The Genetic Polymorphisms of CYP2C9 and VKORC1 in the Saudi Population and Their Impact on Anticoagulant Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Polymerase Chain Reaction (PCR)
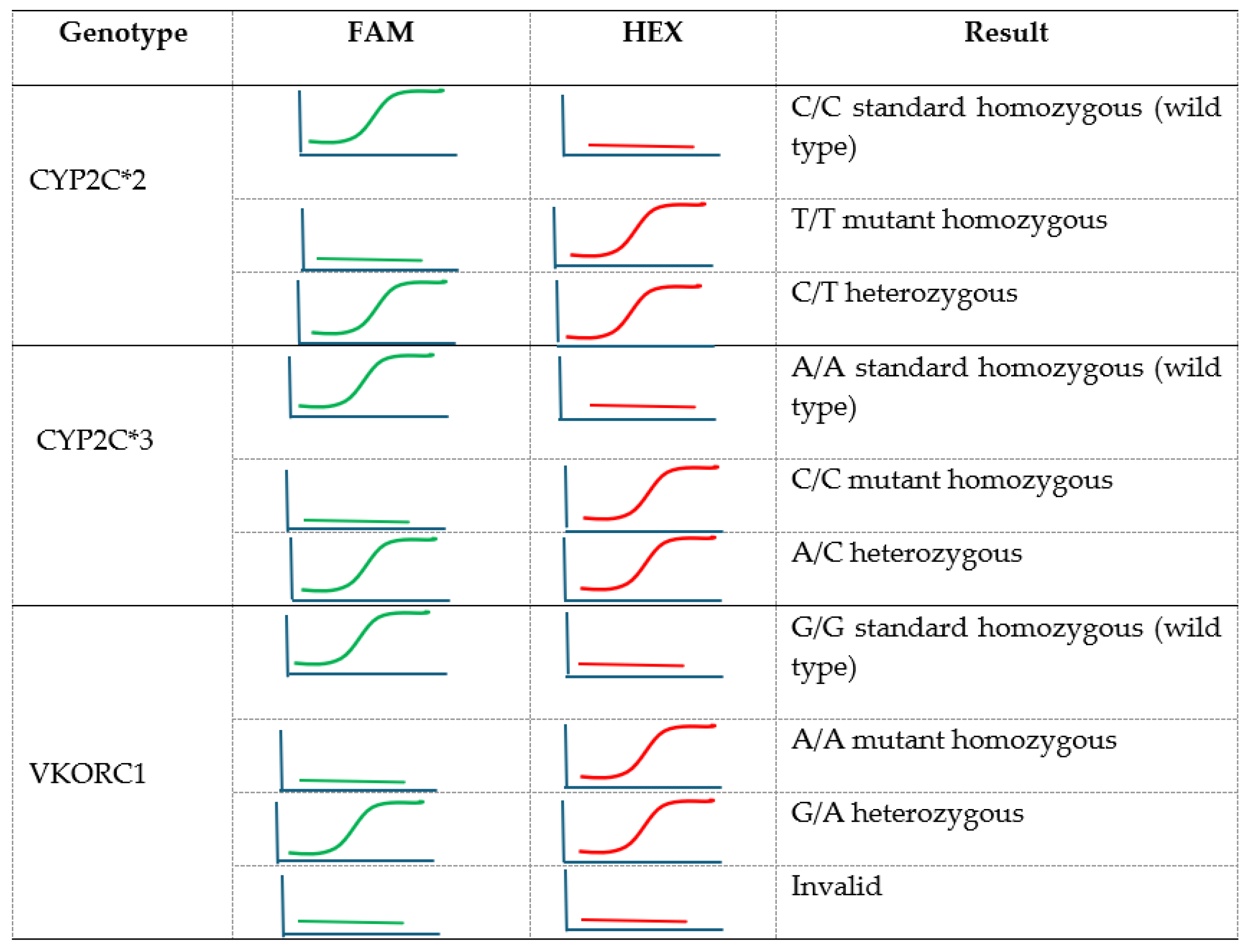
2.3. VKORC1 and CYP2C9 Variants
2.4. Statistical Analysis
3. Results
3.1. CYP2C9 and VKORC1 Genotypes
3.2. Association Between Genotypes and Clinical Outcomes
3.3. The Impact of VKORC1 and CYP2C9 Genotype Variants on the Weekly Average Warfarin Dose
3.4. Genetic Diversity in VKORC1 and CYP2C9 Among Different Ethnicities
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| INR | International normalized ratio |
| PCR | Polymerase chain reaction |
| LD | linkage disequilibrium |
| BMI | Body mass index |
| VKORC1 | Vitamin K epoxide reductase multiprotein complex |
| HRM | high-resolution melting analysis |
| NTC | No-template controls |
References
- Biswas, M.; Bendkhale, S.R.; Deshpande, S.P.; Thaker, S.J.; Kulkarni, D.V.; Bhatia, S.J.; Rajadhyaksha, A.G.; Gogtay, N.J.; Thatte, U.M. Association between genetic polymorphisms of CYP2C9 and VKORC1 and safety and efficacy of warfarin: Results of a 5 years audit. Indian Heart J. 2018, 70 (Suppl. S3), S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Witt, D.M.; Sadler, M.A.; Shanahan, R.L.; Mazzoli, G.; Tillman, D.J. Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapy. Chest 2005, 127, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, A.M.; Pereira, J.A.; Labiris, R.; McDonald, H.; Douketis, J.D.; Crowther, M.; Wells, P.S. Systematic overview of warfarin and its drug and food interactions. Arch. Intern. Med. 2005, 165, 1095–1106. [Google Scholar] [CrossRef]
- Iwuchukwu, O.F.; Ramirez, A.H.; Shi, Y.; Bowton, E.A.; Kawai, V.K.; Schildcrout, J.S.; Roden, D.M.; Denny, J.C.; Stein, C.M. Genetic determinants of variability in warfarin response after the dose-titration phase. Pharmacogenet Genom. 2016, 26, 510–516. [Google Scholar] [CrossRef]
- Wang, X.; Liao, X.; Yang, C.; Huang, K.; Yu, T.; Yu, L.; Han, C.; Zhu, G.; Zeng, X.; Liu, Z.; et al. Identification of prognostic biomarkers for patients with hepatocellular carcinoma after hepatectomy. Oncol. Rep. 2019, 41, 1586–1602. [Google Scholar] [CrossRef]
- Sconce, E.A.; Khan, T.I.; Wynne, H.A.; Avery, P.; Monkhouse, L.; King, B.P.; Wood, P.; Kesteven, P.; Daly, A.K.; Kamali, F. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: Proposal for a new dosing regimen. Blood 2005, 106, 2329–2333. [Google Scholar] [CrossRef]
