Effects of Lower Limb-Focused Low-Intensity Resistance Exercise Using Slow Movements on Locomotive Syndrome in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics Statement
2.3. Physical Exercise Protocol
2.4. Clinical Evaluations
2.5. Statistical Analysis
3. Results
3.1. Change in HbA1c and Body Weight
3.2. Change in LS Severity and Improvement in LS Risk Test Outcomes
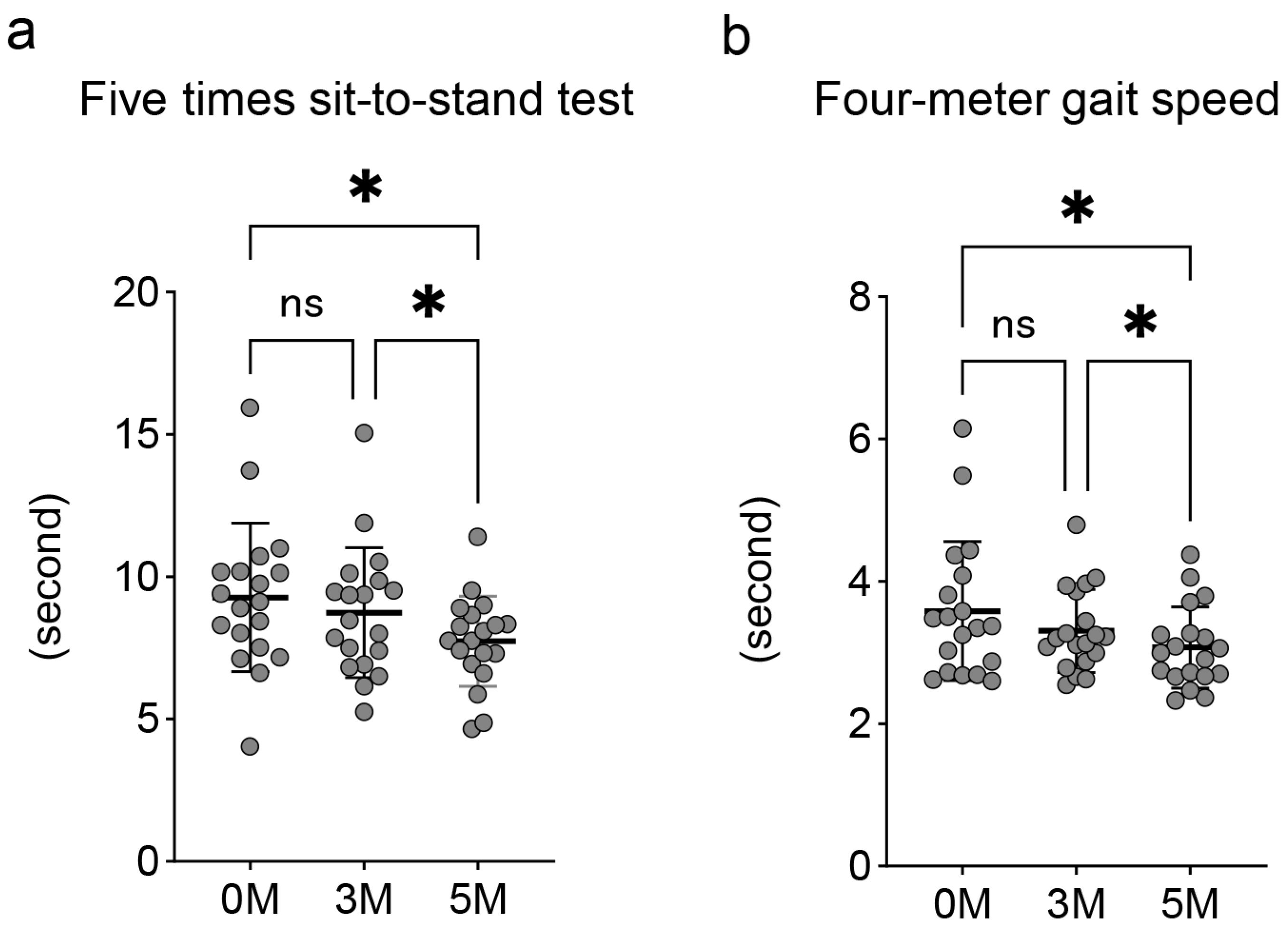
3.3. Changes in Lower Limb Function Indicators
3.4. Changes in Grip Strength and Skeletal Muscle Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LS | locomotive syndrome |
| QOL | quality of life |
| T2DM | type 2 diabetes mellitus |
| 1RM | one-repetition maximum |
| HbA1c | hemoglobin A1c |
| GLFS-25 | the 25-question Geriatric Locomotive Function Scale |
| SMI | skeletal muscle mass index |
| PhA | phase angle |
References
- Yoshimura, N.; Muraki, S.; Nakamura, K.; Tanaka, S. Epidemiology of the locomotive syndrome: The research on osteoarthritis/osteoporosis against disability study 2005–2015. Mod. Rheumatol. 2017, 27, 1–7. [Google Scholar] [CrossRef]
- Nakamura, K. A “super-aged” society and the “locomotive syndrome”. J. Orthop. Sci. 2008, 13, 1–2. [Google Scholar] [CrossRef]
- Hisamoto, K.; Okubo, N.; Fukushima, H.; Yamanaka, T.; Okizuka, Y.; Matsui, T.; Shinjo, H.; Morihara, T.; Takahashi, K. Can the measurement of Locomo Age improve motivation for exercise in fitness club users? Geriatr. Gerontol. Int. 2023, 23, 589–594. [Google Scholar] [CrossRef]
- Yoshimura, N.; Iidaka, T.; Horii, C.; Mure, K.; Muraki, S.; Oka, H.; Kawaguchi, H.; Akune, T.; Ishibashi, H.; Ohe, T.; et al. Epidemiology of locomotive syndrome using updated clinical decision limits: 6-year follow-ups of the ROAD study. J. Bone Miner. Metab. 2022, 40, 623–635. [Google Scholar] [CrossRef]
- Kasukawa, Y.; Miyakoshi, N.; Hongo, M.; Ishikawa, Y.; Kudo, D.; Kimura, R.; Ono, Y.; Shimada, Y. Locomotive Syndrome Is Associated with Health-Related Quality of Life and Low Back Pain in the Elderly, Including Individuals More Than 80 Years Old. Prog. Rehabil. Med. 2020, 5, 20200029. [Google Scholar] [CrossRef]
- Parthasarathy, B.; Kirubhakaran, K.; Selvam, S.P. A study of sarcopenia in patients with type 2 diabetes mellitus of more than 10 years duration and its association with bone mineral density. Ir. J. Med. Sci. 2025. [Google Scholar] [CrossRef]
- Kitagawa, N.; Okamura, T.; Kitagawa, N.; Hashimoto, Y.; Hamaguchi, M.; Fukui, M. Handgrip measurement as a useful benchmark for locomotive syndrome in patients with type 2 diabetes mellitus: A KAMOGAWA-DM cohort study. J. Diabetes Investig. 2020, 11, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Koistinen, H.A.; Zierath, J.R. Regulation of glucose transport in human skeletal muscle. Ann. Med. 2002, 34, 410–418. [Google Scholar] [CrossRef]
