Psychological and Behavioral Predictors of Postpartum Lumbopelvic Pain: A Multivariate Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Inclusion and Exclusion Criteria
2.3. Instruments and Data Collection
2.4. Study Variables
2.5. Statistical Analysis
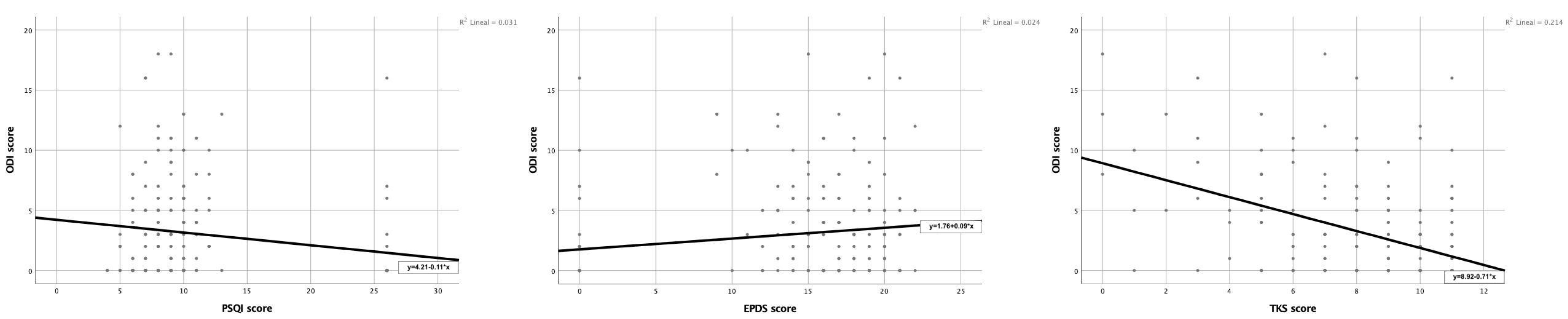
3. Results
4. Discussion
Limitations of the Study and Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Sayegh, N.A.; Salem, M.; Dashti, L.; Al-Sharrah, S.; Kalakh, S.; Al-Rashidi, R. Pregnancy-related lumbopelvic pain: Prevalence, risk factors, and profile in Kuwait. Pain Med. 2012, 13, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Burani, E.; Marruganti, S.; Giglioni, G.; Bonetti, F.; Ceron, D.; Cozzi Lepri, A. Predictive Factors for Pregnancy-Related Persistent Pelvic Girdle Pain (PPGP): A Systematic Review. Medicina 2023, 59, 2123. [Google Scholar] [CrossRef] [PubMed]
- Gausel, A.; Malmqvist, S.; Andersen, K. Subjective recovery from pregnancy-related pelvic girdle pain during the first 6 weeks after delivery: A prospective longitudinal cohort study. Eur. Spine J. 2020, 29, 556–563. [Google Scholar] [CrossRef]
- Inoue-Hirakawa, T.; Iguchi, S.; Matsumoto, D.; Kajiwara, Y.; Aoyama, T.; Kawabe, R.; Sugiura, H.; Uchiyama, Y. Gap between the prevalence of and consultation rate for lumbopelvic pain in postnatal Japanese women. J. Orthop. Sci. 2024, 29, 1353–1358. [Google Scholar] [CrossRef]
- Elden, H.; Gutke, A.; Kjellby-Wendt, G.; Fagevik-Olsen, M.; Östgaard, H.C. Predictors and consequences of long-term pregnancy-related pelvic girdle pain: A longitudinal follow-up study. BMC Musculoskelet. Disord. 2016, 17, 276. [Google Scholar] [CrossRef]
- Verstraete, E.H.; Vanderstraeten, G.; Parewijck, W. Pelvic girdle pain during or after pregnancy: A review of recent evidence and a clinical care path proposal. Facts Views Vis. Obgyn. 2013, 5, 33–43. [Google Scholar]
- Simonds, A.H.; Abraham, K.; Spitznagle, T. Clinical Practice Guidelines for Pelvic Girdle Pain in the Postpartum Population. J. Women’s Health Phys. Ther. 2022, 46, E1–E38. [Google Scholar] [CrossRef]
- Ceballos-Rivera, M.; González-González, Y.; Alonso-Calvete, A.; Justo-Cousiño, L.A.; Da Cuña-Carrera, I. Fisioterapia en las secuelas del parto por cesárea: Una revisión sistemática. Rev. Esp. Salud Publica 2023, 97, e202301002. [Google Scholar]
- Madzivhandila, P.M.; Cochrane, M.E.; Nkuna, R.D. Prevalence and Characteristics of Women with Persistent LBP Postpartum. Open Pain J. 2023, 16, e187638632308010. [Google Scholar] [CrossRef]
- Gutke, A.; Lundberg, M.; Östgaard, H.C.; Öberg, B. Impact of postpartum lumbopelvic pain on disability, pain intensity, health-related quality of life, activity level, kinesiophobia, and depressive symptoms. Eur. Spine J. 2011, 20, 440–448. [Google Scholar] [CrossRef]
