Scapular Dyskinesis and Associated Factors in Adult Elite Swimmers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
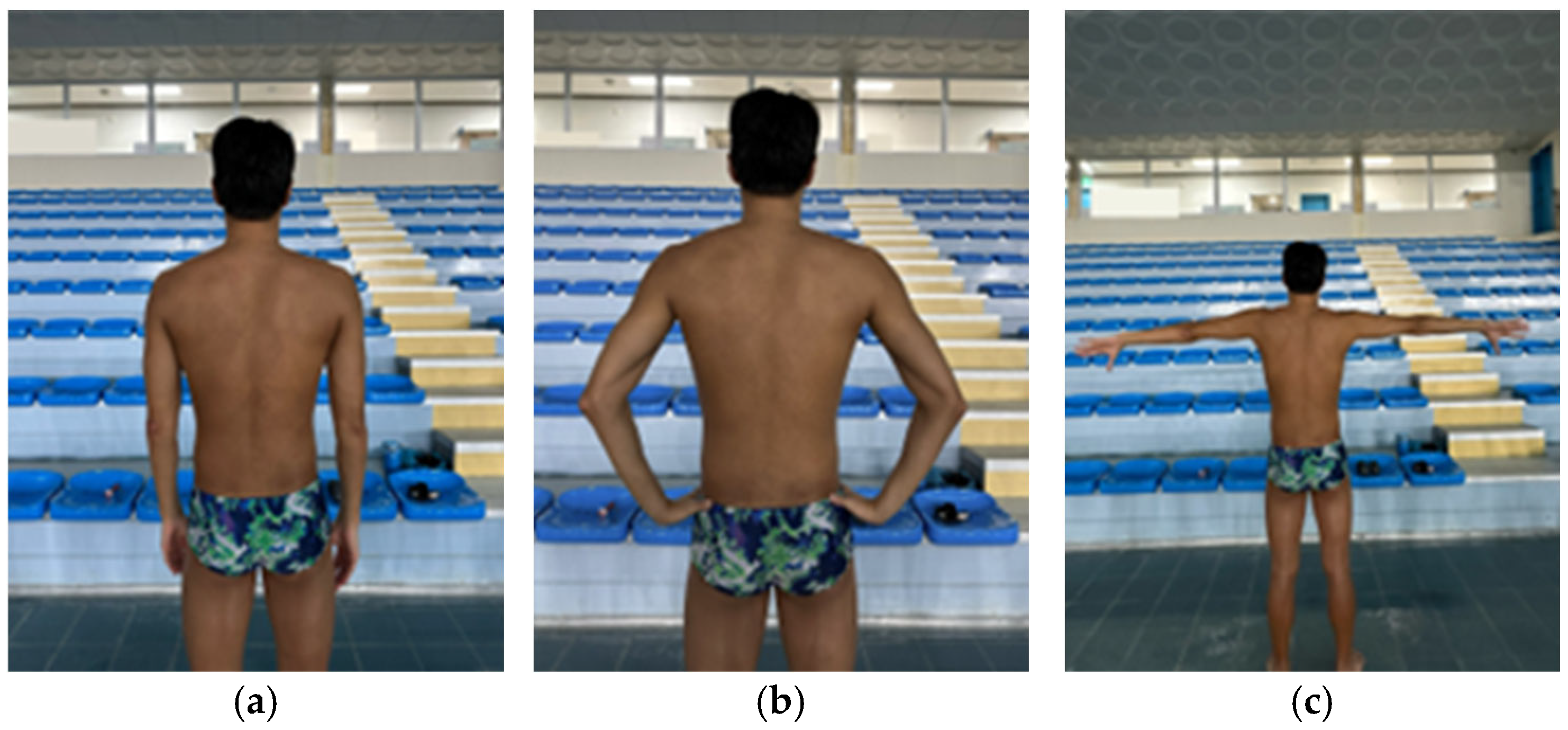
2.3. Scapular Dyskinesis Test
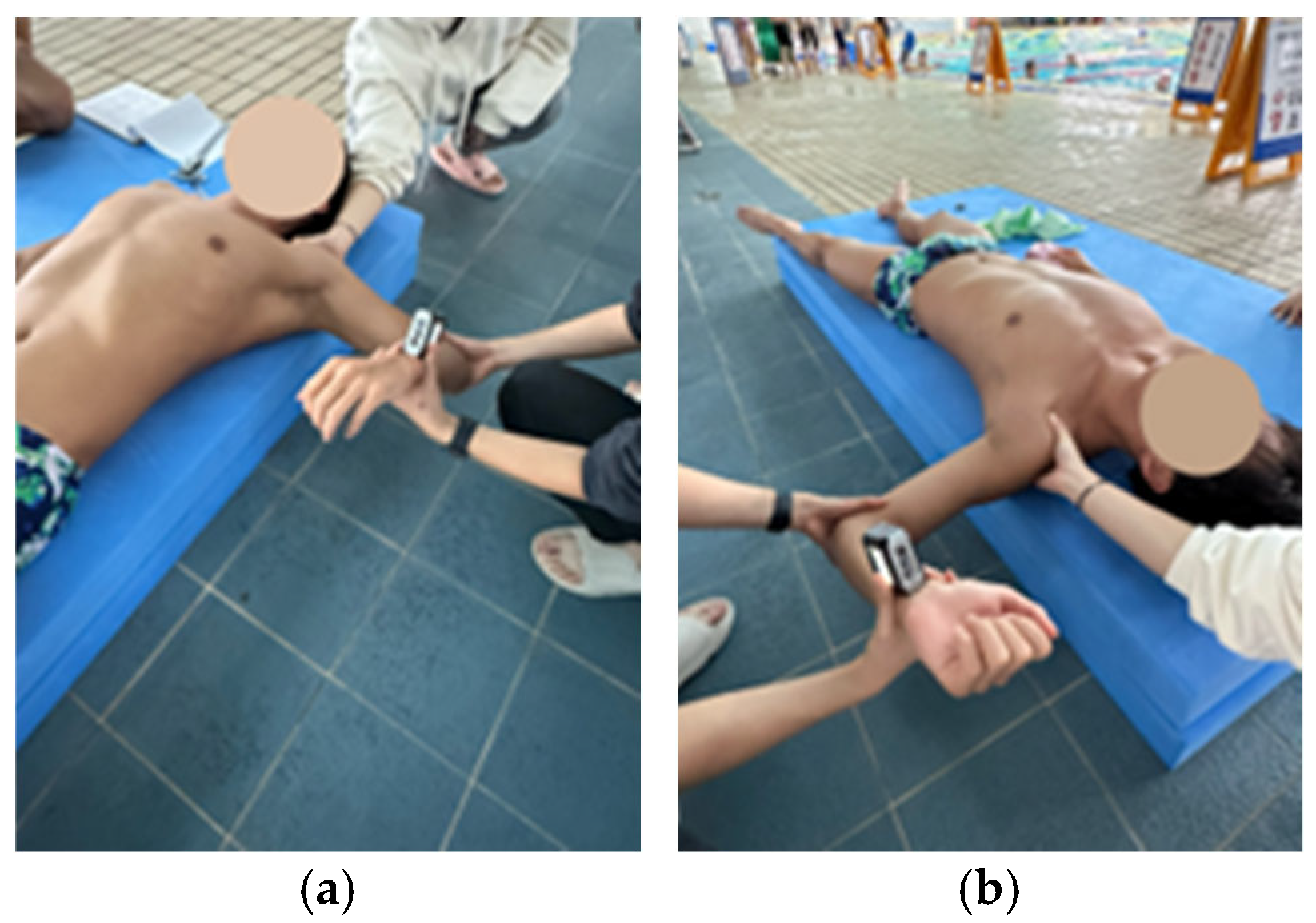
2.4. Lateral Scapular Slide Test
2.5. Shoulder Internal and External Rotation Range of Motion
2.6. Pectoralis Minor Length
2.7. Penn Shoulder Score
2.8. Statistical Analysis
3. Results
3.1. Anthropometric Data and Swimming Characteristics
3.2. The Prevalence of SD
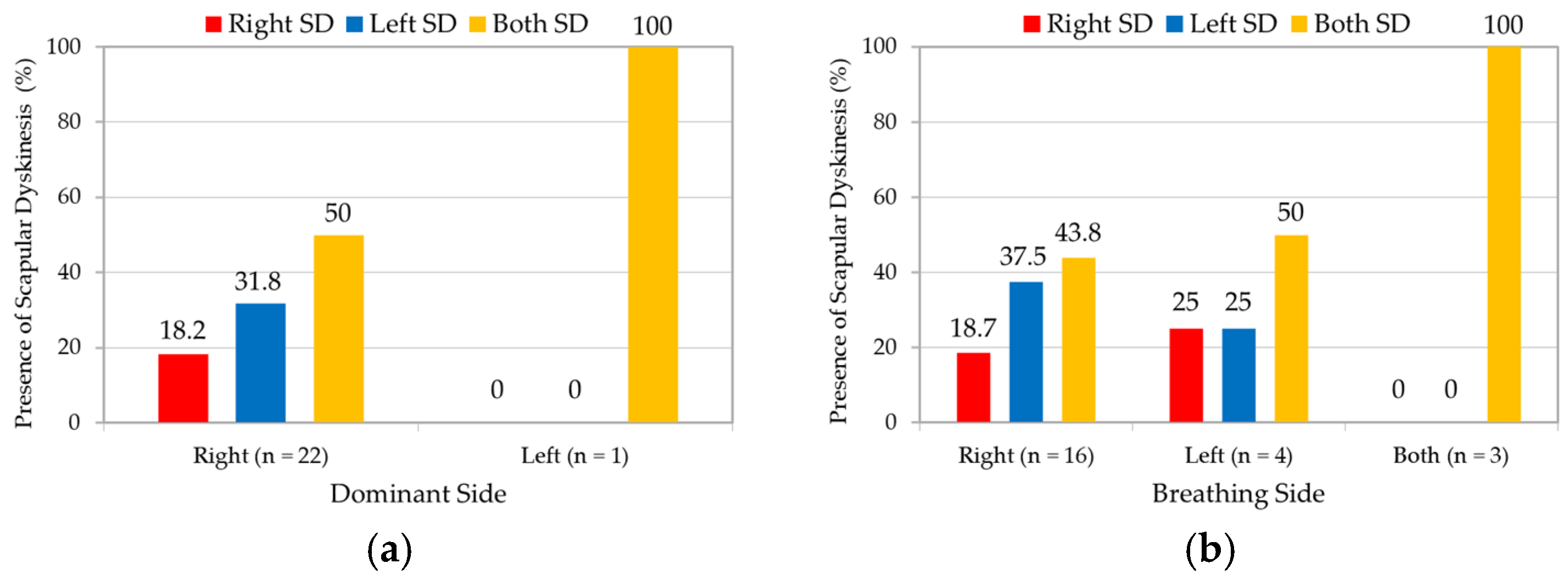
3.3. Association with Dominant and Breathing Side
3.4. Association with Years of Experience
3.5. Comparison by Main Stroke Type
3.6. Comparison of LSST Length, Pectoralis Minor Length, and IR-ROM
3.7. Comparison of Shoulder Pain
3.8. Comparison of Penn Shoulder Score
3.9. Association Between Penn Shoulder Score and SDT Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SD | Scapular dyskinesis |
| LSST | Lateral scapular slide test |
| IR | Internal rotation |
| ROM | Range of motion |
| SDT | Scapular dyskinesis test |
References
- Sein, M.L.; Walton, J.; Linklater, J.; Appleyard, R.; Kirkbride, B.; Kuah, D.; Murrell, G.A. Shoulder pain in elite swimmers: Primarily due to swim-volume-induced supraspinatus tendinopathy. Br. J. Sports Med. 2010, 44, 105–113. [Google Scholar] [CrossRef]
- Standoli, T.P.; Fratalocchi, F.; Standoli, J.P. Swimmers’ shoulder pain and scapula dyskinesis: A narrative review. Rev. Cuba. Ortop. Traumatol. 2025, 39, e946. [Google Scholar]
- Tavares, N.; Dias, G.; Carvalho, P.; Vilas-Boas, J.P.; Castro, M.A. Effectiveness of therapeutic exercise in musculoskeletal risk factors related to swimmer’s shoulder. Eur. J. Investig. Health Psychol. Educ. 2022, 12, 601–615. [Google Scholar] [CrossRef]
- Struyf, F.; Tate, A.; Kuppens, K.; Feijen, S.; Michener, L.A. Musculoskeletal dysfunctions associated with swimmers’ shoulder. Br. J. Sports Med. 2017, 51, 775–780. [Google Scholar] [CrossRef]
- Öztürk, F.; Özçadırcı, A.; Cinemre, Ş.A.; Kınıklı, G.İ. Is Scapular Dyskinesis effective on Shoulder Strength Profiles in Asymptomatic Young Swimmers? J. Basic Clin. Health Sci. 2021, 6, 694–702. [Google Scholar] [CrossRef]
