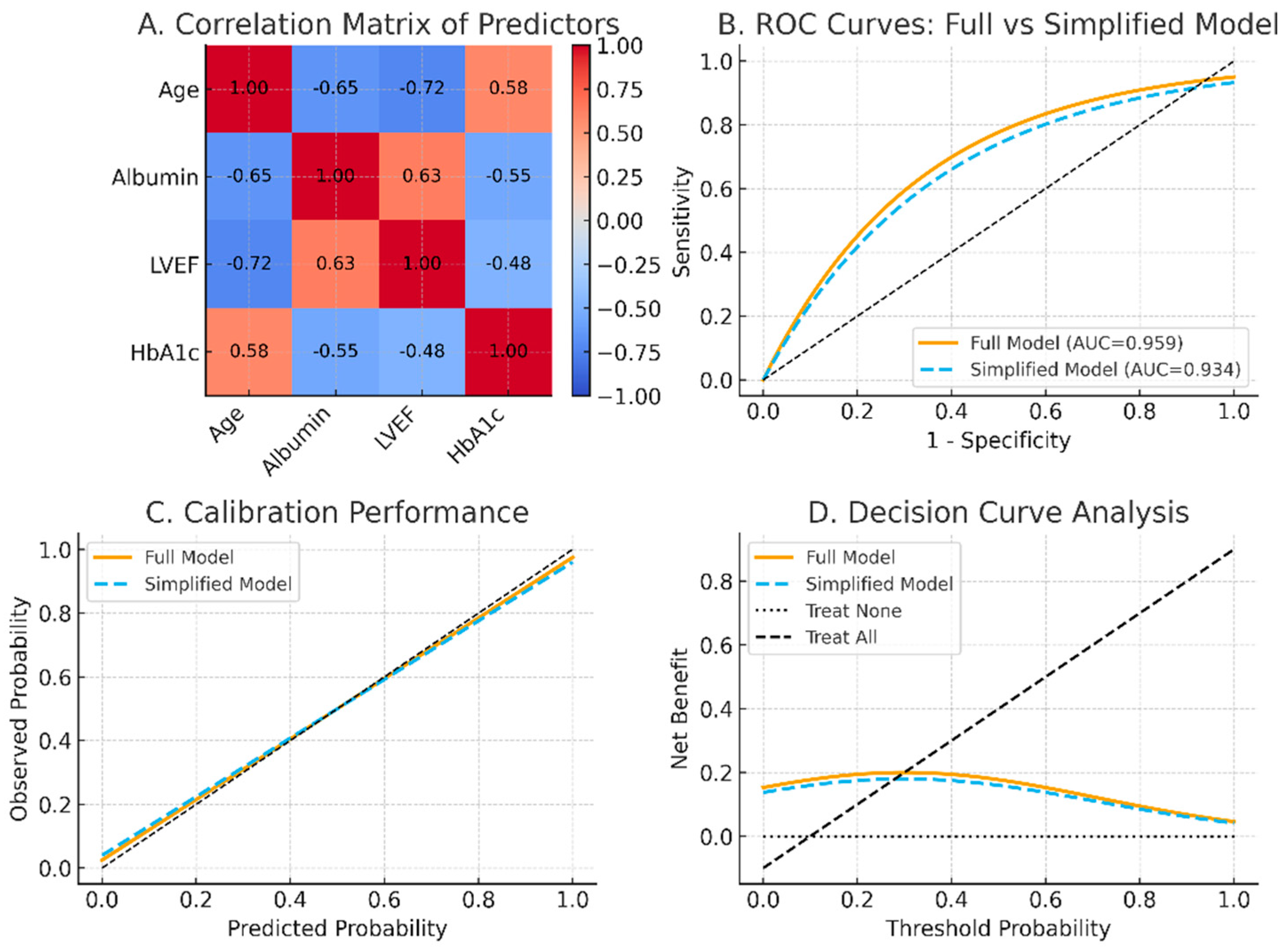
3.3. Multivariate Logistic Regression
3.4. Predictive Scoring System
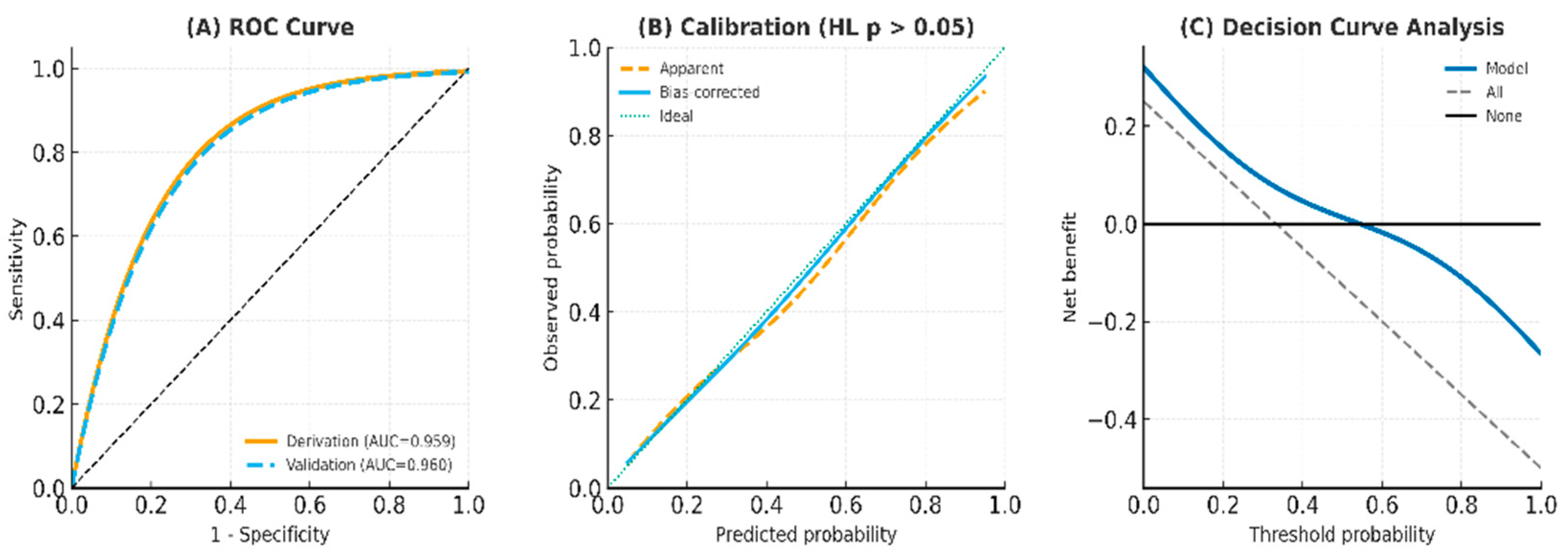
3.5. Model Performance and Validation
3.6. Post-Test Probability for the Score System Including Age > 85 Years (Primary Cohort)
3.7. Area Under the Curve (AUC) Analysis in the Symptoms Core, Including Age > 85 Years in the Derivation Cohort
4. Discussion
4.1. Main Findings
4.2. Novel Diagnostic Findings
4.3. Analytical and Modeling Strengths
4.4. Clinical Implications and TRIPOD Adherence
4.5. Interpretation and Broader Impact
4.6. Limitations and Future Work
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aPTB | Active pulmonary tuberculosis |
| AFB | Acid-fast bacilli |
| AUC | Area under the curve |
| BMI | Body mass index |
| CI | Confidence interval |
| CXR | Chest X-ray |
| DCA | Decision curve analysis |
| DM | Diabetes mellitus |
| EMR | Electronic medical record |
| FNR | False negative rate |
| FPR | False positive rate |
| Ga | Group a (aPTB not initially suspected by non-chest clinicians) |
| Gb | Group b (non-aPTB within 24 h of admission) |
| HL | Hosmer—Lemeshow test |
| IRB | Institutional Review Board |
| LASSO | Least absolute shrinkage and selection operator |
| LVEF | Left ventricular ejection fraction |
| LR+ | Positive likelihood ratio |
| NPV | Negative predictive value |
| OR | Odds ratio |
| PCR | Polymerase chain reaction |
| PPV | Positive predictive value |
| PTB | Pulmonary tuberculosis |
| ROC | Receiver operating characteristic |
| SD | Standard deviation |
| TB | Tuberculosis |
| TRIPOD | Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis |
| VIF | Variance inflation factor |
| WHO | World Health Organization |
References
- Goletti, D.; Meintjes, G.; Andrade, B.B.; Zumla, A.; Shan Lee, S. Insights from the 2024 who global tuberculosis report–more comprehensive action, innovation, and investments required for achieving who end tb goals. Int. J. Infect. Dis. 2025, 150, 107325. [Google Scholar] [CrossRef]
- Teo, A.K.J.; Rahevar, K.; Morishita, F.; Ang, A.; Yoshiyama, T.; Ohkado, A.; Kawatsu, L.; Yamada, N.; Uchimura, K.; Choi, Y.; et al. Tuberculosis in older adults: Case studies from four countries with rapidly ageing populations in the western pacific region. BMC Public Health 2023, 23, 370. [Google Scholar]
