Reappraising Use of Flecainide for Atrial Fibrillation and Ventricular Arrhythmias in Structural Heart Disease Patients
Abstract
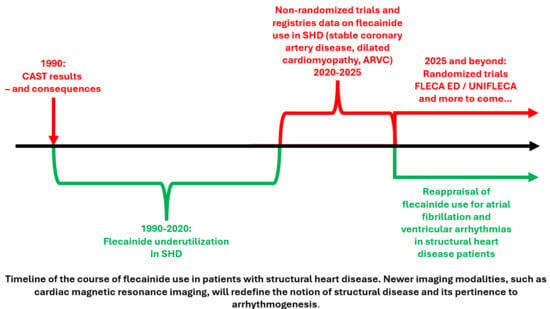
1. Introduction
1.1. Old and New Evidence
1.1.1. CAST: Overgeneralization to Structural Heart Disease
1.1.2. Flecainide in Patients with AF and CAD
1.1.3. Flecainide Use in Premature Ventricular Contractions and Associated Cardiomyopathy
1.1.4. Flecainide in ARVC
1.1.5. Flecainide in LV Hypertrophy (LVH)
1.1.6. Pro-Arrhythmic Effect of Long-Term Flecainide Use
2. Discussion
Future Directions
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Echt, D.S.; Liebson, P.R.; Mitchell, L.B.; Peters, R.W.; Obias-Manno, D.; Barker, A.H.; Arensberg, D.; Baker, A.; Friedman, L.; Greene, H.L.; et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991, 324, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, C.; Trivigno, S.; Vetta, G.; Magnocavallo, M.; Mariani, M.V.; Santini, L.; Forleo, G.B.; Grimaldi, M.; Badagliacca, R.; Lanata, L.; et al. Flecainide in Ventricular Arrhythmias: From Old Myths to New Perspectives. J. Clin. Med. 2021, 10, 3696. [Google Scholar] [CrossRef]
- Sangpornsuk, N.; Rungpradubvong, V.; Tiensantisuk, T.; Leelapatana, P.; Chokesuwattanaskul, R.; Prechawat, S. Flecainide use in arrhythmic patients who have structural heart disease. Ther. Adv. Drug Saf. 2025, 16, 20420986251316462. [Google Scholar] [CrossRef] [PubMed]
- Burnham, T.S.; May, H.T.; Bair, T.L.; Anderson, J.A.; Crandall, B.G.; Cutler, M.J.; Day, J.D.; Freedman, R.A.; Knowlton, K.U.; Muhlestein, J.B.; et al. Long-term outcomes in patients treated with flecainide for atrial fibrillation with stable coronary artery disease. Am. Heart J. 2022, 243, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Gaine, S.; Rolland, T.; Asatryan, B.; Laredo, M.; Sampognaro, J.; Carrick, R.T.; Peretto, G.; Muller, S.; Villatore, A.; Murray, B.; et al. Long-Term Follow-Up Data on Flecainide Use as an Antiarrhythmic in Arrhythmogenic Right Ventricular Cardiomyopathy: A Multicenter Study. JACC Clin. Electrophysiol. 2025, 11, 1159–1170. [Google Scholar] [CrossRef]
- Tsioufis, P.; Tsiachris, D.; Argyriou, N.; Doundoulakis, I.; Kordalis, A.; Antoniou, C.K.; Botis, M.; Zamanis, I.; Matthaiopoulos, G.; Tsioufis, K.; et al. Flecainide vs Amiodarone for the cardioversion of Atrial Fibrillation in the Emergency Department in patients with Coronary Artery Disease and preserved ejection fraction. FLECA-ED study. EP Eur. 2025, 27 (Suppl. S1), euaf085.325. [Google Scholar] [CrossRef]
- Tsioufis, P.; Tsiachris, D.; Doundoulakis, I.; Kordalis, A.; Antoniou, C.K.; Vlachakis, P.K.; Theofilis, P.; Manta, E.; Gatzoulis, K.A.; Parissis, J.; et al. Rationale and Design of a Randomized Controlled Clinical Trial on the Safety and Efficacy of Flecainide versus Amiodarone in the Cardioversion of Atrial Fibrillation at the Emergency Department in Patients with Coronary Artery Disease (FLECA-ED). J. Clin. Med. 2023, 12, 3961. [Google Scholar] [CrossRef]
- Anderson, J.L.; Platia, E.V.; Hallstrom, A.; Henthorn, R.W.; Buckingham, T.A.; Carlson, M.D.; Carson, P.E. Interaction of baseline characteristics with the hazard of encainide, flecainide, and moricizine therapy in patients with myocardial infarction. A possible explanation for increased mortality in the Cardiac Arrhythmia Suppression Trial (CAST). Circulation 1994, 90, 2843–2852. [Google Scholar] [CrossRef]