- McQuade, K.J.; Dawson, J.; Smidt, G.L. Scapulothoracic muscle fatigue associated with alterations in scapulohumeral rhythm kinematics during maximum resistive shoulder elevation. J. Orthop. Sports Phys. Ther. 1998, 28, 74–80. [Google Scholar] [CrossRef]
- Kibler, B.W.; Sciascia, A.; Wilkes, T. Scapular dyskinesis and its relation to shoulder injury. JAAOS J. Am. Acad. Orthop. Surg. 2012, 20, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Kibler, W.B.; Sciascia, A. Current concepts: Scapular dyskinesis. Br. J. Sports Med. 2010, 44, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, S.S.; Morgan, C.D.; Kibler, W.B. The disabled throwing shoulder: Spectrum of pathology Part III: The SICK scapula, scapular dyskinesis, the kinetic chain, and rehabilitation. Arthroscopy 2003, 19, 641–661. [Google Scholar] [CrossRef]
- Burkhart, S.S.; Morgan, C.D.; Kibler, W.B. The disabled throwing shoulder: Spectrum of pathology Part I: Pathoanatomy and biomechanics. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Habermeyer, P.; Magosch, P.; Pritsch, M.; Scheibel, M.T.; Lichtenberg, S. Anterosuperior impingement of the shoulder as a result of pulley lesions: A prospective arthroscopic study. J. Shoulder Elb. Surg. 2004, 13, 5–12. [Google Scholar] [CrossRef]
- Burn, M.B.; McCulloch, P.C.; Lintner, D.M.; Liberman, S.R.; Harris, J.D. Prevalence of scapular dyskinesis in overhead and nonoverhead athletes: A systematic review. Orthop. J. Sports Med. 2016, 4, 2325967115627608. [Google Scholar] [CrossRef]
- Preziosi Standoli, J.; Fratalocchi, F.; Candela, V.; Preziosi Standoli, T.; Giannicola, G.; Bonifazi, M.; Gumina, S. Scapular dyskinesis in young, asymptomatic elite swimmers. Orthop. J. Sports Med. 2018, 6, 2325967117750814. [Google Scholar] [CrossRef]
- Welbeck, A.; Amilo, N.; Le, D.; Killelea, C.; Kirsch, A.; Zarzour, R.; Burgi, C.; Sell, T.; Faherty, M. Examining the link between thoracic rotation and scapular dyskinesis and shoulder pain amongst college swimmers. Phys. Ther. Sport 2019, 40, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Riemann, B.L.; Witt, J.; Davies, G.J. Glenohumeral joint rotation range of motion in competitive swimmers. J. Sports Sci. 2011, 29, 1191–1199. [Google Scholar] [CrossRef]
