Breast Lesions of Uncertain Malignant Potential and Risk of Breast Cancer Development: A Single-Center Experience on 10,531 Consecutive Biopsies
Abstract
1. Introduction
2. Materials and Methods
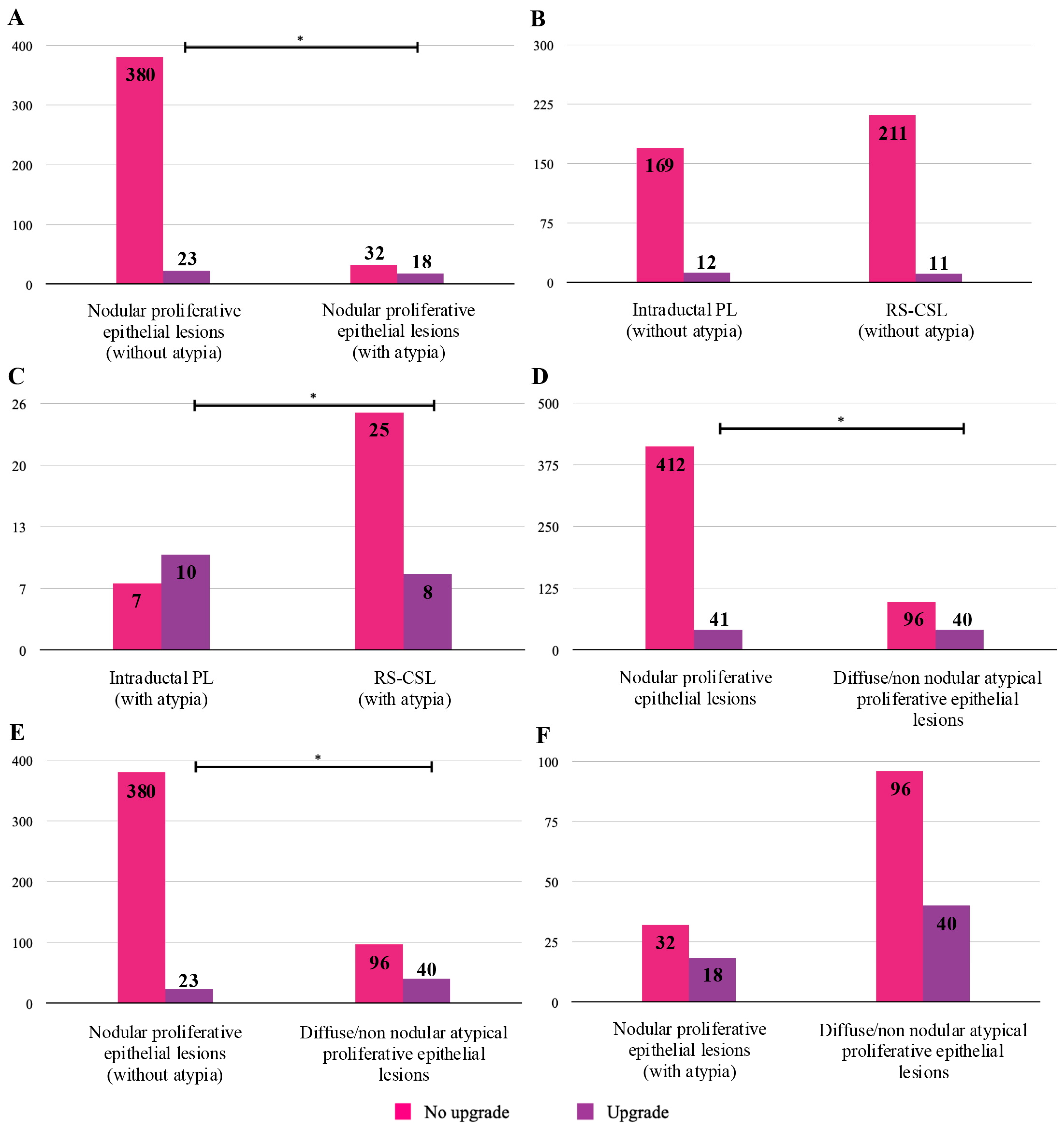
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ollila, D.W.; Hwang, E.S.; Brenin, D.R.; Kuerer, H.M.; Yao, K.; Feldman, S. The Changing Paradigms for Breast Cancer Surgery: Performing Fewer and Less-Invasive Operations. Ann. Surg. Oncol. 2018, 25, 2807–2812. [Google Scholar] [CrossRef]
- Hartmann, L.C.; Degnim, A.C.; Santen, R.J.; Dupont, W.D.; Ghosh, K. Atypical hyperplasia of the breast--risk assessment and management options. N. Engl. J. Med. 2015, 372, 78–89. [Google Scholar] [CrossRef]
- Sheikh, S.E.; Rathbone, M.; Chaudhary, K.; Joshi, A.; Lee, J.; Muthukumar, S.; Mylona, E.; Roxanis, I.; Rees, J. Rates and Outcomes of Breast Lesions of Uncertain Malignant Potential (B3) benchmarked against the National Breast Screening Pathology Audit; Improving Performance in a High Volume Screening Unit. Clin. Breast Cancer 2022, 22, 381–390. [Google Scholar] [CrossRef]
- Houssami, N.; Ciatto, S.; Bilous, M.; Vezzosi, V.; Bianchi, S. Borderline breast core needle histology: Predictive values for malignancy in lesions of uncertain malignant potential (B3). Br. J. Cancer 2007, 96, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.E.; Rakha, E.A.; Reed, J.; Lee, A.H.; Evans, A.J.; Ellis, I.O. Predictive value of needle core biopsy diagnoses of lesions of uncertain malignant potential (B3) in abnormalities detected by mammographic screening. Histopathology 2008, 53, 650–657. [Google Scholar] [CrossRef]
- Bianchi, S.; Caini, S.; Renne, G.; Cassano, E.; Ambrogetti, D.; Cattani, M.G.; Saguatti, G.; Chiaramondia, M.; Bellotti, E.; Bottiglieri, R.; et al. Positive predictive value for malignancy on surgical excision of breast lesions of uncertain malignant potential (B3) diagnosed by stereotactic vacuum-assisted needle core biopsy (VANCB): A large multi-institutional study in Italy. Breast 2011, 20, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Saladin, C.; Haueisen, H.; Kampmann, G.; Oehlschlegel, C.; Seifert, B.; Rageth, L.; Rageth, C.; Stadlmann, S.; Kubik-Huch, R.A.; Group, M. Lesions with unclear malignant potential (B3) after minimally invasive breast biopsy: Evaluation of vacuum biopsies performed in Switzerland and recommended further management. Acta Radiol. 2016, 57, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Richter-Ehrenstein, C.; Maak, K.; Roger, S.; Ehrenstein, T. Lesions of “uncertain malignant potential” in the breast (B3) identified with mammography screening. BMC Cancer 2018, 18, 829. [Google Scholar] [CrossRef]
