Use of AI Histopathology in Breast Cancer Diagnosis
Abstract
1. Introduction
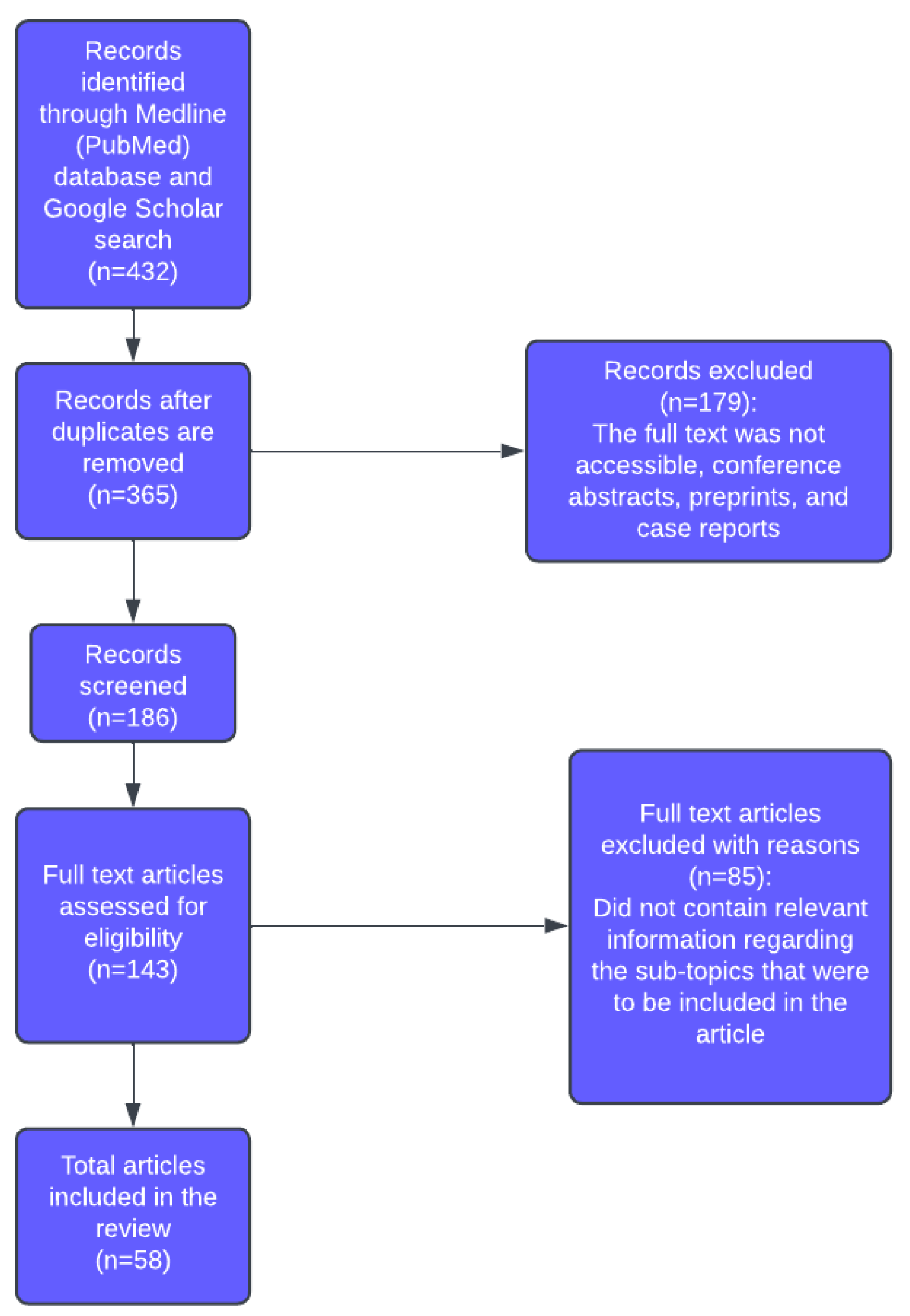
2. Materials and Methods
- Original research articles, systematic reviews, or meta-analyses related to BC in the context of AI, machine learning, or digital pathology.
- Studies published in English.