- Madsen, P.H.; Bak, K.; Jensen, S.; Welter, U. Training induces scapular dyskinesis in pain-free competitive swimmers: A reliability and observational study. Clin. J. Sport Med. 2011, 21, 109–113. [Google Scholar] [CrossRef]
- Rodeo, S.A.; Nguyen, J.T.; Cavanaugh, J.T.; Patel, Y.; Adler, R.S. Clinical and ultrasonographic evaluations of the shoulders of elite swimmers. Am. J. Sports Med. 2016, 44, 3214–3221. [Google Scholar] [CrossRef] [PubMed]
- Harrington, S.; Meisel, C.; Tate, A. A cross-sectional study examining shoulder pain and disability in Division I female swimmers. J. Sport Rehabil. 2014, 23, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Maor, M.B.; Ronin, T.; Kalichman, L. Scapular dyskinesis among competitive swimmers. J. Bodyw. Mov. Ther. 2017, 21, 633–636. [Google Scholar] [CrossRef]
- McClure, P.; Tate, A.R.; Kareha, S.; Irwin, D.; Zlupko, E. A clinical method for identifying scapular dyskinesis, part 1: Reliability. J. Athl. Train. 2009, 44, 160–164. [Google Scholar] [CrossRef]
- Rossi, D.M.; Pedroni, C.R.; Martins, J.; de Oliveira, A.S. Intrarater and interrater reliability of three classifications for scapular dyskinesis in athletes. PLoS ONE 2017, 12, e0181518. [Google Scholar] [CrossRef]
- Shadmehr, A.; Sarafraz, H.; Blooki, M.H.; Jalaie, S.; Morais, N. Reliability, agreement, and diagnostic accuracy of the Modified Lateral Scapular Slide test. Man. Ther. 2016, 24, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ben Kibler, W. The role of the scapula in athletic shoulder function. Am. J. Sports Med. 1998, 26, 325–337. [Google Scholar] [CrossRef]
- Ozunlu, N.; Tekeli, H.; Baltaci, G. Lateral scapular slide test and scapular mobility in volleyball players. J. Athl. Train. 2011, 46, 438–444. [Google Scholar] [CrossRef]
- Shadmehr, A.; Bagheri, H.; Ansari, N.N.; Sarafraz, H. The reliability measurements of lateral scapular slide test at three different degrees of shoulder joint abduction. Br. J. Sports Med. 2010, 44, 289–293. [Google Scholar] [CrossRef]