- Beales, D.; Lutz, A.; Thompson, J.; Wand, B.M.; O’Sullivan, P. Disturbed body perception, reduced sleep, and kinesiophobia in subjects with pregnancy-related persistent lumbopelvic pain and moderate levels of disability: An exploratory study. Man. Ther. 2016, 21, 69–75. [Google Scholar] [CrossRef]
- Remus, A.; Smith, V.; Gutke, A.; Mena, J.J.S.; Mørkved, S.; Wikmar, L.N.; Öberg, B.; Olsson, C.; Robinson, H.S.; Stuge, B.; et al. A core outcome set for research and clinical practice in women with pelvic girdle pain: PGP-COS. PLoS ONE 2021, 16, e0247466. [Google Scholar] [CrossRef]
- Ogundimu, E.O.; Altman, D.G.; Collins, G.S. Adequate sample size for developing prediction models is not simply related to events per variable. J. Clin. Epidemiol. 2016, 76, 175–182. [Google Scholar] [CrossRef]
- Starzec-Proserpio, M.; Węgrzynowska, M.; Sys, D.; Kajdy, A.; Rongies, W.; Baranowska, B. Prevalence and factors associated with postpartum pelvic girdle pain among women in Poland: A prospective, observational study. BMC Musculoskelet. Disord. 2022, 23, 928. [Google Scholar] [CrossRef]
- Selva-Sevilla, C.; Ferrara, P.; Gerónimo-Pardo, M. Psychometric Properties Study of the Oswestry Disability Index in a Spanish Population with Previous Lumbar Disc Surgery: Homogeneity and Validity. Spine (Phila Pa 1976) 2019, 44, E430–E437. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Martínez-López, E.; Latorre-Román, P.A.; Garrido, F.; Santos, M.A.; Martínez-Amat, A. Reliability and validity of the Spanish version of the Pittsburgh Sleep Quality Index (PSQI) in patients with fibromyalgia. Rheumatol. Int. 2014, 34, 929–936. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.I. Predictive validity of the Edinburgh Postnatal Depression Scale and other tools for screening depression in pregnant and postpartum women: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2023, 307, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Zotes, A.; Gallardo-Pujol, D.; Labad, J.; Martin-Santos, R.; Garcia-Esteve, L.; Gelabert, E.; Jover, M.; Guillamat, R.; Mayoral, F.; Gornemann, I.; et al. Factor structure of the Spanish version of the Edinburgh Postnatal Depression Scale. Actas Esp. Psiquiatr. 2018, 46, 174–182. [Google Scholar] [PubMed]
- Gómez-Pérez, L.; López-Martínez, A.E.; Ruiz-Párraga, G.T. Psychometric properties of the Spanish version of the Tampa Scale for Kinesiophobia (TSK). J. Pain. 2011, 12, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Sjödahl, J.; Gutke, A.; Öberg, B. Predictors for long-term disability in women with persistent postpartum pelvic girdle pain. Eur. Spine J. 2013, 22, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Tavares, P.; Barrett, J.; Hogg-Johnson, S.; Ho, S.; Corso, M.; Batley, S.; Wishloff, K.; Weis, C.A. Prevalence of Low Back Pain, Pelvic Girdle Pain, and Combination Pain in a Postpartum Ontario Population. J. Obstet. Gynaecol. Can. 2020, 42, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Guinn Dunn, M.J.; Egger, M.J.; Shaw, J.M.; Yang, J.; Bardsley, T.; Powers, E.; Nygaard, I.E. Trajectories of lower back, upper back, and pelvic girdle pain during pregnancy and early postpartum in primiparous women. Women’s Health Issues 2019, 29, 454–461. [Google Scholar]