- Articles reporting clear methods and results relevant to diagnostic or prognostic applications.
- Non-English publications.
- The full text was not accessible.
- Conference abstracts, preprints, and case reports.
3. Results
3.1. Histology
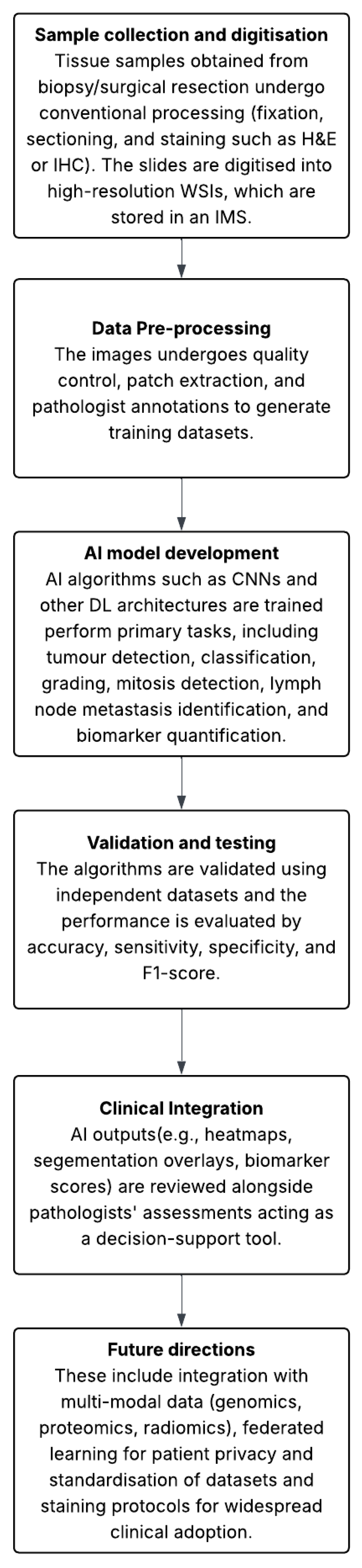
3.2. Digital Pathology
3.3. Artificial Intelligence in Breast Cancer Diagnosis
3.4. Role of AI in Diagnosing Lymph Node Metastasis in Breast Cancer
3.5. Role of AI in the Histological Grading of Breast Cancer
Comparative Perspectives on AI Architectures
3.6. Molecular Pathology
4. Discussion
4.1. Ethical, Legal, and Privacy Issues
4.2. Standardisation and Interoperability
4.3. Research Gaps and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast Cancer |
| MRI | Magnetic Resonance Imaging |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
| WSI | Whole Slide Image |
| IMS | Image Management System |
| NN | Neural Network |
| CNN | Convolutional Neural Network |
| ROI | Region of Interest |
| FRBCNN | Faster Region Based Convolutional Neural Network |
| FFDM | Full Field Digital Mammography |
| FPI | False Positive per Image |
| TPR | True Positive Rate |
| INbreast | Siemens scanner dataset |
| mFPI | mean False Positive Indications per image |
| AUC | Area Under the Curve |
| YOLO | You Only Look Once |
| LOGO | Local Global |
| IoU | Intersection over Union |
| SLN | Sentinel Lymph Node |
| DCIS | Ductal Carcinoma In Situ |
| IDC | Invasive Ductal Carcinoma |
| VIS | Visiopharm Integrator System |
| NPV | Negative Predictive Value |
| DIA | Digital Image Analysis |
| VDS | Virtual Double Staining |
| CK | Cytokeratin |
| CBIS | Curated Breast Imaging subset |
| CAMELYON16 | Cancer Metastases in Lymph Nodes Challenge 2016 |
| IHC | Immunohistochemistry/Immunohistochemical |
| AMIDA13 | Assessment of Mitosis Detection Algorithms 2013 |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| GLOBOCAN | Global Cancer Observatory |
| SVM | Support Vector Machine |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2022; Available online: https://gco.iarc.fr/today (accessed on 5 October 2025).