- Moreno-Pérez, V.; Elvira, J.; Fernandez-Fernandez, J.; Vera-Garcia, F. A comparative study of passive shoulder rotation range of motion, isometric rotation strength and serve speed between elite tennis players with and without history of shoulder pain. Int. J. Sports Phys. Ther. 2018, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Wilk, K.E.; Reinold, M.M.; Macrina, L.C.; Porterfield, R.; Devine, K.M.; Suarez, K.; Andrews, J.R. Glenohumeral internal rotation measurements differ depending on stabilization techniques. Sports Health 2009, 1, 131–136. [Google Scholar] [CrossRef]
- Clarsen, B.; Bahr, R.; Andersson, S.H.; Munk, R.; Myklebust, G. Reduced glenohumeral rotation, external rotation weakness and scapular dyskinesis are risk factors for shoulder injuries among elite male handball players: A prospective cohort study. Br. J. Sports Med. 2014, 48, 1327–1333. [Google Scholar] [CrossRef]
- Cools, A.M.; De Wilde, L.; Van Tongel, A.; Ceyssens, C.; Ryckewaert, R.; Cambier, D.C. Measuring shoulder external and internal rotation strength and range of motion: Comprehensive intra-rater and inter-rater reliability study of several testing protocols. J. Shoulder Elb. Surg. 2014, 23, 1454–1461. [Google Scholar] [CrossRef]
- Borstad, J.D. Measurement of pectoralis minor muscle length: Validation and clinical application. J. Orthop. Sports Phys. Ther. 2008, 38, 169–174. [Google Scholar] [CrossRef]
- Cools, A.M.; Johansson, F.R.; Cambier, D.C.; Vande Velde, A.; Palmans, T.; Witvrouw, E.E. Descriptive profile of scapulothoracic position, strength and flexibility variables in adolescent elite tennis players. Br. J. Sports Med. 2010, 44, 678–684. [Google Scholar] [CrossRef]
- Yeşilyaprak, S.S.; Yüksel, E.; Kalkan, S. Influence of pectoralis minor and upper trapezius lengths on observable scapular dyskinesis. Phys. Ther. Sport 2016, 19, 7–13. [Google Scholar] [CrossRef]
- Borstad, J.D.; Ludewig, P.M. The effect of long versus short pectoralis minor resting length on scapular kinematics in healthy individuals. J. Orthop. Sports Phys. Ther. 2005, 35, 227–238. [Google Scholar] [CrossRef]