- Pinder, S.E.; Shaaban, A.; Deb, R.; Desai, A.; Gandhi, A.; Lee, A.H.S.; Pain, S.; Wilkinson, L.; Sharma, N. NHS Breast Screening multidisciplinary working group guidelines for the diagnosis and management of breast lesions of uncertain malignant potential on core biopsy (B3 lesions). Clin. Radiol. 2018, 73, 682–692. [Google Scholar] [CrossRef]
- Elsharkawy, M.; Vestring, T.; Raatschen, H.J. A ten-year, single-center experience: Concordance between breast core needle biopsy/vacuum-assisted biopsy and postoperative histopathology in B3 and B5a cases. PLoS ONE 2020, 15, e0233574. [Google Scholar] [CrossRef]
- Griffiths, R.; Kaur, C.; Alarcon, L.; Szollosi, Z. Three-year Trends in Diagnosis of B3 Breast Lesions and Their Upgrade Rates to Malignant Lesions. Clin. Breast Cancer 2020, 20, e353–e357. [Google Scholar] [CrossRef]
- Lucioni, M.; Rossi, C.; Lomoro, P.; Ballati, F.; Fanizza, M.; Ferrari, A.; Garcia-Etienne, C.A.; Boveri, E.; Meloni, G.; Sommaruga, M.G.; et al. Positive predictive value for malignancy of uncertain malignant potential (B3) breast lesions diagnosed on vacuum-assisted biopsy (VAB): Is surgical excision still recommended? Eur. Radiol. 2021, 31, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.T.; Wyld, L.; Marotti, L.; Athanasiou, A.; Regitnig, P.; Catanuto, G.; Schoones Ma, J.W.; Zambon, M.; Camps, J.; Santini, D.; et al. European guidelines for the diagnosis, treatment and follow-up of breast lesions with uncertain malignant potential (B3 lesions) developed jointly by EUSOMA, EUSOBI, ESP (BWG) and ESSO. Eur. J. Surg. Oncol. 2023, 50, 107292. [Google Scholar] [CrossRef] [PubMed]
- Rageth, C.J.; O’Flynn, E.A.; Comstock, C.; Kurtz, C.; Kubik, R.; Madjar, H.; Lepori, D.; Kampmann, G.; Mundinger, A.; Baege, A.; et al. First International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res. Treat. 2016, 159, 203–213. [Google Scholar] [CrossRef]
- Rageth, C.J.; O’Flynn, E.A.M.; Pinker, K.; Kubik-Huch, R.A.; Mundinger, A.; Decker, T.; Tausch, C.; Dammann, F.; Baltzer, P.A.; Fallenberg, E.M.; et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res. Treat. 2019, 174, 279–296. [Google Scholar] [CrossRef]
- Elfgen, C.; Leo, C.; Kubik-Huch, R.A.; Muenst, S.; Schmidt, N.; Quinn, C.; McNally, S.; van Diest, P.J.; Mann, R.M.; Bago-Horvath, Z.; et al. Third International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Virchows Arch. 2023, 483, 5–20. [Google Scholar] [CrossRef]