- Wadelius, M.; Chen, L.Y.; Downes, K.; Ghori, J.; Hunt, S.; Eriksson, N.; Wallerman, O.; Melhus, H.; Wadelius, C.; Bentley, D.; et al. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005, 5, 262–270. [Google Scholar] [CrossRef]
- Eichelbaum, M.; Ingelman-Sundberg, M.; Evans, W.E. Pharmacogenomics and individualized drug therapy. Annu. Rev. Med. 2006, 57, 119–137. [Google Scholar] [CrossRef]
- Farzamikia, N.; Sakhinia, E.; Afrasiabirad, A. Pharmacogenetics-Based Warfarin Dosing in Patients With Cardiac Valve Replacement: The Effects of CYP2C9 and VKORC1 Gene Polymorphisms. Lab. Med. 2017, 49, 25–34. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; D’Ambrosio, R.L.; Di Perna, P.; Chetta, M.; Santacroce, R.; Brancaccio, V.; Grandone, E.; Margaglione, M. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood 2005, 105, 645–649. [Google Scholar] [CrossRef]
- Muszkat, M.; Blotnik, S.; Elami, A.; Krasilnikov, I.; Caraco, Y. Warfarin metabolism and anticoagulant effect: A prospective, observational study of the impact of CYP2C9 genetic polymorphism in the presence of drug-disease and drug-drug interactions. Clin. Ther. 2007, 29, 427–437. [Google Scholar] [CrossRef]
- Ruano, G.; Thompson, P.D.; Villagra, D.; Bower, B.; Kocherla, M.; Yazdanpanah, G.; Seip, R.L.; Windemuth, A.; White, C.M.; Duconge, J.; et al. High carrier prevalence of combinatorial CYP2C9 and VKORC1 genotypes affecting warfarin dosing. Pers. Med. 2008, 5, 225–232. [Google Scholar] [CrossRef]
- Limdi, N.; Goldstein, J.; Blaisdell, J.; Beasley, T.; Rivers, C.; Acton, R. Influence of CYP2C9 Genotype on warfarin dose among African American and European Americans. Pers. Med. 2007, 4, 157–169. [Google Scholar] [CrossRef]
- Oldenburg, J.; Bevans, C.G.; Muller, C.R.; Watzka, M. Vitamin K epoxide reductase complex subunit 1 (VKORC1): The key protein of the vitamin K cycle. Antioxid. Redox Signal 2006, 8, 347–353. [Google Scholar] [CrossRef]
- Wu, S.; Chen, X.; Jin, D.Y.; Stafford, D.W.; Pedersen, L.G.; Tie, J.K. Warfarin and vitamin K epoxide reductase: A molecular accounting for observed inhibition. Blood 2018, 132, 647–657. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Hayashi, H.; Tashiro, Y.; Sakawa, S.; Moriwaki, H.; Akimoto, T.; Doi, O.; Kimura, M.; Kawarasaki, Y.; Inoue, K.; et al. Effect of VKORC1-1639 G>A polymorphism, body weight, age, and serum albumin alterations on warfarin response in Japanese patients. Thromb. Res. 2009, 124, 161–166. [Google Scholar] [CrossRef]