- Leggin, B.G.; Michener, L.A.; Shaffer, M.A.; Brenneman, S.K.; Iannotti, J.P.; Williams Jr, G.R. The Penn shoulder score: Reliability and validity. J. Orthop. Sports Phys. Ther. 2006, 36, 138–151. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Oxfordshire, UK, 2013. [Google Scholar] [CrossRef]
- McCabe, C.B.; Sanders, R.H.; Psycharakis, S.G. Upper limb kinematic differences between breathing and non-breathing conditions in front crawl sprint swimming. J. Biomech. 2015, 48, 3995–4001. [Google Scholar] [CrossRef] [PubMed]
- Myer, G.D.; Jayanthi, N.; Difiori, J.P.; Faigenbaum, A.D.; Kiefer, A.W.; Logerstedt, D.; Micheli, L.J. Sport specialization, part I: Does early sports specialization increase negative outcomes and reduce the opportunity for success in young athletes? Sports Health 2015, 7, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Pink, M.M.; Edelman, G.T.; Mark, R.; Rodeo, S.A. Applied biomechanics of swimming. In Athletic and Sport Issues in Musculoskeletal Rehabilitation; Saunders Elsevier: St. Louis, MO, USA, 2011; pp. 331–349. [Google Scholar]
- Matsuura, Y.; Matsunaga, N.; Akuzawa, H.; Kojima, T.; Oshikawa, T.; Iizuka, S.; Okuno, K.; Kaneoka, K. Difference in muscle synergies of the butterfly technique with and without swimmer’s shoulder. Sci. Rep. 2022, 12, 14546. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, P.M.; Cook, T.M. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys. Ther. 2000, 80, 276–291. [Google Scholar] [CrossRef]
- Kibler, W.B.; Ludewig, P.M.; McClure, P.W.; Michener, L.A.; Bak, K.; Sciascia, A.D. Clinical implications of scapular dyskinesis in shoulder injury: The 2013 consensus statement from the ‘Scapular Summit’. Br. J. Sports Med. 2013, 47, 877–885. [Google Scholar] [CrossRef]
- Wolf, B.R.; Ebinger, A.E.; Lawler, M.P.; Britton, C.L. Injury patterns in Division I collegiate swimming. Am. J. Sports Med. 2009, 37, 2037–2042. [Google Scholar] [CrossRef]
- Moon, S.E.; Kim, Y.K. Neck and shoulder pain with scapular dyskinesis in computer office workers. Medicina 2023, 59, 2159. [Google Scholar] [CrossRef]