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Dromain, C.; Boyer, B.; Ferré, R.; Canale, S.; Delaloge, S.; Balleyguier, C. Computed-aided diagnosis (CAD) in the detection of breast cancer. Eur. J. Radiol. 2013, 82, 417–423. [Google Scholar] [CrossRef]
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef]
- Paci, E.; Broeders, M.; Hofvind, S.; Puliti, D.; Duffy, S.W.; the EUROSCREEN Working Group. European breast cancer service screening outcomes: A first balance sheet of the benefits and harms. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.J.; Gøtzsche, P.C. Overdiagnosis in publicly organised mammography screening programmes: Systematic review of incidence trends. BMJ 2009, 339, b2587. [Google Scholar] [CrossRef]
- Pharoah, P.D.P.; Sewell, B.; Fitzsimmons, D.; Bennett, H.S.; Pashayan, N. Cost effectiveness of the NHS breast screening programme: Life table model. BMJ 2013, 346, f2618. [Google Scholar] [CrossRef]
- Bond, M.; Pavey, T.; Welch, K.; Cooper, C.; Garside, R.; Dean, S.; Hyde, C. Systematic review of the psychological consequences of false-positive screening mammograms. Heal. Technol. Assess. 2013, 17, 1–170. [Google Scholar] [CrossRef]
- Gomes, D.S.; Porto, S.S.; Balabram, D.; Gobbi, H. Inter-observer variability between general pathologists and a specialist in breast pathology in the diagnosis of lobular neoplasia, columnar cell lesions, atypical ductal hyperplasia and ductal carcinoma in situ of the breast. Diagn. Pathol. 2014, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.J.; Brown, R.W.; Bui, M.M.; Chlipala, E.A.; Lacchetti, C.; Milner, D.A.; Pantanowitz, L.; Parwani, A.V.; Reid, K.; Riben, M.W.; et al. Validating Whole Slide Imaging Systems for Diagnostic Purposes in Pathology. Arch. Pathol. Lab. Med. 2021, 146, 440–450. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Sinard, J.H.; Henricks, W.H.; Fatheree, L.A.; Carter, A.B.; Contis, L.; Beckwith, B.A.; Evans, A.J.; Lal, A.; Parwani, A.V.; et al. Validating whole slide imaging for diagnostic purposes in pathology: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch. Pathol. Lab. Med. 2013, 137, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Aleskandarani, M.; Toss, M.S.; Green, A.R.; Ball, G.; Ellis, I.O.; Dalton, L.W. Breast cancer histologic grading using digital microscopy: Concordance and outcome association. J. Clin. Pathol. 2018, 71, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Ahmed, M.A.; Aleskandarany, M.A.; Hodi, Z.; Lee, A.H.; Pinder, S.E.; Ellis, I.O. Diagnostic concordance of breast pathologists: Lessons from the National Health Service Breast Screening Programme Pathology External Quality Assurance Scheme. Histopathology 2016, 70, 632–642. [Google Scholar] [CrossRef]
- Rakha, E.A.; Bennett, R.L.; Coleman, D.; Pinder, S.E.; Ellis, I.O.; UK National Coordinating Committee for Breast Pathology (EQA Scheme Steering Committee). Review of the national external quality assessment (EQA) scheme for breast pathology in the UK. J. Clin. Pathol. 2017, 70, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bayareh-Mancilla, R.; Medina-Ramos, L.A.; Toriz-Vázquez, A.; Hernández-Rodríguez, Y.M.; Cigarroa-Mayorga, O.E. Automated Computer-Assisted Medical Decision-Making System Based on Morphological Shape and Skin Thickness Analysis for Asymmetry Detection in Mammographic Images. Diagnostics 2023, 13, 3440. [Google Scholar] [CrossRef]
- Jiang, B.; Bao, L.; He, S.; Chen, X.; Jin, Z.; Ye, Y. Deep learning applications in breast cancer histopathological imaging: Diagnosis, treatment, and prognosis. Breast Cancer Res. 2024, 26, 137. [Google Scholar] [CrossRef]