- Jiang, H.H.; Liu, J.; Wang, Y.C.; Ye, H.M.; Li, X.; Zhou, Y.X.; Zhang, W.; Wang, L.S. The Impact of Gene Polymorphisms on Anticoagulation Control With Warfarin. Clin. Appl. Thromb. Hemost. 2018, 24, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Khasawneh, R.; Peter, I.; Kornreich, R.; Desnick, R.J. Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics 2010, 11, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Caudle, K.E.; Gong, L.; Whirl-Carrillo, M.; Stein, C.M.; Scott, S.A.; Lee, M.T.; Gage, B.F.; Kimmel, S.E.; Perera, M.A.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clin. Pharmacol. Ther. 2017, 102, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gage, B.F.; Bass, A.R.; Lin, H.; Woller, S.C.; Stevens, S.M.; Al-Hammadi, N.; Li, J.; Rodriguez, T., Jr.; Miller, J.P.; McMillin, G.A.; et al. Effect of Genotype-Guided Warfarin Dosing on Clinical Events and Anticoagulation Control Among Patients Undergoing Hip or Knee Arthroplasty: The GIFT Randomized Clinical Trial. JAMA 2017, 318, 1115–1124. [Google Scholar] [CrossRef]
- Santos, P.C.; Dinardo, C.L.; Schettert, I.T.; Soares, R.A.; Kawabata-Yoshihara, L.; Bensenor, I.M.; Krieger, J.E.; Lotufo, P.A.; Pereira, A.C. CYP2C9 and VKORC1 polymorphisms influence warfarin dose variability in patients on long-term anticoagulation. Eur. J. Clin. Pharmacol. 2013, 69, 789–797. [Google Scholar] [CrossRef]
- Al-Eitan, L.N.; Almasri, A.Y.; Khasawneh, R.H. Impact of CYP2C9 and VKORC1 Polymorphisms on Warfarin Sensitivity and Responsiveness in Jordanian Cardiovascular Patients during the Initiation Therapy. Genes 2018, 9, 578. [Google Scholar] [CrossRef]
- Kneeland, P.P.; Fang, M.C. Current issues in patient adherence and persistence: Focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Prefer. Adherence 2010, 4, 51–60. [Google Scholar]
- Asiimwe, I.G.; Zhang, E.J.; Osanlou, R.; Jorgensen, A.L.; Pirmohamed, M. Warfarin dosing algorithms: A systematic review. Br. J. Clin. Pharmacol. 2021, 87, 1717–1729. [Google Scholar] [CrossRef]
- McWilliam, A.; Lutter, R.; Nardinelli, C. Healthcare impact of personalized medicine using genetic testing: An exploratory analysis for warfarin. Pers. Med. 2008, 5, 279–284. [Google Scholar] [CrossRef]
- Hamdy, S.I.; Hiratsuka, M.; Narahara, K.; El-Enany, M.; Moursi, N.; Ahmed, M.S.; Mizugaki, M. Allele and genotype frequencies of polymorphic cytochromes P450 (CYP2C9, CYP2C19, CYP2E1) and dihydropyrimidine dehydrogenase (DPYD) in the Egyptian population. Br. J. Clin. Pharmacol. 