- Molin, B.; Zwedberg, S.; Berger, A.K.; Sand, A.; Georgsson, S. “The ignored pain”—Experiences of encounters with healthcare from the perspective of women with pain persisting after childbirth—A qualitative study. Sex Reprod. Healthc. 2024, 39, 100929. [Google Scholar] [CrossRef]
- Míguez, M.C.; Vázquez, M.B. Prevalence of postpartum major depression and depressive symptoms in Spanish women: A longitudinal study up to 1 year postpartum. Midwifery 2023, 126, 103808. [Google Scholar] [CrossRef]
- Flor-Alemany, M.; Migueles, J.H.; Alemany-Arrebola, I.; Aparicio, V.A.; Baena-García, L. Exercise, Mediterranean Diet Adherence or Both during Pregnancy to Prevent Postpartum Depression—GESTAFIT Trial Secondary Analyses. Int. J. Environ. Res. Public Health 2022, 19, 14450. [Google Scholar] [CrossRef]
- Niwa, M.; Lockhart, S.; Wood, D.J.; Yang, K.; Francis-Oliveira, J.; Kin, K.; Ahmed, A.; Wand, G.S.; Kano, S.I.; Payne, J.L.; et al. Prolonged HPA axis dysregulation in postpartum depression associated with adverse early life experiences: A cross-species translational study. Nat. Ment. Health 2024, 2, 593–604. [Google Scholar] [CrossRef]
- Virgara, R.; Maher, C.; Van Kessel, G. The comorbidity of low back pelvic pain and risk of depression and anxiety in pregnancy in primiparous women. BMC Pregnancy Childbirth 2018, 18, 288. [Google Scholar] [CrossRef]
- Long, G.; Yao, Z.Y.; Na, Y.; Ping, Y.; Wei, S.; Mingsheng, T. Different types of low back pain in relation to pre- and post-natal maternal depressive symptoms. BMC Pregnancy Childbirth 2020, 20, 551. [Google Scholar] [CrossRef]
- Gómez-Pérez, L.; Cumsille, P.; Román, C. Bidirectional relationship between physical health symptoms and depressive symptoms in the pre- and postpartum period. J. Psychosom. Res. 2020, 139, 110280. [Google Scholar] [CrossRef]
- Lundberg, M.; Larsson, M.; Ostlund, H.; Styf, J. Kinesiophobia among patients with musculoskeletal pain in primary healthcare. J. Rehabil. Med. 2006, 38, 37–43. [Google Scholar] [CrossRef]
- Nijs, J.; Roussel, N.; Paul van Wilgen, C.; Köke, A.; Smeets, R. Thinking beyond muscles and joints: Therapists’ and patients’ attitudes and beliefs regarding chronic musculoskeletal pain are key to applying effective treatment. Man. Ther. 2013, 18, 96–102. [Google Scholar] [CrossRef]
- Rashidi Fakari, F.; Simbar, M.; Saei Ghare Naz, M. The Relationship between Fear-Avoidance Beliefs and Pain in Pregnant Women with Pelvic Girdle Pain: A Cross-Sectional Study. Int. J. Community Based Nurs. Midwifery 2018, 6, 305–313. [Google Scholar]