- van Arkel, C.; Storms, I.; Kurver, L.; Smeenk, F.; Wielders, P.; Hoefsloot, W.; Carpaij, N.; Boeree, M.J.; van Crevel, R.; van Laarhoven, A.; et al. Elderly patients with tuberculosis in a low-incidence country: Clinical characteristics, inflammation and outcome. J. Infect. 2024, 89, 106200. [Google Scholar] [CrossRef]
- Saukkonen, J.J.; Duarte, R.; Munsiff, S.S.; Winston, C.A.; Mammen, M.J.; Abubakar, I.; Acuña-Villaorduña, C.; Barry, P.M.; Bastos, M.L.; Carr, W.; et al. Updates on the treatment of drug-susceptible and drug-resistant tuberculosis: An official ats/cdc/ers/idsa clinical practice guideline. Am. J. Respir. Crit. Care Med. 2025, 211, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Guo, J.; Gong, F. Construction and validation of a diagnostic scoring system for predicting active pulmonary tuberculosis in patients with positive t-spot based on indicators associated with coagulation and inflammation: A retrospective cross-sectional study. Infect. Drug Resist. 2023, 16, 5755–5764. [Google Scholar] [CrossRef] [PubMed]
- Gebregergs, G.B.; Berhe, G.; Gebrehiwot, K.G.; Mulugeta, A. Development and validation of a risk prediction model for pulmonary tuberculosis in presumptive tuberculosis patients in tigray, northern ethiopia. Sci. Rep. 2025, 15, 32270. [Google Scholar] [CrossRef]
- Woodruff, R.; Pratt, R.; Kolasa, M. Progress toward tuberculosis elimination and tuberculosis program performance—National tuberculosis indicators project, 2016–2022. MMWR Surveill. Summ. 2024, 73, 1–18. [Google Scholar] [CrossRef]
- Scott, I.A.; Crock, C.; Twining, M. Too much versus too little: Looking for the “sweet spot” in optimal use of diagnostic investigations. Med. J. Aust. 2024, 220, 67–70. [Google Scholar] [CrossRef]
- Choi, H.; Shin, J.; Jung, J.-H.; Han, K.; Choi, W.; Lee, H.R.; Yoo, J.E.; Yeo, Y.; Lee, H.; Shin, D.W. Tuberculosis and osteoporotic fracture risk: Development of individualized fracture risk estimation prediction model using a nationwide cohort study. Front. Public Health 2024, 12, 1358010. [Google Scholar] [CrossRef]
- Mohamad Zani, R.A.; Ahmad Yusof, H.; Azizan, N.; Hyder Ali, I.A.; Ismail, S.; Mohd Shariff, N. Sarcopenia and it’s influencing factors among adults with asthma, chronic obstructive pulmonary disease, and tuberculosis in penang, malaysia. BMC Public Health 2025, 25, 1572. [Google Scholar] [CrossRef] [PubMed]