- Akiyama, T.; Pawitan, Y.; Greenberg, H.; Kuo, C.S.; Reynolds-Haertle, R.A. Increased risk of death and cardiac arrest from encainide and flecainide in patients after non-Q-wave acute myocardial infarction in the Cardiac Arrhythmia Suppression Trial. CAST Investigators. Am. J. Cardiol. 1991, 68, 1551–1555. [Google Scholar] [CrossRef]
- Greenberg, H.M.; Dwyer, E.M., Jr.; Hochman, J.S.; Steinberg, J.S.; Echt, D.S.; Peters, R.W. Interaction of ischaemia and encainide/flecainide treatment: A proposed mechanism for the increased mortality in CAST I. Br. Heart J. 1995, 74, 631–635. [Google Scholar] [CrossRef]
- Nattel, S. Experimental evidence for proarrhythmic mechanisms of antiarrhythmic drugs. Cardiovasc. Res. 1998, 37, 567–577. [Google Scholar] [CrossRef]
- Tsiachris, D.; Doundoulakis, I.; Pagkalidou, E.; Kordalis, A.; Deftereos, S.; Gatzoulis, K.A.; Tsioufis, K.; Stefanadis, C. Pharmacologic Cardioversion in Patients with Paroxysmal Atrial Fibrillation: A Network Meta-Analysis. Cardiovasc. Drugs Ther. 2021, 35, 293–308. [Google Scholar] [CrossRef]
- Tsiachris, D.; Doundoulakis, I.; Tsioufis, P.; Pagkalidou, E.; Antoniou, C.K.; Zafeiropoulos, S.M.; Gatzoulis, K.A.; Tsioufis, K.; Stefanadis, C. Reappraising the role of class Ic antiarrhythmics in atrial fibrillation. Eur. J. Clin. Pharmacol. 2022, 78, 1039–1045. [Google Scholar] [CrossRef]
- Donovan, K.D.; Dobb, G.J.; Coombs, L.J.; Lee, K.Y.; Weekes, J.N.; Murdock, C.J.; Clarke, G.M. Efficacy of flecainide for the reversion of acute onset atrial fibrillation. Am. J. Cardiol. 1992, 70, A50–A55. [Google Scholar] [CrossRef] [PubMed]
- Donovan, K.D.; Power, B.M.; Hockings, B.E.; Dobb, G.J.; Lee, K.Y. Intravenous flecainide versus amiodarone for recent-onset atrial fibrillation. Am. J. Cardiol. 1995, 75, 693–697. [Google Scholar] [CrossRef]
- Kirchhof, P.; Andresen, D.; Bosch, R.; Borggrefe, M.; Meinertz, T.; Parade, U.; Ravens, U.; Samol, A.; Steinbeck, G.; Treszl, A.; et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): A prospective, randomised, open-label, blinded endpoint assessment trial. Lancet 2012, 380, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Rillig, A.; Eckardt, L.; Borof, K.; Camm, A.J.; Crijns, H.; Goette, A.; Breithardt, G.; Lemoine, M.D.; Metzner, A.; Rottner, L.; et al. Safety and efficacy of long-term sodium channel blocker therapy for early rhythm control: The EAST-AFNET 4 trial. Europace 2024, 26, euae121. [Google Scholar] [CrossRef]
- Meinertz, T.; Lip, G.Y.; Lombardi, F.; Sadowski, Z.P.; Kalsch, B.; Camez, A.; Hewkin, A.; Eberle, S. Efficacy and safety of propafenone sustained release in the prophylaxis of symptomatic paroxysmal atrial fibrillation (The European Rythmol/Rytmonorm Atrial Fibrillation Trial [ERAFT] Study). Am. J. Cardiol. 2002, 90, 1300–1306. [Google Scholar] [CrossRef]
- Ashraf, H.; Ko, N.K.; Ladia, V.; Agasthi, P.; Prendiville, T.; O’Herlihy, F.; Pujari, S.H.; Mulpuru, S.K.; Scott, L.; Sorajja, D. Use of Flecainide in Stable Coronary Artery Disease: An Analysis of Its Safety in Both Nonobstructive and Obstructive Coronary Artery Disease. Am. J. Cardiovasc. Drugs 2021, 21, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Sayegh, M.N.; Ibrahim, R.; Bhatia, N.K.; Merchant, F.M.; Shah, A.D.; Westerman, S.B.; De Lurgio, D.B.; Patel, A.M.; Thompkins, C.M.; et al. The Feasibility and Safety of Flecainide Use Among Patients With Varying Degrees of Coronary Disease. JACC Clin. Electrophysiol. 2023, 9, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Kotoulas, S.; Tsiachris, D.; Botis, M.; Kordalis, A.; Varvarousis, D.; Leventopoulos, G.; Kallergis, E.; Doundoulakis, I.; Poulimenos, L.E.; Tsioufis, K. Effect of Flecainide in Idiopathic Premature Ventricular Contractions and the Induced Cardiomyopathy-UNIFLECA: A Single Arm, Non-Randomized Trial: Review of the Literature and Initial Results. J. Pers. Med. 2025, 15, 132. [Google Scholar] [CrossRef]