- Okun, M.L.; Mancuso, R.A.; Hobel, C.J.; Schetter, C.D.; Coussons-Read, M. Poor sleep quality increases symptoms of depression and anxiety in postpartum women. J. Behav. Med. 2018, 41, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.A.; Gjerdingen, D.; Schuver, K.; Avery, M.; Marcus, B.H. The effect of sleep pattern changes on postpartum depressive symptoms. BMC Womens Health 2018, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Nasreen, H.E.; Kabir, Z.N.; Forsell, Y.; Edhborg, M. Low birth weight in offspring of women with depressive and anxiety symptoms during pregnancy: Results from a population based study in Bangladesh. BMC Public Health 2010, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Jarde, A.; Morais, M.; Kingston, D.; Giallo, R.; MacQueen, G.M.; Giglia, L.; Beyene, J.; Wang, Y.; McDonald, S.D. Neonatal Outcomes in Women with Untreated Antenatal Depression Compared with Women Without Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2016, 73, 826–837. [Google Scholar] [CrossRef]
- Accortt, E.E.; Cheadle, A.C.; Dunkel Schetter, C. Prenatal depression and adverse birth outcomes: An updated systematic review. Matern. Child Health J. 2015, 19, 1306–1337. [Google Scholar] [CrossRef]
- Dadi, A.F.; Akalu, T.Y.; Wolde, H.F.; Baraki, A.G. Effect of perinatal depression on birth and infant health outcomes: A systematic review and meta-analysis of observational studies from Africa. Arch. Public Health 2022, 80, 34. [Google Scholar] [CrossRef]
- Netsi, E.; Pearson, R.M.; Murray, L.; Cooper, P.; Craske, M.G.; Stein, A. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry 2018, 75, 247–253. [Google Scholar] [CrossRef]
- Beales, D.; Slater, H.; Palsson, T.; O’Sullivan, P. Understanding and managing pelvic girdle pain from a person-centred biopsychosocial perspective. Musculoskelet. Sci. Pract. 2020, 48, 102152. [Google Scholar] [CrossRef] [PubMed]
- Deegan, O.; Fullen, B.M.; Segurado, R.; Doody, C. The effectiveness of a combined exercise and psychological treatment programme on measures of nervous system sensitisation in adults with chronic musculoskeletal pain—A systematic review and meta-analysis. BMC Musculoskelet. 2024, 25, 140. [Google Scholar] [CrossRef] [PubMed]
- Field, T. Postpartum depression effects on early interactions, parenting, and safety practices: A review. Infant Behav. Dev. 2010, 33, 1–6. [Google Scholar] [CrossRef]
- Vesting, S.; Gutke, A.; Fagevik Olsén, M.; Praetorius Björk, M.; Rembeck, G.; Larsson, M.E.H. Can clinical postpartum muscle assessment help predict the severity of postpartum pelvic girdle pain? A prospective cohort study. Phys. Ther. 2022, 103, pzac152. [Google Scholar] [CrossRef] [PubMed]
- Vesting, S.; Gutke, A.; de Baets, L. Educating women to prevent and treat low back and pelvic girdle pain during and after pregnancy: A systematized narrative review. Ann. Med. 2025, 57, 2476046. [Google Scholar] [CrossRef]
- Kazeminia, M.; Rajati, F.; Rajati, M. The effect of pelvic floor muscle-strengthening exercises on low back pain: A systematic review and meta-analysis on randomized clinical trials. Neurol. Sci. 2023, 44, 859–872. [Google Scholar] [CrossRef]