- Shen, Y.; Li, M.; Wang, K.; Qi, G.; Liu, H.; Wang, W.; Ji, Y.; Chang, M.; Deng, C.; Xu, F.; et al. Diabetic Muscular Atrophy: Molecular Mechanisms and Promising Therapies. Front. Endocrinol. 2022, 13, 917113. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, S.; Sun, J. The Relationship Between Lower Limb Muscle Strength with Fall Risk in Elderly Women with and Without Type 2 Diabetes: 2535. Med. Sci. Sports Exerc. 2022, 54, 488. [Google Scholar] [CrossRef]
- Sanz-Cánovas, J.; López-Sampalo, A.; Cobos-Palacios, L.; Ricci, M.; Hernández-Negrín, H.; Mancebo-Sevilla, J.J.; Álvarez-Recio, E.; López-Carmona, M.D.; Pérez-Belmonte, L.M.; Gómez-Huelgas, R.; et al. Management of Type 2 Diabetes Mellitus in Elderly Patients with Frailty and/or Sarcopenia. Int. J. Environ. Res. Public Health 2022, 19, 8677. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Takahashi, F.; Okamura, T.; Hamaguchi, M.; Fukui, M. Diet, exercise, and pharmacotherapy for sarcopenia in people with diabetes. Metabolism 2023, 144, 155585. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S86–S127. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone, M.A.; Marks, E.C.; Ryan, N.D.; Meredith, C.N.; Lipsitz, L.A.; Evans, W.J. High-intensity strength training in nonagenarians. Effects on skeletal muscle. J. Am. Med. Assoc. 1990, 263, 3029–3034. [Google Scholar] [CrossRef]
- Frontera, W.R.; Meredith, C.N.; O’Reilly, K.P.; Knuttgen, H.G.; Evans, W.J. Strength conditioning in older men: Skeletal muscle hypertrophy and improved function. J. Appl. Physiol. 1988, 64, 1038–1044. [Google Scholar] [CrossRef]
- Hurst, C.; Robinson, S.M.; Witham, M.D.; Dodds, R.M.; Granic, A.; Buckland, C.; De Biase, S.; Finnegan, S.; Rochester, L.; Skelton, D.A.; et al. Resistance exercise as a treatment for sarcopenia: Prescription and delivery. Age Ageing 2022, 51, afac003. [Google Scholar] [CrossRef]
- Hansen, D.; Niebauer, J.; Cornelissen, V.; Barna, O.; Neunhäuserer, D.; Stettler, C.; Tonoli, C.; Greco, E.; Fagard, R.; Coninx, K.; et al. Exercise Prescription in Patients with Different Combinations of Cardiovascular Disease Risk Factors: A Consensus Statement from the EXPERT Working Group. Sports Med. 2018, 48, 1781–1797. [Google Scholar] [CrossRef]
- Piva, S.R.; Susko, A.M.; Khoja, S.S.; Josbeno, D.A.; Fitzgerald, G.K.; Toledo, F.G. Links between osteoarthritis and diabetes: Implications for management from a physical activity perspective. Clin. Geriatr. Med. 2015, 31, 67–87. [Google Scholar] [CrossRef]