- Tsiachris, D.; Botis, M.; Doundoulakis, I.; Bartsioka, L.I.; Tsioufis, P.; Kordalis, A.; Antoniou, C.K.; Tsioufis, K.; Gatzoulis, K.A. Electrocardiographic Characteristics, Identification, and Management of Frequent Premature Ventricular Contractions. Diagnostics 2023, 13, 3094. [Google Scholar] [CrossRef] [PubMed]
- Hyman, M.C.; Mustin, D.; Supple, G.; Schaller, R.D.; Santangeli, P.; Arkles, J.; Lin, D.; Muser, D.; Dixit, S.; Nazarian, S.; et al. Class IC antiarrhythmic drugs for suspected premature ventricular contraction–induced cardiomyopathy. Heart Rhythm. 2018, 15, 159–163. [Google Scholar] [CrossRef]
- Raad, M.; Yogasundaram, H.; Oranefo, J.; Guandalini, G.; Markman, T.; Hyman, M.; Schaller, R.; Supple, G.; Deo, R.; Nazarian, S.; et al. Class 1C Antiarrhythmics for Premature Ventricular Complex Suppression in Nonischemic Cardiomyopathy With Implantable Cardioverter-Defibrillators. JACC Clin. Electrophysiol. 2024, 10, 846–853. [Google Scholar] [CrossRef]
- Corrado, D.; Wichter, T.; Link, M.S.; Hauer, R.N.; Marchlinski, F.E.; Anastasakis, A.; Bauce, B.; Basso, C.; Brunckhorst, C.; Tsatsopoulou, A.; et al. Treatment of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: An International Task Force Consensus Statement. Circulation 2015, 132, 441–453. [Google Scholar] [CrossRef]
- Haverkamp, W.; Martinez-Rubio, A.; Hief, C.; Lammers, A.; Mühlenkamp, S.; Wichter, T.; Breithardt, G.; Borggrefe, M. Efficacy and safety of d,l-sotalol in patients with ventricular tachycardia and in survivors of cardiac arrest. J. Am. Coll. Cardiol. 1997, 30, 487–495. [Google Scholar] [CrossRef]
- Marcus, G.; Glidden, D.; Polonsky, B.; Zareba, W.; Smith, L.; Cannom, D.; Estes, N.A.M.; Marcus, F.; Scheinman, M. Efficacy of Antiarrhythmic Drugs in Arrhythmogenic Right Ventricular Cardiomyopathy A Report From the North American ARVC Registry. J. Am. Coll. Cardiol. 2009, 54, 609–615. [Google Scholar] [CrossRef]
- Wichter, T.; Borggrefe, M.; Haverkamp, W.; Chen, X.; Breithardt, G. Efficacy of antiarrhythmic drugs in patients with arrhythmogenic right ventricular disease. Results in patients with inducible and noninducible ventricular tachycardia. Circulation 1992, 86, 29–37. [Google Scholar] [CrossRef]
- Wichter, T.; Paul, T.M.; Eckardt, L.; Gerdes, P.; Kirchhof, P.; Böcker, D.; Breithardt, G. Arrhythmogenic right ventricular cardiomyopathy. Antiarrhythmic drugs, catheter ablation, or ICD? Herz 2005, 30, 91–101. [Google Scholar] [CrossRef]
- Ermakov, S.; Gerstenfeld, E.P.; Svetlichnaya, Y.; Scheinman, M.M. Use of flecainide in combination antiarrhythmic therapy in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2017, 14, 564–569. [Google Scholar] [CrossRef]