- Micoogullari, M.; Uygur, S.F.; Yosmaoglu, H.B. Effect of scapular stabilizer muscles strength on scapular position. Sports Health 2023, 15, 349–356. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, Y.K. Scapular dyskinesis in elite boxers with neck disability and shoulder malfunction. Medicina 2021, 57, 1347. [Google Scholar] [CrossRef]
- Laudner, K.G.; Wenig, M.; Selkow, N.M.; Williams, J.; Post, E. Forward shoulder posture in collegiate swimmers: A comparative analysis of muscle-energy techniques. J. Athl. Train. 2015, 50, 1133–1139. [Google Scholar] [CrossRef]
- Kibler, W.B.; Sciascia, A. The shoulder at risk: Scapular dyskinesis and altered glenohumeral rotation. Oper. Tech. Sports Med. 2016, 24, 162–169. [Google Scholar] [CrossRef]
- Kijima, T.; Matsuki, K.; Ochiai, N.; Yamaguchi, T.; Sasaki, Y.; Hashimoto, E.; Sasaki, Y.; Yamazaki, H.; Kenmoku, T.; Yamaguchi, S. In vivo 3-dimensional analysis of scapular and glenohumeral kinematics: Comparison of symptomatic or asymptomatic shoulders with rotator cuff tears and healthy shoulders. J. Shoulder Elb. Surg. 2015, 24, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.M.A.d.; Silva, H.A.d.; Pitangui, A.C.R.; Passos, M.H.P.d.; Araújo, R.C.d. Scapular dyskinesis was not associated with pain and function in male adolescent athletes. BrJP 2018, 1, 40–45. [Google Scholar] [CrossRef]
- Struyf, F.; Nijs, J.; Meeus, M.; Roussel, N.A.; Mottram, S.; Truijen, S.; Meeusen, R. Does scapular positioning predict shoulder pain in recreational overhead athletes? Int. J. Sports Med. 2014, 35, 75–82. [Google Scholar] [CrossRef]
- McKenna, L.; Straker, L.; Smith, A. Can scapular and humeral head position predict shoulder pain in adolescent swimmers and non-swimmers? J. Sports Sci. 2012, 30, 1767–1776. [Google Scholar] [CrossRef]
- Hickey, D.; Solvig, V.; Cavalheri, V.; Harrold, M.; Mckenna, L. Scapular dyskinesis increases the risk of future shoulder pain by 43% in asymptomatic athletes: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 102–110. [Google Scholar] [CrossRef]
- Salamh, P.A.; Hanney, W.J.; Boles, T.; Holmes, D.; McMillan, A.; Wagner, A.; Kolber, M.J. Is it time to normalize scapular dyskinesis? The incidence of scapular dyskinesis in those with and without symptoms: A systematic review of the literature. Int. J. Sports Phys. Ther. 2023, 18, 558. [Google Scholar] [CrossRef] [PubMed]