| Total Sample (n = 192) | PLPP | PPD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present (n = 81) | Absent (n = 62) | p-Value | Present (n = 115) | Absent (n = 28) | p-Value | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age (years) | 35.61 | 4.77 | 35.37 | 4.77 | 35.92 | 4.78 | 0.497 a | 35.71 | 4.98 | 35.18 | 3.82 | 0.597 a |
| BMI (kg/m2) | 23.50 | 4.45 | 23.28 | 3.91 | 23.78 | 5.08 | 0.508 a | 23.34 | 4.01 | 24.15 | 5.96 | 0.386 a |
| Maternal education level | ||||||||||||
| Primary (n.%) | 1 | 0.7% | 0 | 0 | 1 | 1.6% | 0.151 b | 0 | 0 | 1 | 3.6% | 0.598 b |
| Secondary school (n.%) | 2 | 1.4% | 0 | 0 | 2 | 3.2% | 2 | 1.7% | 0 | 0 | ||
| High school (n.%) | 3 | 2.1% | 2 | 2.5% | 1 | 1.6% | 3 | 2.6% | 0 | 0 | ||
| Vocational training (n.%) | 17 | 11.9% | 11 | 13.6% | 6 | 9.6% | 14 | 12.2% | 3 | 10.7% | ||
| University (n.%) | 120 | 83.9% | 68 | 84.0% | 52 | 83.8% | 96 | 83.5% | 24 | 85.7% | ||
| Maternal employment status | ||||||||||||
| On maternity leave | 48 | 33.6% | 28 | 34.6% | 20 | 32.26% | 0.095 b | 42 | 36.5% | 6 | 21.4% | 0.799 b |
| Unemployed | 8 | 5.6% | 3 | 3.7% | 5 | 8% | 6 | 5.2% | 2 | 7.1% | ||
| Housewife | 1 | 0.7% | 0 | 0 | 1 | 1.6% | 1 | 0.9% | 0 | 0 | ||
| Student | 3 | 2.1% | 3 | 3.7% | 0 | 0 | 3 | 2.6% | 0 | 0 | ||
| Student and working | 3 | 2.1% | 3 | 3.7% | 0 | 0 | 3 | 2.6% | 0 | 0 | ||
| Currently working | 80 | 55.9% | 44 | 54.3% | 36 | 58% | 60 | 52.2% | 20 | 71.4% | ||
| Lifestyle | ||||||||||||
| Smoke (yes (n.%)) | 6 | 4.2% | 3 | 3.7% | 3 | 4.8% | 0.208 b | 4 | 3.5% | 2 | 7.1% | 0.612 b |
| Alcohol (yes (n.%)) | 84 | 58.7% | 45 | 55.6% | 39 | 62.9% | 0.197 b | 66 | 57.4% | 18 | 64.3% | 0.734 b |
| Walking | ||||||||||||
| Hours/day | 1.32 | 1.11 | 1.26 | 0.99 | 1.44 | 1.42 | 0.384 a | 1.34 | 1.16 | 1.35 | 1.38 | 0.989 a |
| Days/week | 3.93 | 2.58 | 3.75 | 2.60 | 4.12 | 2.41 | 0.401 a | 3.87 | 2.48 | 4.13 | 2.67 | 0.650 a |
| Sports practice | ||||||||||||
| Hours/day | 0.84 | 0.74 | 0.86 | 0.78 | 1.02 | 1.19 | 0.344 a | 0.9 | 0.76 | 1.04 | 1.62 | 0.514 a |
| Days/week | 1.66 | 1.54 | 1.69 | 1.51 | 1.85 | 1.62 | 0.566 a | 1.81 | 1.5 | 1.52 | 1.77 | 0.447 a |
| Obstetric data | ||||||||||||
| Gestational age | 0.663 b | 0.546 b | ||||||||||
| 28 to 37 weeks | 7 | 5.6% | 4 | 4.9% | 3 | 4.8% | 7 | 6.1% | 0 | 0 | ||
| 38 to 41 weeks | 111 | 88.8% | 55 | 67.9% | 56 | 90.3% | 90 | 78.3% | 21 | 75% | ||
| 42 weeks or more | 7 | 5.6% | 3 | 3.7% | 4 | 6.5% | 6 | 5.2% | 1 | 3.6% | ||
| Type of delivery | 0.685 b | 0.924 b | ||||||||||
| Vaginal delivery | 57 | 45.6% | 27 | 33.3% | 30 | 48.4% | 45 | 39.1% | 12 | 42.9% | ||
| Instrumental vaginal delivery | 9 | 7.2% | 6 | 7.4% | 3 | 4.8% | 8 | 7% | 1 | 3.6% | ||
| Induced vaginal delivery | 24 | 19.2% | 10 | 12.3% | 14 | 22.6% | 19 | 16.5% | 5 | 17.9% | ||