2002, 53, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Bazan, N.S.; Sabry, N.A.; Rizk, A.; Mokhtar, S.; Badary, O.A. Factors affecting warfarin dose requirements and quality of anticoagulation in adult Egyptian patients: Role of gene polymorphism. Ir. J. Med. Sci. 2014, 183, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Alrashid, M.H.; Al-Serri, A.; Alshemmari, S.H.; Koshi, P.; Al-Bustan, S.A. Association of Genetic Polymorphisms in the VKORC1 and CYP2C9 Genes with Warfarin Dosage in a Group of Kuwaiti Individuals. Mol. Diagn. Ther. 2016, 20, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Pathare, A.; Al Khabori, M.; Alkindi, S.; Al Zadjali, S.; Misquith, R.; Khan, H.; Lapoumeroulie, C.; Paldi, A.; Krishnamoorthy, R. Warfarin pharmacogenetics: Development of a dosing algorithm for Omani patients. J. Hum. Genet. 2012, 57, 665–669. [Google Scholar] [CrossRef]
- Johnson, J.A.; Gong, L.; Whirl-Carrillo, M.; Gage, B.F.; Scott, S.A.; Stein, C.M.; Anderson, J.L.; Kimmel, S.E.; Lee, M.T.; Pirmohamed, M.; et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin. Pharmacol. Ther. 2011, 90, 625–629. [Google Scholar] [CrossRef]
- Selim, T.E.; Azzam, H.A.; Ghoneim, H.R.; Mohamed, A.A.; El Wakeel, H.; Abu Bakr, H.M. Pharmacogenetic Warfarin Dosing Algorithms: Validity in Egyptian Patients. Acta Haematol. 2018, 139, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, A.M.; Bardissy, A.E.; Saad, M.O.; Fares, A.; Sadek, A.; Elshafei, M.N.; Eltahir, A.; Mohamed, A.; Elewa, H. Accuracy of an internationally validated genetic-guided warfarin dosing algorithm compared to a clinical algorithm in an Arab population. Curr. Probl. Cardiol. 2024, 49, 102865. [Google Scholar] [CrossRef]
- Ayesh, B.M.; Abu Shaaban, A.S.; Abed, A.A. Evaluation of CYP2C9- and VKORC1-based pharmacogenetic algorithm for warfarin dose in Gaza-Palestine. Future Sci. OA 2018, 4, FSO276. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, M.; Fang, K.; Xing, Q.; Feng, G.; Shen, L.; He, L.; Qin, S. A systematic genetic polymorphism analysis of the CYP2C9 gene in four different geographical Han populations in mainland China. Genomics 2011, 97, 277–281. [Google Scholar] [CrossRef]
- Gaikwad, T.; Ghosh, K.; Shetty, S. VKORC1 and CYP2C9 genotype distribution in Asian countries. Thromb. Res. 2014, 134, 537–544. [Google Scholar] [CrossRef]
- Dean, L. Warfarin Therapy and VKORC1 and CYP Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kattman, B.L., Malheiro, A.J., Eds.; Bethesda: Rockville, MD, USA, 2012. [Google Scholar]
- Yuan, H.Y.; Chen, J.J.; Lee, M.T.; Wung, J.C.; Chen, Y.F.; Charng, M.J.; Lu, M.J.; Hung, C.R.; Wei, C.Y.; Chen, C.H.; et al. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum. Mol. Genet. 2005, 14, 1745–1751. [Google Scholar] [CrossRef]