- Elliott, K.; McQuaid, S.; Salto-Tellez, M.; Maxwell, P. Immunohistochemistry should undergo robust validation equivalent to that of molecular diagnostics. J. Clin. Pathol. 2015, 68, 766–770. [Google Scholar] [CrossRef]
- Bankhead, P.; Fernández, J.A.; McArt, D.G.; Boyle, D.P.; Li, G.; Loughrey, M.B.; Irwin, G.W.; Harkin, D.P.; James, J.A.; McQuaid, S.; et al. Integrated tumor identification and automated scoring minimizes pathologist involvement and provides new insights to key biomarkers in breast cancer. Lab. Investig. 2018, 98, 15–26. [Google Scholar] [CrossRef]
- Ong, S.H.; Jin, X.C.; Jayasooriah Sinniah, R. Image analysis of tissue sections. Comput. Biol. Med. 1996, 26, 269–279. [Google Scholar] [CrossRef]
- Colling, R.; Pitman, H.; Oien, K.; Rajpoot, N.; Macklin, P.; CM-Path AI in Histopathology Working Group; Snead, D.; Sackville, T.; Verrill, C. Artificial intelligence in digital pathology: A roadmap to routine use in clinical practice. J. Pathol. 2019, 249, 143–150. [Google Scholar] [CrossRef]
- Hamet, P.; Tremblay, J. Artificial intelligence in medicine. Metabolism 2017, 69S, S36–S40. [Google Scholar] [CrossRef]
- Ahern, T.P.; Beck, A.H.; A Rosner, B.; Glass, B.; Frieling, G.; Collins, L.C.; Tamimi, R.M. Continuous measurement of breast tumour hormone receptor expression: A comparison of two computational pathology platforms. J. Clin. Pathol. 2017, 70, 428–434. [Google Scholar] [CrossRef]
- Shafi, S.; Kellough, D.A.; Lujan, G.; Satturwar, S.; Parwani, A.V.; Li, Z. Integrating and validating automated digital imaging analysis of estrogen receptor immunohistochemistry in a fully digital workflow for clinical use. J. Pathol. Inform. 2022, 13, 100122. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.J.; Hanby, A.; Millican-Slater, R.; Nijhawan, A.; Verghese, E.; Treanor, D. Digital pathology for the primary diagnosis of breast histopathological specimens: An innovative validation and concordance study on digital pathology validation and training. Histopathology 2018, 72, 662–671. [Google Scholar] [CrossRef]
- Williams, B.; Hanby, A.; Millican-Slater, R.; Verghese, E.; Nijhawan, A.; Wilson, I.; Besusparis, J.; Clark, D.; Snead, D.; Rakha, E.; et al. Digital pathology for primary diagnosis of screen-detected breast lesions-experimental data, validation and experience from four centres. Histopathology 2020, 76, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, D.P.; Fu, T.; Yang, J.C.; Ma, D.; Zhu, X.Z.; Wang, X.X.; Jiao, Y.P.; Jin, X.; Xiao, Y.; et al. Single-cell morphological and topological atlas reveals the ecosystem diversity of human breast cancer. Nat. Commun. 2023, 14, 6796. [Google Scholar] [CrossRef] [PubMed]
- Díaz, O.; Rodríguez-Ruíz, A.; Sechopoulos, I. Artificial Intelligence for breast cancer detection: Technology, challenges, and prospects. Eur. J. Radiol. 2024, 175, 111457. [Google Scholar] [CrossRef]
- Li, J.; Cheng, J.H.; Shi, J.Y.; Huang, F. Brief Introduction of Back Propagation (BP) Neural Network Algorithm and its Improvement. In Advances in Computer Science and Information Engineering; Jin, D., Lin, S., Eds.; Advances in Intelligent and Soft Computing; Springer: Berlin/Heidelberg, Germany, 2012; pp. 553–558. [Google Scholar]
- Acs, B.; Rantalainen, M.; Hartman, J. Artificial intelligence as the next step towards precision pathology. J. Intern. Med. 2020, 288, 62–81. [Google Scholar] [CrossRef]