- Rolland, T.; Badenco, N.; Maupain, C.; Duthoit, G.; Waintraub, X.; Laredo, M.; Himbert, C.; Frank, R.; Hidden-Lucet, F.; Gandjbakhch, E. Safety and efficacy of flecainide associated with beta-blockers in arrhythmogenic right ventricular cardiomyopathy. EP Eur. 2022, 24, 278–284. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Katritsis, D.; Rowland, E.; O’Nunain, S.; Shakespeare, C.F.; Poloniecki, J.; Camm, A.J. Effect of flecainide on atrial and ventricular refractoriness and conduction in patients with normal left ventricle. Implications for possible antiarrhythmic and proarrhythmic mechanisms. Eur. Heart J. 1995, 16, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Cros, C.; Skinner, M.; Moors, J.; Lainee, P.; Valentin, J.P. Detecting drug-induced prolongation of the QRS complex: New insights for cardiac safety assessment. Toxicol. Appl. Pharmacol. 2012, 265, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Almroth, H.; Andersson, T.; Fengsrud, E.; Friberg, L.; Linde, P.; Rosenqvist, M.; Englund, A. The safety of flecainide treatment of atrial fibrillation: Long-term incidence of sudden cardiac death and proarrhythmic events. J. Intern. Med. 2011, 270, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Rubin, J.M.; Perez, D.; Gomez, J.; Florez, J.P.; Avanzas, P.; Garcia-Ruiz, J.M.; de la Hera, J.M.; Reguero, J.; Coto, E.; et al. Time-dependent responses to provocative testing with flecainide in the diagnosis of Brugada syndrome. Heart Rhythm. 2015, 12, 350–357. [Google Scholar] [CrossRef]
- Oguayo, K.N.; Oyetayo, O.O.; Costa, S.M.; Mixon, T.A. An unusual case of flecainide-induced QT prolongation leading to cardiac arrest. Pharmacotherapy 2014, 34, e30–e33. [Google Scholar] [CrossRef]

| SHD Type | Study—Year | Analysis Type | Enrolled | On Flecainide | Patients with SHD | Follow-Up | Mortality/Discontinuation | Arrhythmia-Related Events |
|---|---|---|---|---|---|---|---|---|
| Ischemic Heart Disease (CAD/MI) | CAST [3], 1989 | Original RCT | 2309 | 730 | 730—Post-MI with LV dysfunction | Mean 10 months | ↑ All-cause mortality (56 arrhythmic deaths)—Non-Q Wave related MI | Excess ventricular arrhythmias |
| Tsiachris et al., 2021 [14,15] | Network meta-analysis & systematic review | 3310 | 580 | 113—Ischemic heart disease | N/A | None | 2 VT, 4 bradycardia, 31 hypotension | |
| Burnham et al., 2022 [6] | Retrospective cohort (AF + CAD) | 3445 | 328 | 196—Stable CAD. | Median: 3 yrs | Stable CAD: 18 deaths. | Stable CAD: 11 VT. | |
| 134—Post PCI/CABG | Post PCI/CABG: 28 deaths | Post PCI/CABG: 15 VT | ||||||
| FLECA-ED [8,9], 2023 | Prospective RCT (AF cardioversion in ED) | 25 | 10 | 10—Stable CAD with LVEF >35% | Acute | TBA | TBA | |
| FLEC-SL [18] | Prospective Randomized (AF Pharmacologic Cardioversion) | 635 | 601 | 37—CAD | Up to 6 months | 1 Event | No excess VT/VF | |
| 86—Valvular Heart Disease | ||||||||
| EAST-AFNET 4, 2020–24 [20] | RCT subanalysis (early rhythm control) | 2789 | 689 (Class IC) | 41—Stable CAD; 177—HFpEF | Median 5 years | Part of 34 composite events (death/stroke/RC-related); no excess in Class IC subgroup | No excess VT/VF | |
| Ashraf et al., 2022 [22] | Retrospective cohort (AF + CAD) | 348 | 348 | 196—Obstructive CAD (>70% stenosis or PCI/CABG) | Mean 6.3 years | 15 deaths/cardiac arrests | No increase in proarrhythmia overall—VT/VF in 15 patients | |
| 152—Non-obstructive CAD (<50% stenosis) | 10 deaths/cardiac arrests | VT/VF in 10 patients | ||||||
| Kiani et al., 2023 [23] | Multicenter retrospective (Class IC vs. Class III) | 5661 | 3445 (Class IC) | Subgroup: obstructive CAD, LVH | Long-term | Worse survival in obstructive CAD subgroup than Class III AADs | ↑ MACEs in High-Risk CAD subgroup | |