- Hasunuma, T.; Tohkin, M.; Kaniwa, N.; Jang, I.J.; Yimin, C.; Kaneko, M.; Saito, Y.; Takeuchi, M.; Watanabe, H.; Yamazoe, Y.; et al. Absence of ethnic differences in the pharmacokinetics of moxifloxacin, simvastatin, and meloxicam among three East Asian populations and Caucasians. Br. J. Clin. Pharmacol. 2016, 81, 1078–1090. [Google Scholar] [CrossRef]
- Silan, C.; Dogan, O.T.; Silan, F.; Kukulguven, F.M.; Asgun, H.F.; Ozdemir, S.; Uludag, A.; Atik, S.; Gungor, B.; Akdur, S.; et al. The prevalence of VKORC1 1639 G>A and CYP2C9*2*3 genotypes in patients that requiring anticoagulant therapy in Turkish population. Mol. Biol. Rep. 2012, 39, 11017–11022. [Google Scholar] [CrossRef]
- Abdelhedi, R.; Bouayed, N.A.; Alfadhli, S.; Abid, L.; Rebai, A.; Kharrat, N. Characterization of drug-metabolizing enzymes CYP2C9, CYP2C19 polymorphisms in Tunisian, Kuwaiti and Bahraini populations. J. Genet. 2015, 94, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, K.; Al Banna, R.; Malalla, Z.; Husain, A.; Sater, M.; Jassim, G.; Otoom, S. Influence of CYP2C9, VKORC1, and CYP4F2 polymorphisms on the pharmacodynamic parameters of warfarin: A cross-sectional study. Pharmacol. Rep. 2021, 73, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Holail, J.; Mobarak, R.; Al-Ghamdi, B.; Aljada, A.; Fakhoury, H. Association of VKORC1 and CYP2C9 single-nucleotide polymorphisms with warfarin dose adjustment in Saudi patients. Drug Metab. Pers. Ther. 2022, 37, 353–359. [Google Scholar] [CrossRef]
- Jokhab, S.; AlRasheed, M.M.; Bakheet, D.; AlMomen, A.; AlAboud, N.; Kamali, F. The impact of CYP2C9, VKORC1, and CYP4F2 polymorphisms on warfarin dose requirement in Saudi patients. Front. Pharmacol. 2025, 16, 1547142. [Google Scholar] [CrossRef]
- Chang, W.C.; Hung, S.I.; Carleton, B.C.; Chung, W.H. An update on CYP2C9 polymorphisms and phenytoin metabolism: Implications for adverse effects. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 723–734. [Google Scholar] [CrossRef]
- Park, Y.A.; Song, Y.B.; Yee, J.; Yoon, H.Y.; Gwak, H.S. Influence of CYP2C9 Genetic Polymorphisms on the Pharmacokinetics of Losartan and Its Active Metabolite E-3174: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Ammari, M.A.; Almuzzaini, B.; Al Sulaiman, K.; AlBalwi, M.; Sultana, K.; Alabdulkareem, I.B.; Almakhlafi, N.S.; Humoud, A.A.; Waheeby, M.; Balla, M.; et al. Targeted next-generation sequencing of genes involved in Warfarin Pharmacodynamics and pharmacokinetics pathways using the Saudi Warfarin Pharmacogenetic study (SWAP). Pharmacogenomics J. 2023, 23, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Paludetto, M.N.; Kurkela, M.; Kahma, H.; Neuvonen, M.; Xiang, X.; Cai, W.; Backman, J.T. In vitro assessment of inhibitory effects of kinase inhibitors on CYP2C9, 3A and 1A2: Prediction of drug-drug interaction risk with warfarin and direct oral anticoagulants. Eur. J. Pharm. Sci. 2024, 203, 106884. [Google Scholar] [CrossRef] [PubMed]