| Instrumental and induced vaginal delivery | 7 | 5.6% | 3 | 3.7% | 4 | 6.5% | 7 | 6.1% | 0 | 0 | ||
| Scheduled cesarean | 13 | 10.4% | 7 | 8.6% | 6 | 9.7% | 11 | 9.6% | 2 | 7.1% | ||
| Emergency cesarean | 15 | 12% | 9 | 11.1% | 6 | 9.7% | 13 | 11.3% | 2 | 7.1% | ||
| Complications | ||||||||||||
| Episiotomy (yes (n.%)) | 17 | 13.7% | 8 | 9.9% | 9 | 14.5% | 0.434 b | 15 | 13% | 2 | 7.1% | 0.453 b |
| Tear (yes (n.%)) | 43 | 34.7% | 21 | 25.9% | 22 | 35.5% | 0.441 b | 35 | 30.4% | 8 | 28.6% | 0.515 b |
| Postpartum hemorrhage (yes (n.%)) | 28 | 22.6% | 15 | 18.5% | 13 | 21% | 0.554 b | 23 | 20% | 5 | 17.9% | 0.444 b |
| Parity | ||||||||||||
| Primiparous (n.%) | 70 | 56% | 33 | 40.7% | 37 | 59.7% | 0.712 b | 60 | 52.2% | 10 | 35.7% | 0.358 b |
| Multiparous (n.%) | 55 | 44% | 29 | 35.8% | 26 | 41.9% | 43 | 37.4% | 12 | 42.9% | ||
| Number of children | 1.19 | 0.77 | 1.15 | 0.74 | 1.23 | 0.79 | 0.576 a | 1.16 | 0.758 | 1.32 | 0.84 | 0.381 a |
| Latest birth data | ||||||||||||
| Birth weight (kg) | 3.25 | 0.52 | 3.26 | 0.57 | 3.24 | 0.47 | 0.833 a | 3.22 | 0.53 | 3.39 | 0.44 | 0.155 a |
| Birth length (cm) | 49.66 | 4.13 | 49.13 | 4.9 | 50.19 | 3.13 | 0.150 a | 49.46 | 4.43 | 50.59 | 2.08 | 0.246 a |
| ODI score | 2.99 | 4.12 | 5.68 | 4.12 | 0 c | 0 c | <0.001 a | 3.31 | 4.12 | 1.72 | 3.89 | 0.028 a |
| PSQI score | 11.54 | 6.82 | 9.71 | 4.15 | 13.57 | 8.46 | <0.001 a | 8.52 | 1.87 | 23.41 | 6.2 | <0.001 a |
| EPDS score | 13.62 | 7.14 | 15.5 | 5.15 | 11.54 | 8.38 | <0.001 a | 16.97 | 2.7 | 0.46 | 2.01 | <0.001 a |
| TSK score | 8.42 | 2.7 | 7.47 | 2.84 | 9.47 | 2.09 | <0.001 a | 7.9 | 2.52 | 10.44 | 2.45 | <0.001 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-de-Ory, I.; Mazur, A.; Oliva-Pascual-Vaca, Á.; Benito-de-Pedro, M.; Fernández-Rodríguez, T.; Rodríguez-López, E.S. Psychological and Behavioral Predictors of Postpartum Lumbopelvic Pain: A Multivariate Analysis. Medicina 2025, 61, 1869. https://doi.org/10.3390/medicina61101869
Jiménez-de-Ory I, Mazur A, Oliva-Pascual-Vaca Á, Benito-de-Pedro M, Fernández-Rodríguez T, Rodríguez-López ES. Psychological and Behavioral Predictors of Postpartum Lumbopelvic Pain: A Multivariate Analysis. Medicina. 2025; 61(10):1869. https://doi.org/10.3390/medicina61101869
Chicago/Turabian StyleJiménez-de-Ory, Ignacio, Angelika Mazur, Ángel Oliva-Pascual-Vaca, María Benito-de-Pedro, Tomás Fernández-Rodríguez, and Elena Sonsoles Rodríguez-López. 2025. "Psychological and Behavioral Predictors of Postpartum Lumbopelvic Pain: A Multivariate Analysis" Medicina 61, no. 10: 1869. https://doi.org/10.3390/medicina61101869
APA StyleJiménez-de-Ory, I., Mazur, A., Oliva-Pascual-Vaca, Á., Benito-de-Pedro, M., Fernández-Rodríguez, T., & Rodríguez-López, E. S. (2025). Psychological and Behavioral Predictors of Postpartum Lumbopelvic Pain: A Multivariate Analysis. Medicina, 61(10), 1869. https://doi.org/10.3390/medicina61101869