- IARC. WHO Classification of Tumours Editorial Board Breast Tumors, 5th ed.; World Health Organization: Lyon, France, 2019; Volume 2. [Google Scholar]
- Gagnon, N.; Martel, E.; Cadrin-Chenevert, A.; Ledoux, E.; Racicot, C.; Villiard, R. Upgrade Rate of Atypical Ductal Hyperplasia: Ten Years Experience and Predictive Factors. J. Surg. Res. 2021, 266, 311–318. [Google Scholar] [CrossRef]
- Mohrmann, S.; Maier-Bode, A.; Dietzel, F.; Reinecke, P.; Krawczyk, N.; Kaleta, T.; Kreimer, U.; Antoch, G.; Fehm, T.N.; Roth, K.S. Malignancy Rate and Malignancy Risk Assessment in Different Lesions of Uncertain Malignant Potential in the Breast (B3 Lesions): An Analysis of 192 Cases from a Single Institution. Breast Care 2022, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, V.; Feudale, E.; Foschini, M.P.; Micheli, A.; Conti, A.; Riva, C.; Di Palma, S.; Rilke, F. Long-term follow-up of in situ carcinoma of the breast. Semin. Diagn. Pathol. 1994, 11, 223–235. [Google Scholar] [PubMed]
- Renshaw, A.A.; Gould, E.W. Long term clinical follow-up of atypical ductal hyperplasia and lobular carcinoma in situ in breast core needle biopsies. Pathology 2016, 48, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.M.; Sharma, N. Management of B3 Lesions—Practical Issues. Curr. Breast Cancer Rep. 2019, 11, 83–88. [Google Scholar] [CrossRef]
- Ellis, O.; Humphreys, S.; Michell, M.; Pinder, S.E.; Wells, C.A.; Zakhour, H.D. Guidelines for Non-Operative Diagnostic Procedures and Reporting in Breast Cancer Screening; The Royal College of Pathologists: London, UK, 2021. [Google Scholar]

| Diagnosis | Nr. Patients | Percentage (%) | |
|---|---|---|---|
| B1: Non-diagnostic/unsatisfactory or normal tissue only | 311 | 2.95 | |
| B2: Benign lesion | 5480 | 52.03 | |
| B3: Lesion of uncertain malignant potential | 1045 | 9.93 | |
| B4: Suspicion of malignancy | 48 | 0.45 | |
| B5a: Malignant (non-invasive) | 446 | 4.24 | 34.64 |
| B5b: Malignant (invasive) | 3103 | 29.47 | |
| B5c: Malignant (in situ/invasion not assessable) | 77 | 0.73 | |
| B5d: Malignant (non-epithelial, metastatic) | 21 | 0.2 | |
| Total 10,531 | |||
| Nr. Patients | Percentage (%) | |
|---|---|---|
| Radiological feature | 900 | 86.1 |
| 652 | 62.39 (72.5) |
| 189 | 18.09 (21.1) |
| 34 | 3.21 (3.7) |
| 25 | 2.39 (2.7) |
| Radiological feature not indicated | 145 | 13.9 |
| BI-RADS category | 956 | 91.5 |
| 1 | 0.1 (0.1) |
| 266 | 25.25 (27.8) |
| 673 | 64.40 (70.43) |
| 16 | 1.53 (1.67) |
| BI-RADS category not indicated | 89 | 8.5 |
| Type of biopsy | ||