- Cruz-Roa, A.; Gilmore, H.; Basavanhally, A.; Feldman, M.; Ganesan, S.; Shih, N.; Tomaszewski, J.; Madabhushi, A.; González, F. High-throughput adaptive sampling for whole-slide histopathology image analysis (HASHI) via convolutional neural networks: Application to invasive breast cancer detection. PLoS ONE 2018, 13, e0196828. [Google Scholar] [CrossRef]
- Han, Z.; Wei, B.; Zheng, Y.; Yin, Y.; Li, K.; Li, S. Breast Cancer Multi-classification from Histopathological Images with Structured Deep Learning Model. Sci. Rep. 2017, 7, 4172. [Google Scholar] [CrossRef]
- Agarwal, R.; Díaz, O.; Yap, M.H.; Lladó, X.; Martí, R. Deep learning for mass detection in Full Field Digital Mammograms. Comput. Biol. Med. 2020, 121, 103774. [Google Scholar] [CrossRef]
- Al-Masni, M.A.; Al-Antari, M.A.; Park, J.M.; Gi, G.; Kim, T.Y.; Rivera, P.; Valarezo, E.; Choi, M.T.; Han, S.M.; Kim, T.S. Simultaneous detection and classification of breast masses in digital mammograms via a deep learning YOLO-based CAD system. Comput. Methods Programs Biomed. 2018, 157, 85–94. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Q.; Xie, W.; Hu, P. YOLO-LOGO: A transformer-based YOLO segmentation model for breast mass detection and segmentation in digital mammograms. Comput. Methods Programs Biomed. 2022, 221, 106903. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.H.; Goyal, M.; Osman, F.; Martí, R.; Denton, E.; Juette, A.; Zwiggelaar, R. Breast ultrasound region of interest detection and lesion localisation. Artif. Intell. Med. 2020, 107, 101880. [Google Scholar] [CrossRef] [PubMed]
- Ueda, D.; Yamamoto, A.; Onoda, N.; Takashima, T.; Noda, S.; Kashiwagi, S.; Morisaki, T.; Fukumoto, S.; Shiba, M.; Morimura, M.; et al. Development and validation of a deep learning model for detection of breast cancers in mammography from multi-institutional datasets. PLoS ONE 2022, 17, e0265751. [Google Scholar] [CrossRef] [PubMed]
- Ehteshami Bejnordi, B.; Veta, M.; Johannes van Diest, P.; van Ginneken, B.; Karssemeijer, N.; Litjens, G.; van der Laak, J.A.W.M.; the CAMELYON16 Consortium; Hermsen, M.; Manson, Q.F.; et al. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women With Breast Cancer. JAMA 2017, 318, 2199–2210. [Google Scholar] [CrossRef]
- Holten-Rossing, H.; Talman, M.M.; Jylling, A.M.B.; Lænkholm, A.; Kristensson, M.; Vainer, B. Application of automated image analysis reduces the workload of manual screening of sentinel lymph node biopsies in breast cancer. Histopathology 2017, 71, 866–873. [Google Scholar] [CrossRef]
- Steiner, D.F.; MacDonald, R.; Liu, Y.; Truszkowski, P.; Hipp, J.D.; Gammage, C.; Thng, F.; Peng, L.; Stumpe, M.C. Impact of Deep Learning Assistance on the Histopathologic Review of Lymph Nodes for Metastatic Breast Cancer. Am. J. Surg. Pathol. 2018, 42, 1636–1646. [Google Scholar] [CrossRef]
- Liu, Y.; Kohlberger, T.; Norouzi, M.; Dahl, G.E.; Smith, J.L.; Mohtashamian, A.; Olson, N.; Peng, L.H.; Hipp, J.D.; Stumpe, M.C. Artificial Intelligence–Based Breast Cancer Nodal Metastasis Detection: Insights Into the Black Box for Pathologists. Arch. Pathol. Lab. Med. 2019, 143, 859–868. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Javed, Z.; Sadia, H.; Qureshi, I.A.; Irshad, A.; Ahmed, R.; Malik, K.; Raza, S.; Abbas, A.; Pezzani, R.; et al. Clinical applications of artificial intelligence and machine learning in cancer diagnosis: Looking into the future. Cancer Cell Int. 2021, 21, 270. [Google Scholar] [CrossRef]
- Wang, R.; Gu, Y.; Zhang, T.; Yang, J. Fast cancer metastasis location based on dual magnification hard example mining network in whole-slide images. Comput. Biol. Med. 2023, 158, 106880. [Google Scholar] [CrossRef]