| Genotype and Alleles | No. of Patients (Frequency%) |
|---|---|
| VKORC1 genotype and alleles | |
| GG | 54 (54%) |
| AG | 30 (30%) |
| AA | 16 (16%) |
| G | 69% |
| A | 31% |
| CYP2C9 genotype and alleles | |
| *1/*1 | 71 (71%) |
| *1/*2 | 14 (14%) |
| *2/*2 | 1 (1%) |
| *1/*3 | 11 (11%) |
| *2/*3 | 2 (2%) |
| *3/*3 | 1 (1%) |
| *1 | 83% |
| *2 | 9% |
| *3 | 8% |
| Genotypes and Alleles | Number | Mean of INR | 95% CI | p Value |
|---|---|---|---|---|
| CYP2C9 *1*1 | 71 | 2.436 | (2.115, 2.757) | |
| CYP2C9 *1*2 | 14 | 2.226 | (1.507, 2.944) | |
| CYP2C9 *1*3 | 11 | 2.138 | (1.328, 2.949) | |
| CYP2C9 *2*2 | 1 | 2.400 | (−0.288, 5.088) | 0.976 |
| CYP2C9 *2*3 | 2 | 2.075 | (0.174, 3.976) | |
| CYP2C9 *3*3 | 1 | 2.040 | (−0.648, 4.728) | |
| VKORC1 GG | 54 | 2.2805 | (2.099, 3.005) | |
| VKORC1 GA | 30 | 2.2805 | (1.8777, 2.6832) | |
| VKORC1 AA | 16 | 2.2264 | (1.6633, 2.7894) | 0.584 |
| Term | Coef | SE Coef | T-Value | p-Value | VIF |
|---|---|---|---|---|---|
| Constant | 7.05 | 1.47 | 4.78 | 0.000 | |
| Age | −0.0285 | 0.0222 | −1.28 | 0.203 | 1.05 |
| VKORC1 variants | |||||
| GA | 3.111 | 0.919 | 3.39 | 0.001 | 1.83 |
| GG | 6.234 | 0.949 | 6.57 | 0.000 | 1.82 |
| CYP2C9 variants | |||||
| *1*2 | −1.568 | 0.991 | −1.58 | 0.117 | 1.05 |
| *1*3 | −3.55 | 1.13 | −3.15 | 0.002 | 1.10 |
| *2*2 | −4.22 | 3.49 | −1.21 | 0.229 | 1.07 |
| *2*3 | −2.19 | 2.45 | −0.89 | 0.373 | 1.04 |
| *3*3 | −4.08 | 3.43 | −1.19 | 0.238 | 1.03 |
| Gender | |||||
| Male | −1.971 | 0.819 | −2.41 | 0.018 | 1.17 |
| Genotype | Present Study (Saudi Patients) (mg/day) | FDA Guidelines (mg/day) [36] |
|---|---|---|
| VKORC1 GG and CYP2C9 *1/*1 | 10.6 | 5–7 |
| VKORC1 GG and CYP2C9 *1/*2, *1/*3 | 6.2–7.3 | 3–4 |
| VKORC1 GG and CYP2C9 *2/*2, *2/*3, *3/*3 | 0.5–2 | 0.5–2 |
| VKORC1 GA and CYP2C9 *1/*1 | 7.4 | 5–7 |
| VKORC1 GA and CYP2C9 *1/*2, *1/*3 | 5.1–5.6 | 3–4 |
| VKORC1 GA and CYP2C9 *2/*2, *2/*3, *3/*3 | 0.5–2 | 0.5–2 |
| VKORC1 AA and CYP2C9 *1/*1 | 4.2 | 3–4 |
| VKORC1 AA and CYP2C9 *1/*2, *1/*3 | 3.4–3.8 | 0.5–2 |
| VKORC1 AA and CYP2C9 *2/*2, *2/*3, *3/*3 | 0.5–2 | 0.5–2 |
| Gene Variants | No. of Patients (Frequency %) | Average Weekly Warfarin Initial Dose (mg) | p-Value |
|---|---|---|---|