| 804 | 76.94 |
| 241 | 23.06 |
| Type of excision | 892 | 85.36 |
| 877 | 83.92 (98.32) |
| 15 | 1.44 (1.68) |
| No excision | 153 | 14.64 |
| Nr. Lesions | Percentage (%) | |
|---|---|---|
| Nodular proliferative epithelial lesions | 606 | 57.99 |
| 537 | 51.4 (88.61) |
| 69 | 6.6 (11.39) |
| Diffuse/non-nodular atypical proliferative epithelial lesions | 196 | 18.75 |
| Adenomyoepitheliomas | 13 | 1.25 |
| Fibroepithelial lesions | 220 | 21.05 |
| 176 | 16.84 (80.0) |
| 16 | 1.53 (7.27) |
| 27 | 2.58 (12.25) |
| 1 | 0.1 (0.45) |
| Stromal lesions | 10 | 0.95 |
| 3 | 0.285 (30.0) |
| 5 | 0.475 (50.0) |
| 2 | 0.19 (20.0) |
| Nr. Lesions | Percentage (%) | |
|---|---|---|
| Nodular proliferative epithelial lesions (without atypia) | 537 | 51.4 |
| 251 | 24 (46.7) |
| 286 | 27.4 (53.3) |
| Nodular proliferative epithelial lesions (with atypia) | 69 | 6.6 |
| 26 | 2.5 (37.6) |
| 43 | 4.1 (62.4) |
| Diffuse/non-nodular atypical proliferative epithelial lesions | 196 | 18.75 |
| 92 | 8.8 (47) |
| 16 | 1.53 (8.2) |
| 65 | 6.22 (33.2) |
| 21 | 2 (10.6) |
| 1 | 0.1 (0.5) |
| 1 | 0.1 (0.5) |
| B3 Lesions | Resection | Diagnostic Concordance | Diagnosis of Malignancy on Resection | Upgrade | ||||
|---|---|---|---|---|---|---|---|---|
| Type | Subtype | Nr. | Percentage (%) | Invasive Carcinoma | Ductal Carcinoma In Situ | Number Upgraded | Upgrade Rate (95% CI) | |
| Nodular proliferative epithelial lesions (without atypia) | Intraductal PL | 181 | 143 | 79.01 | 0 | 12 * | 12 | 6.63 (3.5–11.3) |
| RS-CSL | 222 | 132 | 59.46 | 5 | 6 | 11 | 4.95 (2.5–8.7) | |
| Subtotal | 403 | 275 | 68.24 | 5 | 18 | 23 | 5.71 (3.7–8.4) | |
| Nodular proliferative epithelial lesions (with atypia) | Intraductal PL | 17 | 7 | 41.18 | 1 | 9 ** | 10 | 58.82 (32.9–81.6) |
| RS-CSL | 33 | 19 | 57.58 | 2 | 6 *** | 8 | 24.24 (11.1–42.3) | |
| Subtotal | 50 | 26 | 52.0 | 3 | 15 | 18 | 36.0 (22.9–50.8) | |
| Diffuse/non-nodular atypical proliferative epithelial lesions | ADH | 65 | 17 | 26.15 | 5 | 20 | 25 | 38.46 (26.7–51.4) |
| FEA | 11 | 3 | 27.27 | 1 | 3 | 4 | 36.36 (10.9–69.2) | |
| ALH | 46 | 32 | 69.57 | 5 | 3 *** | 8 | 17.39 (7.8–31.4) | |
| LCIS | 12 | 10 | 83.33 | 2 | 0 | 2 | 16.67 (2.1–48.4) | |
| AAA | 1 | 1 | 100 | |||||
| AMGA | 1 | 0 | 1 | 1 | 100 | |||
| Subtotal | 136 | 63 | 46.32 | 14 | 26 | 40 | 29.41 (21.9–37.8) | |
| B3 Lesions | Resection | Diagnostic Concordance | Outcome * | Upgrade | ||||
|---|---|---|---|---|---|---|---|---|
| Type | Subtype | Nr. | Percentage (%) | Borderline PT | Malignant PT | Number Upgraded | Upgrade Rate (95% CI) | |