| Male (n = 38) | Female (n = 12) | Total (N = 50) | |
|---|---|---|---|
| Age (y) | 23.39 ± 2.55 | 25.75 ± 4.16 | 23.96 ± 3.14 |
| Height (cm) | 179.39 ± 5.58 | 165.67 ± 4.66 | 176.10 ± 7.96 |
| Weight (kg) | 75.18 ± 5.01 | 58.50 ± 5.68 | 71.18 ± 8.83 |
| Hand dominance (n, %) | |||
| Right | 35 (92.1) | 12 (100.0) | 47 (94.0) |
| Left | 3 (7.9) | 0 (0.0) | 3 (6.0) |
| Male (n = 38) | Female (n = 12) | Total (N = 50) | |
|---|---|---|---|
| Years of experience (y) | 13.26 ± 3.17 | 14.67 ± 5.20 | 13.60 ± 3.74 |
| Breathing side (n, %) | |||
| Right | 30 (78.9) | 8 (66.7) | 38 (76.0) |
| Left | 3 (7.9) | 1 (8.3) | 4 (8.0) |
| Both | 5 (13.2) | 3 (25.0) | 8 (16.0) |
| Stroke (n, %) | |||
| Butterfly | 8 (19.1) | 3 (21.4) | 11 (19.6) |
| Backstroke | 8 (19.1) | 0 (0.0) | 8 (14.3) |
| Breaststroke | 8 (19.1) | 1 (7.2) | 9 (16.1) |
| Freestyle | 15 (35.6) | 7 (50.0) | 22 (39.3) |
| Individual Medley | 3 (7.1) | 3 (21.4) | 6 (10.7) |
| Distance (n, %) | |||
| Sprint | 26 (46.4) | 4 (18.2) | 30 (38.4) |
| Mid-distance | 24 (42.9) | 12 (54.5) | 36 (46.2) |
| Long-distance | 6 (10.7) | 6 (27.3) | 12 (15.4) |
| Normal | Subtle | Obvious | |
|---|---|---|---|
| Dominant arm (n = 50) | 34 (68.0%) | 10 (20.0%) | 6 (12.0%) |
| Non-dominant arm (n = 50) | 31 (62.0%) | 16 (32.0%) | 3 (6.0%) |
| Total (N = 100) | 65 (65.0%) | 26 (26.0%) | 9 (9.0%) |
| r | p | |
|---|---|---|
| Years of experience and SDT score | 0.127 | 0.378 |
| Normal | Subtle | Obvious | Mean Rank | Kruskal–Wallis H | p | |
|---|---|---|---|---|---|---|
| Butterfly(n = 12) | 4 (33.3%) | 4 (33.3%) | 4 (33.3%) | 32.92 | 8.323 | 0.080 |
| Backstroke (n = 14) | 12 (85.7%) | 2 (14.3%) | 0 (0.0%) | 17.86 | ||
| Breaststroke (n = 18) | 15 (83.3%) | 3 (16.7%) | 0 (0.0%) | 17.50 | ||
| Freestyle (n = 38) | 23 (60.5%) | 11 (29.0%) | 4 (10.5%) | 23.89 | ||
| Individual Medley (n = 6) | 5 (83.3%) | 1 (16.7%) | 0 (0.0%) | 18.67 |
| Normal a (n = 65) | Subtle b (n = 26) | Obvious c (n = 9) | F | p | Effect Size (η2p) | Bonferroni | |