- Nesti, L.; Pugliese, N.R.; Sciuto, P.; Natali, A. Type 2 diabetes and reduced exercise tolerance: A review of the literature through an integrated physiology approach. Cardiovasc. Diabetol. 2020, 19, 134. [Google Scholar] [CrossRef]
- Watanabe, Y.; Madarame, H.; Ogasawara, R.; Nakazato, K.; Ishii, N. Effect of very low-intensity resistance training with slow movement on muscle size and strength in healthy older adults. Clin. Physiol. Funct. Imaging 2014, 34, 463–470. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tanimoto, M.; Ohgane, A.; Sanada, K.; Miyachi, M.; Ishii, N. Increased muscle size and strength from slow-movement, low-intensity resistance exercise and tonic force generation. J. Aging Phys. Act. 2013, 21, 71–84. [Google Scholar] [CrossRef]
- Takenami, E.; Iwamoto, S.; Shiraishi, N.; Kato, A.; Watanabe, Y.; Yamada, Y.; Yamada, S.; Ishii, N. Effects of low-intensity resistance training on muscular function and glycemic control in older adults with type 2 diabetes. J. Diabetes Investig. 2019, 10, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Ito, Y.M.; Akagi, M.; Chosa, E.; Fuji, T.; Hirano, K.; Ikeda, S.; Ishibashi, H.; Ishibashi, Y.; Ishijima, M.; et al. Reference values for the locomotive syndrome risk test quantifying mobility of 8681 adults aged 20-89 years: A cross-sectional nationwide study in Japan. J. Orthop. Sci. 2020, 25, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Morimoto, T.; Otani, K.; Mawatari, M. Locomotive Syndrome and Lumbar Spine Disease: A Systematic Review. J. Clin. Med. 2022, 11, 1304. [Google Scholar] [CrossRef] [PubMed]
- Seichi, A.; Hoshino, Y.; Doi, T.; Akai, M.; Tobimatsu, Y.; Iwaya, T. Development of a screening tool for risk of locomotive syndrome in the elderly: The 25-question Geriatric Locomotive Function Scale. J. Orthop. Sci. 2012, 17, 163–172. [Google Scholar] [CrossRef]
- Sardinha, L.B. Physiology of exercise and phase angle: Another look at BIA. Eur. J. Clin. Nutr. 2018, 72, 1323–1327. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Kusakabe, T.; Arai, H.; Yamamoto, Y.; Nakao, K.; Ikeue, K.; Ishihara, Y.; Tagami, T.; Yasoda, A.; Ishii, K.; et al. Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J. Cachexia Sarcopenia Muscle 2022, 13, 180–189. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Heintz, E.C.; Rebello, C.J.; Axelrod, C.L. Exercise in the Prevention and Treatment of Type 2 Diabetes. Compr. Physiol. 2023, 13, 4559–4585. [Google Scholar] [CrossRef]
- Ribeiro, A.; Carvalho, J.P.R.; Bento-Torres, N.V.O. Physical exercise as treatment for adults with type 2 diabetes: A rapid review. Front. Endocrinol. 2023, 14, 1233906. [Google Scholar] [CrossRef]
- Anderson, K.C.; Weeldreyer, N.R.; Leicht, Z.S.; Angadi, S.S.; Liu, Z. Exercise Intolerance in Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2025, 14, e035721. [Google Scholar] [CrossRef]