- Challa, B.; Tahir, M.; Hu, Y.; Kellough, D.; Lujan, G.; Sun, S.; Parwani, A.V.; Li, Z. Artificial Intelligence-Aided Diagnosis of Breast Cancer Lymph Node Metastasis on Histologic Slides in a Digital Workflow. Mod. Pathol. 2023, 36, 100216. [Google Scholar] [CrossRef]
- Fondón, I.; Sarmiento, A.; García, A.I.; Silvestre, M.; Eloy, C.; Polónia, A.; Aguiar, P. Automatic classification of tissue malignancy for breast carcinoma diagnosis. Comput. Biol. Med. 2018, 96, 41–51. [Google Scholar] [CrossRef]
- Sanders, M.E.; Schuyler, P.A.; Dupont, W.D.; Page, D.L. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer 2005, 103, 2481–2484. [Google Scholar] [CrossRef]
- Pattari, S.K.; Dey, P.; Gupta, S.K.; Joshi, K. Myoepithelial cells: Any role in aspiration cytology smears of breast tumors? Cytojournal 2008, 5, 9. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saito, A.; Tateishi, A.; Shimojo, H.; Kanno, H.; Tsuchiya, S.; Ito, K.-I.; Cosatto, E.; Graf, H.P.; Moraleda, R.R.; et al. Quantitative diagnosis of breast tumors by morphometric classification of microenvironmental myoepithelial cells using a machine learning approach. Sci. Rep. 2017, 7, 46732. [Google Scholar] [CrossRef] [PubMed]
- Veta, M.; van Diest, P.J.; Willems, S.M.; Wang, H.; Madabhushi, A.; Cruz-Roa, A.; Gonzalez, F.; Larsen, A.B.; Vestergaard, J.S.; Dahl, A.B.; et al. Assessment of algorithms for mitosis detection in breast cancer histopathology images. Med. Image Anal. 2015, 20, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Veta, M.; Heng, Y.J.; Stathonikos, N.; Bejnordi, B.E.; Beca, F.; Wollmann, T.; Rohr, K.; Shah, M.A.; Wang, D.; Rousson, M.; et al. Predicting breast tumor proliferation from whole-slide images: The TUPAC16 challenge. Med. Image Anal. 2019, 54, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L.; Hartman, D.; Qi, Y.; Cho, E.Y.; Suh, B.; Paeng, K.; Dhir, R.; Michelow, P.; Hazelhurst, S.; Song, S.Y.; et al. Accuracy and efficiency of an artificial intelligence tool when counting breast mitoses. Diagn. Pathol. 2020, 15, 80. [Google Scholar] [CrossRef]
- Romo-Bucheli, D.; Janowczyk, A.; Gilmore, H.; Romero, E.; Madabhushi, A. Automated Tubule Nuclei Quantification and Correlation with Oncotype DX risk categories in ER+ Breast Cancer Whole Slide Images. Sci. Rep. 2016, 6, 32706. [Google Scholar] [CrossRef]
- Veta, M.; Kornegoor, R.; Huisman, A.; Verschuur-Maes, A.H.; Viergever, M.A.; Pluim, J.P.; van Diest, P.J. Prognostic value of automatically extracted nuclear morphometric features in whole slide images of male breast cancer. Mod. Pathol. 2012, 25, 1559–1565. [Google Scholar] [CrossRef]
- Stålhammar, G.; Fuentes Martinez, N.; Lippert, M.; Tobin, N.P.; Mølholm, I.; Kis, L.; Rosin, G.; Rantalainen, M.; Pedersen, L.; Bergh, J.; et al. Digital image analysis outperforms manual biomarker assessment in breast cancer. Mod. Pathol. 2016, 29, 318–329. [Google Scholar] [CrossRef]
- Røge, R.; Riber-Hansen, R.; Nielsen, S.; Vyberg, M. Proliferation assessment in breast carcinomas using digital image analysis based on virtual Ki67/cytokeratin double staining. Breast Cancer Res. Treat. 2016, 158, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lykkegaard Andersen, N.; Brügmann, A.; Lelkaitis, G.; Nielsen, S.; Friis Lippert, M.; Vyberg, M. Virtual Double Staining: A Digital Approach to Immunohistochemical Quantification of Estrogen Receptor Protein in Breast Carcinoma Specimens. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 620–626. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Huang, W.; Zhang, L.; Wu, X.; Zhang, S.; Zhang, B. Radiogenomics: Bridging the gap between imaging and genomics for precision oncology. Medcomm 2024, 5, e722. [Google Scholar] [CrossRef] [PubMed]