| Fibroepithelial lesions | FA with epithelial atypia | 11 | 9 | 81.82 | 0 | 0 | ||
| FA with hypercellular stroma | 148 | 122 | 82.43 | 2 | 2 | 1.35 (0.24–4.79) | ||
| PT | 25 | 19 | 76.0 | 4 | 2 | 6 | 24.0 (9.4–45.1) | |
| Not specified | 1 | 0 | 0 | 0 | ||||
| Subtotal | 185 | 150 | 81.08 | 6 | 2 | 8 | 4.32 (2.21–8.3) | |
| Stromal lesions | Hemangioma | 2 | 2 | 100 | ||||
| Mesenchymal lesion/Fibromatosis | 4 | 4 | 100 | |||||
| PASH | 2 | 1 | 50 | |||||
| Subtotal | 8 | 7 | 87.5 | |||||
| Other lesions | Adenomyoepitheliomas | 13 | 8 | 61.54 | ||||
| ADH | FEA | ALH | LCIS | AMGA | |
|---|---|---|---|---|---|
| ADH | |||||
| FEA | No | ||||
| ALH | Yes, p = 0.0206 | No | |||
| LCIS | No | No | No | ||
| AMGA | No | No | No | No |
| Type | Nr. | Grading | Luminal A | Luminal B | HER2 | TNBC | ||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | ||||||
| Invasive breast carcinomas of no special type | 12 | 7 | 5 | 11 | 1 | 0 | 0 | |
| Invasive lobular carcinomas | 4 | 3 * | 4 | 0 | 0 | 0 | ||
| Mixed invasive ductal lobular carcinomas | 2 | 2 | 2 | |||||
| Tubular carcinomas | 1 | 1 | 1 | |||||
| Carcinomas with apocrine differentiation | 1 | 1 | 1 | |||||
| Mucinous carcinomas | 1 | 1 | 1 | |||||
| Metaplastic carcinomas | 1 | 1 | 1 | |||||
| Total | 22 | 8 | 12 | 1 | 19 | 1 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsaria, M.; Mangogna, A.; Bertoli, M.; Di Loreto, C.; Pegolo, E. Breast Lesions of Uncertain Malignant Potential and Risk of Breast Cancer Development: A Single-Center Experience on 10,531 Consecutive Biopsies. Medicina 2025, 61, 1877. https://doi.org/10.3390/medicina61101877
Orsaria M, Mangogna A, Bertoli M, Di Loreto C, Pegolo E. Breast Lesions of Uncertain Malignant Potential and Risk of Breast Cancer Development: A Single-Center Experience on 10,531 Consecutive Biopsies. Medicina. 2025; 61(10):1877. https://doi.org/10.3390/medicina61101877
Chicago/Turabian StyleOrsaria, Maria, Alessandro Mangogna, Massimo Bertoli, Carla Di Loreto, and Enrico Pegolo. 2025. "Breast Lesions of Uncertain Malignant Potential and Risk of Breast Cancer Development: A Single-Center Experience on 10,531 Consecutive Biopsies" Medicina 61, no. 10: 1877. https://doi.org/10.3390/medicina61101877
APA StyleOrsaria, M., Mangogna, A., Bertoli, M., Di Loreto, C., & Pegolo, E. (2025). Breast Lesions of Uncertain Malignant Potential and Risk of Breast Cancer Development: A Single-Center Experience on 10,531 Consecutive Biopsies. Medicina, 61(10), 1877. https://doi.org/10.3390/medicina61101877