- Sejersted, O.M.; Hargens, A.R. Intramuscular pressures for monitoring different tasks and muscle conditions. Adv. Exp. Med. Biol. 1995, 384, 339–350. [Google Scholar]
- Takada, S.; Okita, K.; Suga, T.; Omokawa, M.; Kadoguchi, T.; Sato, T.; Takahashi, M.; Yokota, T.; Hirabayashi, K.; Morita, N.; et al. Low-intensity exercise can increase muscle mass and strength proportionally to enhanced metabolic stress under ischemic conditions. J. Appl. Physiol. 2012, 113, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ogata, T. Locomotive Syndrome: Definition and Management. Clin. Rev. Bone Miner. Metab. 2016, 14, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Muranaga, S. Development of a convenient way to predict ability to walk, using a two-step test. J. Showa Med. Assoc. 2003, 63, 301–308. [Google Scholar]
- Sakurai, R.; Yasunaga, M.; Nishi, M.; Fukaya, T.; Hasebe, M.; Murayama, Y.; Koike, T.; Matsunaga, H.; Nonaka, K.; Suzuki, H.; et al. Co-existence of social isolation and homebound status increase the risk of all-cause mortality. Int. Psychogeriatr. 2019, 31, 703–711. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morihara, T.; Hisamoto, K.; Okubo, N.; Fukushima, H.; Matsui, T.; Hiramoto, M.; Hamaguchi, M.; Okada, H.; Matsui, T.; Imai, D.; et al. Effects of Lower Limb-Focused Low-Intensity Resistance Exercise Using Slow Movements on Locomotive Syndrome in Patients with Type 2 Diabetes Mellitus. Medicina 2025, 61, 1875. https://doi.org/10.3390/medicina61101875
Morihara T, Hisamoto K, Okubo N, Fukushima H, Matsui T, Hiramoto M, Hamaguchi M, Okada H, Matsui T, Imai D, et al. Effects of Lower Limb-Focused Low-Intensity Resistance Exercise Using Slow Movements on Locomotive Syndrome in Patients with Type 2 Diabetes Mellitus. Medicina. 2025; 61(10):1875. https://doi.org/10.3390/medicina61101875
Chicago/Turabian StyleMorihara, Toru, Kazufumi Hisamoto, Naoki Okubo, Hideki Fukushima, Tomoyuki Matsui, Machiko Hiramoto, Masahide Hamaguchi, Hiroshi Okada, Takaaki Matsui, Dan Imai, and et al. 2025. "Effects of Lower Limb-Focused Low-Intensity Resistance Exercise Using Slow Movements on Locomotive Syndrome in Patients with Type 2 Diabetes Mellitus" Medicina 61, no. 10: 1875. https://doi.org/10.3390/medicina61101875
APA StyleMorihara, T., Hisamoto, K., Okubo, N., Fukushima, H., Matsui, T., Hiramoto, M., Hamaguchi, M., Okada, H., Matsui, T., Imai, D., Fukui, M., & Takahashi, K. (2025). Effects of Lower Limb-Focused Low-Intensity Resistance Exercise Using Slow Movements on Locomotive Syndrome in Patients with Type 2 Diabetes Mellitus. Medicina, 61(10), 1875. https://doi.org/10.3390/medicina61101875