- Che, B.; Zheng, X.; Chen, B.; Lu, Y.; Zhang, Y.; Xu, B. The temporal trend of tuberculosis burden in an aging population in china: A secondary data analysis from the gbd 2019. BMC Pulm. Med. 2024, 24, 476. [Google Scholar] [CrossRef]
- Teo, A.K.J.; Morishita, F.; Islam, T.; Viney, K.; Ong, C.W.M.; Kato, S.; Kim, H.; Liu, Y.; Oh, K.H.; Yoshiyama, T.; et al. Tuberculosis in older adults: Challenges and best practices in the western pacific region. Lancet Reg. Health–West. Pac. 2023, 36, 100770. [Google Scholar]
- Terzi, H.; Aydemir, O.; Karakeçe, E.; Demiray, T.; Koroglu, M.; Altindis, M. Evaluation of the Xpert MTB/RIF test performance in the diagnosis of suspected M. tuberculosis in pulmonary and extrapulmonary clinical specimens. Mediterr. J. Infect. Microbes Antimicrob. 2024, 13, 13. [Google Scholar] [CrossRef]
- Aggarwal, R.; Ruff, C.T.; Virdone, S.; Perreault, S.; Kakkar, A.K.; Palazzolo, M.G.; Dorais, M.; Kayani, G.; Singer, D.E.; Secemsky, E.; et al. Development and validation of the doac score: A novel bleeding risk prediction tool for patients with atrial fibrillation on direct-acting oral anticoagulants. Circulation 2023, 148, 936–946. [Google Scholar] [CrossRef]
- Tan, J.; He, Y.; Zhang, Z.; Liu, J.; Du, J.; Zhao, W.; Liu, Y. Derivation and validation of prediction models for prolonged length of stay and 30-day readmission in elderly patients with type 2 diabetes mellitus: A multicenter study. J. Diabetes Res. 2025, 2025, 3148242. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Ye, W.; Liao, W.; Zheng, Z.; Lin, X.; Chen, F.; Pan, J.; Chen, B.; Chen, R.; et al. Analysis of factors influencing bronchiectasis patients with active pulmonary tuberculosis and development of a nomogram prediction model. Front. Med. 2024, 11, 1457048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, X.; Lv, J.; Dai, B.; Liu, Q.; Ding, X.; Pan, J.; Ding, H.; Lu, W.; Zhu, L.; et al. Prospective cohort study on tuberculosis incidence and risk factors in the elderly population of eastern china. Heliyon 2024, 10, e24507. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.; Chen, S.; Zhang, Y.; Wang, W.; Wu, Q.; Luo, D.; Ling, Y.; Li, Y.; Wang, L.; et al. The asymptomatic tuberculosis proportion among the elderly population: A systematic review and meta-analysis. BMC Public Health 2024, 24, 3551. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Y.; Guan, J.; Liu, Y.; Song, W.; Liu, W.; Yin, X.; Liu, Y.; Li, T.; Jin, L.; et al. Global burden of tuberculosis among adults aged 60 years and older, 1990-2021: Findings from the global burden of disease study 2021. Int. J. Infect. Dis. 2025, 158, 107966. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Xie, Q.; Wei, W.; Huang, Z.; Liu, X.; Ye, Q.; Liu, Y.; Lu, X.; Lu, Y.; Hou, R.; et al. Association between blood inflammatory status and the survival of tuberculosis: A five-year cohort study. Front. Immunol. 2025, 16, 1556857. [Google Scholar] [CrossRef]
- Seid, G.; Alemu, A.; Diriba, G.; Hailu, M.; Wondimu, A.; Tadesse, M.; Tadesse, G.; Mariam, S.H.; Gumi, B. Active tuberculosis in household contacts of bacteriologically confirmed pulmonary tuberculosis patients: A multicenter study finding the ‘missed one’ in central ethiopia. PLoS ONE 2025, 20, e0316903. [Google Scholar] [CrossRef]
- Du, R.; Rao, L.; Xiao, X.; Zhao, Q.; Shen, X.; Xu, B. The impact of population aging on tuberculosis prevention and control in shanghai: A prediction based on age-period-cohort models. Aging Clin. Exp. Res. 2025, 37, 175. [Google Scholar] [CrossRef] [PubMed]
- Gebregergs, G.B.; Berhe, G.; Gebrehiwot, K.G.; Mulugeta, A. Predictors contributing to the estimation of pulmonary tuberculosis among adults in a resource-limited setting: A systematic review of diagnostic predictions. SAGE Open Med. 2024, 12, 20503121241243238. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Huang, L.; Jiao, W.; Wang, Y.; Wang, M.; Zhong, X.; Tong, X.; Jin, J.; Li, Y. Development and validation of a clinical-friendly model for predicting 180-day mortality in the older with community-acquired pneumonia. BMC Geriatr. 2025, 25, 271. [Google Scholar] [CrossRef] [PubMed]
| Variable | Ga (n = 1155) | Gb (n = 4496) | p Value | p (Adjusted) | Effect Size (Cohen’s d or Cramér’s V) |
|---|---|---|---|---|---|
| Age | 90.87 (6.03) | 81.92 (2.50) | <0.001 ** | 0.03 | 2.376 |
| Age > 85 years | 907 (78.5%) | 680 (15.1%) | <0.001 ** | 0.04 | 0.783 |
| Gender (male) | 592 (51.3%) | 2256 (50.1%) | 0.008 ** | 0.32 | 0.054 |
| Initial Smear-negative | 791 (68.4%) | N/A | N/A | N/A | N/A |
| Cough > 2 weeks (2) # | 441 (38.2%) | 1600 (35.6%) | 0.047 * | 1.0 | 0.008 |
| Sputum(including hemoptysis) (2) # | 446 (38.6%) | 2340 (52.0%) | 0.122 | 1.0 | 0.005 |
| Chest pain (1) # | 463 (40.1%) | 1605 (35.7%) | 0.078 | 1.0 | 0.011 |
| Body weight loss (1) # | 437 (37.8%) | 1597 (35.5%) | 0.053 | 1.0 | 0.024 |