| Sangpornsuk et al., 2025 [5] | Retrospective cohort | 336 | 336 | Broad SHD (5—CAD, 13—LVH,12—↓ LVEF, 4—Valvular Heart Disease) | Long-term | 2 Non-Cardiac Deaths | No ↑ VT/VF vs. Non SHD Group | |
| PVC-Induced Cardiomyopathy/NICM | Raad et al., 2018 [26] | Retrospective (PVC-CMP) | 34 | 23 | 34—NICM with ICD | 29 Months mean | 29% Discontinuation | PVC burden ↓ 20 → 6%; LVEF ↑ 33 → 37%; 2 sustained VT, 1 atrial flutter, AF stable in most |
| Hyman et al. [18] | Retrospective (PVC-induced CMP) | 20 | 13 | NICM—PVC induced CMP (Mean EF: 37%) | 3.8 Years Mean | 8/20 discontinued (inefficacy/side effects) | No sustained VA; PVC burden ↓ 36 → 10%; EF ↑ 37 → 49% | |
| Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) | Gain et al., 2025 [7] | Multicenter retrospective | 191 | 191 | 191—ARVC (59% ICD) | Median 4.2 years | 0 deaths; 8% discontinued | ↓ PVC burden; ↓ NSVT; No sustained VA; minor symptoms |
| Ermakov, et al. [33] | Retrospective Case series | 45 | 8 with Sotalol/Metoprolol | ARVC | Median 35.5 Months | No deaths reported; discontinuation/AEs not clearly stated | 6/8 arrhythmia-free; 2/8 recurrent arrhythmia requiring repeat ablation | |
| Roland, et al. [34] | Retrospective Cohort | 100 | 100 | ARVC | Median 47 Months | No deaths: ~10% discontinued (6 inefficacy, 1 AF, 3 side effects) | ↓ PVC burden; ↓ PVS positivity (94% → 40%); sustained VA rate ~5% at 1 yr, ~25% at 5 yr under treatment | |
| Left Ventricular Hypertrophy (LVH) | EAST-AFNET-4 [20] | RCT subanalysis (early rhythm control | 2789 | 689 (Class IC) | 26—LVH (>15 mm) | Median 5 years | No excess mortality in LVH subgroup | No excess VT/VF |
| Sangpornsuk et al., 2025 [5] | Retrospective cohort | 336 | 336 | 13—with LVH > 14 mm | 1 year | No increase in all-cause mortality | No increase in VA compared to non-SHD group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsiachris, D.; Kotoulas, S.C.; Doundoulakis, I.; Antoniou, C.-K.; Botis, M.; Pamporis, K.; Argyriou, N.; Karanikola, A.-E.; Tsioufis, P.; Kordalis, A.; et al. Reappraising Use of Flecainide for Atrial Fibrillation and Ventricular Arrhythmias in Structural Heart Disease Patients. Medicina 2025, 61, 1845. https://doi.org/10.3390/medicina61101845
Tsiachris D, Kotoulas SC, Doundoulakis I, Antoniou C-K, Botis M, Pamporis K, Argyriou N, Karanikola A-E, Tsioufis P, Kordalis A, et al. Reappraising Use of Flecainide for Atrial Fibrillation and Ventricular Arrhythmias in Structural Heart Disease Patients. Medicina. 2025; 61(10):1845. https://doi.org/10.3390/medicina61101845
Chicago/Turabian StyleTsiachris, Dimitrios, Sotirios C. Kotoulas, Ioannis Doundoulakis, Christos-Konstantinos Antoniou, Michail Botis, Konstantinos Pamporis, Nikolaos Argyriou, Aikaterini-Eleftheria Karanikola, Panagiotis Tsioufis, Athanasios Kordalis, and et al. 2025. "Reappraising Use of Flecainide for Atrial Fibrillation and Ventricular Arrhythmias in Structural Heart Disease Patients" Medicina 61, no. 10: 1845. https://doi.org/10.3390/medicina61101845
APA StyleTsiachris, D., Kotoulas, S. C., Doundoulakis, I., Antoniou, C.-K., Botis, M., Pamporis, K., Argyriou, N., Karanikola, A.-E., Tsioufis, P., Kordalis, A., & Tsioufis, K. (2025). Reappraising Use of Flecainide for Atrial Fibrillation and Ventricular Arrhythmias in Structural Heart Disease Patients. Medicina, 61(10), 1845. https://doi.org/10.3390/medicina61101845