- Sammut, S.J.; Crispin-Ortuzar, M.; Chin, S.F.; Provenzano, E.; Bardwell, H.A.; Ma, W.; Cope, W.; Dariush, A.; Dawson, S.J.; Abraham, J.E.; et al. Multi-omic machine learning predictor of breast cancer therapy response. Nature 2021, 601, 623–629. [Google Scholar] [CrossRef]
| Study | Objective | Dataset (Size) | Performance Values | Strengths | Limitations |
|---|---|---|---|---|---|
| Cruz-Roa et al. [31] | Detection of invasive breast cancer in WSI using CNN-based adaptive sampling (HASHI) | ~500 training cases tested on 195 The Cancer Genome Atlas slides | Dice coefficient: 76% | Highly efficient processing ~2000 WSIs/min | Limited generalisability |
| Han et al. [32] | Multi-classification of histopathological breast cancer images using DL model | BreaKHis; 7909 images and 8 sub-classes of breast cancer | Accuracy at patient level: 93.2% Accuracy at image level: 93.8% | Multi-classification Good generalisation | Utilises a single dataset (BreaKHis) Does not report other metrics (accuracy, sensitivity, specificity) |
| Yap et al. [36] | Automated ROI detection and lesion localisation in breast ultrasound images using FRBCNN | Ultrasound dataset A and B | Accuracy recall rate: 0.9236 Precision rate: 0.9408 F1-score: 0.9321 False alarm rate: 0.0621 | Leverages novel object detection algorithm (FRBCNN) Uses transfer learning to overcome the limited dataset available | No standard metrics reported Dataset sizes not clear Focuses on localisation not full lesion classification |
| Ueda et al. [37] | Detecting breast cancers on mammography using DL model | Development dataset: 4636 mammograms Hospital test dataset: 491 images Clinic test dataset: 2821 images | Detected all cancers with a 0.45–0.47 mFPI and partial AUCs of 0.93 in both test datasets | Strong performance across hospital and clinic datasets Used nonmalignant images to help identify normal features Open-source model | Asymmetrical data with more nonmalignant images Difficult detecting malignant lesions in dense breast tissues |
| Su et al. [35] | Simultaneous breast mass detection and segmentation using YOLO and LOGO models | Two independent test sets | True positive rate: 95.7% Mean average precision: 65% Mass segmentation on CBIS-DDSM dataset with F1-score of 74.5% | Simultaneous detection and segmentation Good performance while maintaining segmentation capabilities | Generalisibility to other datasets not fully shown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, V.; Khalid, U.; Gurung, J.; Dimov, R.; Chonov, V.; Uchikov, P.; Kostov, G.; Ivanov, S. Use of AI Histopathology in Breast Cancer Diagnosis. Medicina 2025, 61, 1878. https://doi.org/10.3390/medicina61101878
Ivanov V, Khalid U, Gurung J, Dimov R, Chonov V, Uchikov P, Kostov G, Ivanov S. Use of AI Histopathology in Breast Cancer Diagnosis. Medicina. 2025; 61(10):1878. https://doi.org/10.3390/medicina61101878
Chicago/Turabian StyleIvanov, Valentin, Usman Khalid, Jasmin Gurung, Rosen Dimov, Veselin Chonov, Petar Uchikov, Gancho Kostov, and Stefan Ivanov. 2025. "Use of AI Histopathology in Breast Cancer Diagnosis" Medicina 61, no. 10: 1878. https://doi.org/10.3390/medicina61101878
APA StyleIvanov, V., Khalid, U., Gurung, J., Dimov, R., Chonov, V., Uchikov, P., Kostov, G., & Ivanov, S. (2025). Use of AI Histopathology in Breast Cancer Diagnosis. Medicina, 61(10), 1878. https://doi.org/10.3390/medicina61101878