| VKORC1 GG | 54 (54%) | 68.47 | |
| VKORC1 GA | 30 (30%) | 45.05 | |
| VKORC1 AA | 16 (16%) | 25.88 | p < 0.00001 |
| CYP2C9 *1/*1 | 71 (71%) | 54.2 | |
| CYP2C9 *1/*2 | 14 (14%) | 42.4 | |
| CYP2C9 *1/*3 | 11 (11%) | 34 | |
| CYP2C9 *2/*3 | 2 (2%) | 28 | |
| CYP2C9 *2/*2, CYP2C9 *3/*3 | 2 (2%) | 18 | p < 0.0357 |
| VKORC1 GG CYP2C9 *1/*1 | 26 (26%) | 74.4 | |
| VKORC1 GG CYP2C9 *1/*1, *1/*2, *2/*3, *2/*2, *3/*3 | 9 (9%) | 51.3 | |
| VKORC1 GA CYP2C9 *1/*1 | 29 (29%) | 51.4 | |
| VKORC1 GA, CYP2C9 *1/*2, *2/*3, *2/*2, *3/*3 | 14 (14%) | 31.9 | p < 0.00001 |
| VKORC1 AA CYP2C9 *1*1 | 16 (16%) | 25.2 | |
| VKORC1 AA, CYP2C9 *1/*2, *2/*3, *2/*2, *3/*3 | 5 (5%) | 24.7 |
| Race | VKORC1 | CYP2C9 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GG | GA | AA | *1*1 | *1*2 | *2*2 | *1*3 | *2*3 | *3*3 | |
| Caucasian [37,38] | 39% | 47% | 14% | 67% | 17% | 3% | 13% | 0% | 0% |
| UK [6] | 25% | 56% | 19% | 56% | 22% | 3% | 14% | 4% | <1% |
| Japan [9] | 23% | 58% | 19% | 23% | 4% | 0% | 51% | 17% | 5% |
| Turkey [39] | 21.9% | 75.4% | 2.7% | 56.5% | 23% | 3% | 8.6% | 7.2% | 1.7% |
| Chinese [34] | 2% | 18% | 80% | 91% | 0% | 0% | 7% | 1.5% | 0% |
| India [1] | 69% | 27% | 4% | 74% | 8% | 0% | 16% | 1.5% | 0.05% |
| Egypt [26,27] | 24% | 51% | 25% | 66% | 19% | 2% | 11% | 0% | 4% |
| Tunisia [40] | 9.3% | 66% | 24% | 62% | 19% | 3% | 13% | 3% | 0% |
| Kuwait [28] | 35.2% | 50% | 14.8% | 69.4% | 21.3% | 0% | 6.5% | 2.8% | 0% |
| Oman [29] | 42.7 | 42.7 | 14.6 | 75% | 11.5% | 0% | 8.7% | 2.4% | 1% |
| Bahrain [41] | 43.6% | 41.1% | 15.25 | 68.2% | 18.2% | 1.7% | 10.6 | 0.8% | 0.4% |
| Present study | 54% | 30% | 16% | 71% | 14% | 1% | 11% | 2% | 1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hamad, M. The Genetic Polymorphisms of CYP2C9 and VKORC1 in the Saudi Population and Their Impact on Anticoagulant Management. Medicina 2025, 61, 1872. https://doi.org/10.3390/medicina61101872
Al Hamad M. The Genetic Polymorphisms of CYP2C9 and VKORC1 in the Saudi Population and Their Impact on Anticoagulant Management. Medicina. 2025; 61(10):1872. https://doi.org/10.3390/medicina61101872
Chicago/Turabian StyleAl Hamad, Mohammad. 2025. "The Genetic Polymorphisms of CYP2C9 and VKORC1 in the Saudi Population and Their Impact on Anticoagulant Management" Medicina 61, no. 10: 1872. https://doi.org/10.3390/medicina61101872
APA StyleAl Hamad, M. (2025). The Genetic Polymorphisms of CYP2C9 and VKORC1 in the Saudi Population and Their Impact on Anticoagulant Management. Medicina, 61(10), 1872. https://doi.org/10.3390/medicina61101872