|---|---|---|---|---|---|---|---|
| LSST 1 (cm) | 11.32 ± 0.94 (11.09–11.55) | 11.21 ± 1.28 (10.69–11.73) | 11.27 ± 1.04 (10.47–12.06) | 0.104 | 0.901 | ||
| LSST 2 (cm) | 12.33 ± 0.99 (12.08–12.57) | 12.02 ± 1.42 (11.45–12.59) | 12.21 ± 1.17 (11.31–13.11) | 0.692 | 0.503 | ||
| LSST 3 (cm) | 12.58 ± 1.04 (12.32–12.84) | 12.60 ± 1.11 (12.15–13.04) | 12.56 ± 1.42 (11.47–13.65) | 0.004 | 0.996 | ||
| Pec m length | 9.96 ± 0.58 (9.82–10.11) | 9.65 ± 0.56 (9.42–9.87) | 8.98 ± 0.48 (8.61–9.35) | 13.283 | <0.001 *** | 0.215 | a > b (0.051) b > c (0.008 **) a > c (<0.001 ***) |
| IR ROM (°) | 60.60 ± 9.16 (58.30–62.91) | 66.00 ± 9.32 (62.06–69.94) | 56.19 ± 8.29 (49.26–63.12) | 4.561 | 0.013 * | 0.090 | a < b (0.047 *) b > c (0.029 *) |
| Normal | SD | χ2 | p | |||||
| Shoulder pain (n = 33) | 19 (29.2%) | 14 (40.0%) | 1.193 | 0.275 | ||||
| No Shoulder pain (n = 67) | 46 (70.8%) | 21 (60.0%) | ||||||
| SDT Normal | SDT Subtle | SDT Obvious | χ2 | p | ||||
| Shoulder pain (n = 33) | 19 (29.2%) | 11 (42.3%) | 3 (33.3%) | 1.437 | 0.488 | |||
| No Shoulder pain (n = 67) | 46 (70.8%) | 15 (57.7%) | 6 (66.7%) | |||||
| Mean Rank | Mann–Whitney U | Z | p | |
|---|---|---|---|---|
| Normal (n = 27) | 29.41 | 205 | −2.055 | 0.039 * |
| SD (n = 23) | 20.91 |
| r | p | |
|---|---|---|
| Dominant arm (n = 50) | −0.291 | 0.040 * |
| Non-dominant arm (n = 50) | −0.225 | 0.116 |
| Both (N = 100) | −0.295 | 0.037 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, S.Y.; Kim, Y.K. Scapular Dyskinesis and Associated Factors in Adult Elite Swimmers. Medicina 2025, 61, 1885. https://doi.org/10.3390/medicina61101885
Joo SY, Kim YK. Scapular Dyskinesis and Associated Factors in Adult Elite Swimmers. Medicina. 2025; 61(10):1885. https://doi.org/10.3390/medicina61101885
Chicago/Turabian StyleJoo, Se Young, and Young Kyun Kim. 2025. "Scapular Dyskinesis and Associated Factors in Adult Elite Swimmers" Medicina 61, no. 10: 1885. https://doi.org/10.3390/medicina61101885
APA StyleJoo, S. Y., & Kim, Y. K. (2025). Scapular Dyskinesis and Associated Factors in Adult Elite Swimmers. Medicina, 61(10), 1885. https://doi.org/10.3390/medicina61101885