| Poor appetite (1) # | 459 (39.7%) | 1780 (39.5%) | 0.052 | 1.0 | 0.037 |
| Symptom score ≤ 1 | 797 (69.0%) | 1681 (37.3%) | <0.001 *** | 0.04 | 0.321 |
| Symptom score | 0.89 (0.04) | 2.44 (0.47) | <0.001 *** | 0.04 | −4.679 |
| Weakness | 486 (42.0%) | 2085 (46.3%) | 0.003 ** | 0.12 | 0.023 |
| Fever | 265 (22.9%) | 2024 (45.0%) | <0.001 *** | 0.04 | 0.066 |
| Dyspnea | 481 (41.6%) | 1925 (42.8%) | 0.019 * | 0.76 | 0.023 |
| Night sweating | 107 (9.3%) | 921 (20.4%) | <.0001 *** | 0.004 | 0.100 |
| Diabetes | 925 (80.1%) | 1020 (22.7%) | <0.001 *** | 0.04 | 0.609 |
| Cardiovascular diseases | 847 (73.3%) | 780 (17.3%) | <0.001 *** | 0.04 | 0.594 |
| Chronic renal disease | 857 (74.2%) | 3140 (69.8%) | 0.001 ** | 0.04 | 0.072 |
| Chronic respiratory disease | 974 (84.3%) | 3788 (84.3%) | 0.106 | 1.0 | 0.001 |
| Chronic liver disease | 914 (79.1%) | 3500 (77.8%) | 0.001 ** | 0.04 | 0.066 |
| Gastrectomy | 282 (24.4%) | 730 (16.2%) | <0.001 *** | 0.04 | 0.101 |
| Hematology-related disease | 343 (29.7%) | 1645 (36.6%) | 0.468 | 1.0 | 0.005 |
| Active cancer | 228 (19.7%) | 858 (19.1%) | 0.217 | 1.0 | 0.003 |
| Osteoporosis/sarcopenia | 845 (73.1%) | 751 (16.7%) | <0.001 *** | 0.04 | 0.590 |
| Hypoalbuminemia < 3.5 g/dL | 1059 (91.7%) | 981 (21.8%) | <0.001 *** | 0.04 | 0.784 |
| BMI < 17.5 kg/m2 | 491 (42.5%) | 2066 (46.0%) | 0.202 | 1.0 | 0.061 |
| Mental disorder | 908 (78.6%) | 3087 (68.7%) | <0.001 *** | 0.04 | 0.139 |
| Previous TB | 228 (19.7%) | 925 (20.5%) | 0.394 | 1.0 | 0.014 |
| Immunosuppressants | 575 (49.8%) | 1510 (33.6%) | <0.001 *** | 0.03 | 0.232 |
| Smoking | 495 (42.9%) | 636 (14.2%) | 0.021 * | 0.63 | 0.048 |
| Drinking | 264 (22.9%) | 804 (17.8%) | <0.001 *** | 0.03 | 0.092 |
| Consolidation | 531 (46.0%) | 1752 (38.9%) | <0.001 *** | 0.03 | 0.112 |
| Non-nodular patches | 527 (45.5%) | 1318 (29.3%) | <0.001 *** | 0.03 | 0.104 |
| Cavitation | 102 (8.8%) | 1545 (34.3%) | <0.001 *** | 0.03 | 0.279 |
| Nodules with poorly defined margins | 524 (45.4%) | 1760 (39.1%) | <0.001 *** | 0.03 | 0.113 |
| Interstitial/linear infiltration | 550 (47.6%) | 1314 (21.2%) | <0.001 *** | 0.03 | 0.262 |
| Pleural effusions | 522 (45.2%) | 952 (21.2%) | <0.001 *** | 0.03 | 0.28 |
| Adenopathy | 497 (43.0%) | 1384 (30.8%) | <0.001 *** | 0.03 | 0.102 |
| Predominant lower lung field | 944 (81.7%) | 1376 (30.6%) | <0.001 *** | 0.03 | 0.557 |
| Extrapulmonary diseases | 541 (46.8%) | 1525 (33.9%) | <0.001 *** | 0.03 | 0.108 |
| Variable | β Coefficient (Std. Err.) | Odds Ratio | 95% CI | p Value | Weighting Score |
|---|---|---|---|---|---|
| Score system including patients aged > 85 years (primary cohort) | |||||
| Aged > 85 years | 2.063 (0.106) | 6.31 | [5.31–8.72] | <0.001 *** | 5 |
| Hypoalbuminemia | 1.562 (0.137) | 4.10 | [3.92–7.26] | <0.001 *** | 4 |
| Cardiovascular disease | 1.210 (0.378) | 3.32 | [1.23–5.27] | 0.001 ** | 3 |
| Diabetes | 0.825 (0.386) | 2.03 | [1.32–4.07] | 0.020 * | 2 |
| Lower lung field | 0.508 (0.253) | 1.25 | [1.03–2.44] | 0.014 * | 1 |
| Metric | Derivation Cohort (n = 5651) | 95% CI | Validation Cohort (n = 998) | 95% CI |
|---|---|---|---|---|
| AUC | 0.959 | (0.939–0.966) | 0.960 | (0.932–0.981) |
| Sensitivity | 0.913 | (0.889–0.937) | 0.943 | (0.910–0.971) |
| Specificity | 0.981 | (0.963–0.991) | 0.974 | (0.952–0.988) |
| Positive Predictive Value (PPV) | 0.924 | (0.901–0.946) | 0.804 | (0.742–0.859) |
| Negative Predictive Value (NPV) | 0.978 | (0.962–0.988) | 0.993 | (0.981–0.998) |
| False Positive Rate (FPR) | 0.019 | (0.009–0.037) | 0.026 | (0.012–0.048) |
| False Negative Rate (FNR) | 0.087 | (0.063–0.111) | 0.057 | (0.029–0.090) |
| Positive Likelihood Ratio (LR+) | 47.25 | (31.4–71.0) | 36.9 | (23.8–57.2) |
| Post-test Probability | 0.924 | (0.901–0.946) | 0.804 | (0.742–0.859) |
| Prevalence | 0.204 | N/A | 0.100 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, J.-J.; Chen, J.-H.; Kuo, Y.-L.; Tsai, C.-H.; Ko, Y.-E. A Clinical Prediction Model for Atypical Tuberculosis Manifestations Among Older Adults. Medicina 2025, 61, 1888. https://doi.org/10.3390/medicina61101888
Yeh J-J, Chen J-H, Kuo Y-L, Tsai C-H, Ko Y-E. A Clinical Prediction Model for Atypical Tuberculosis Manifestations Among Older Adults. Medicina. 2025; 61(10):1888. https://doi.org/10.3390/medicina61101888
Chicago/Turabian StyleYeh, Jun-Jun, Jia-Hong Chen, Yi-Ling Kuo, Chieh-Hsuan Tsai, and Yung-En Ko. 2025. "A Clinical Prediction Model for Atypical Tuberculosis Manifestations Among Older Adults" Medicina 61, no. 10: 1888. https://doi.org/10.3390/medicina61101888
APA StyleYeh, J.-J., Chen, J.-H., Kuo, Y.-L., Tsai, C.-H., & Ko, Y.-E. (2025). A Clinical Prediction Model for Atypical Tuberculosis Manifestations Among Older Adults. Medicina, 61(10), 1888. https://doi.org/10.3390/medicina61101888







