Deep Versus Superficial Dry Needling for Neck Pain: A Systematic Review of Randomised Clinical Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Study Selection
2.5. Risk-of-Bias Assessment
2.6. Data Extraction and Synthesis
2.7. Statistical Analysis
3. Results
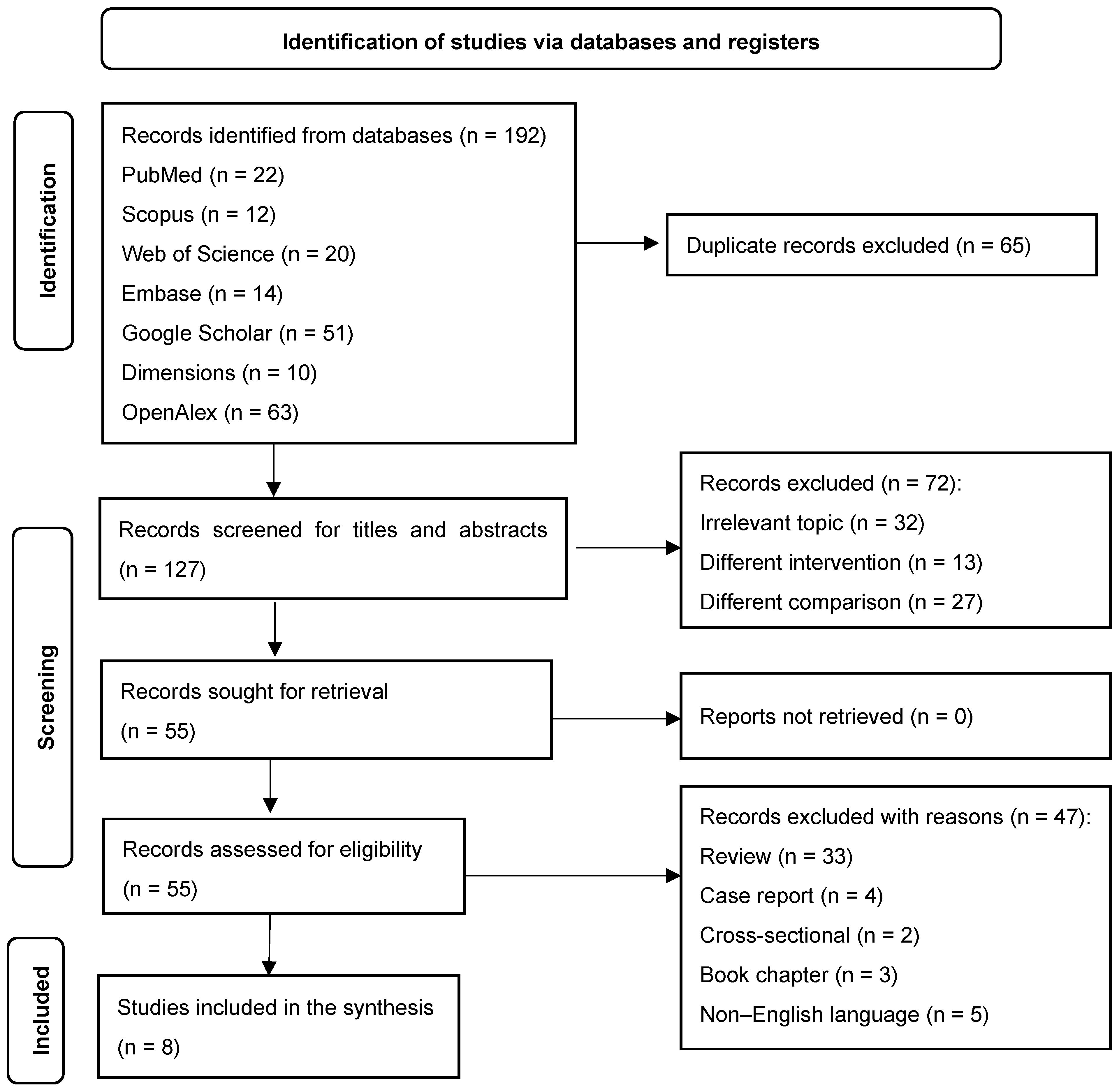
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
3.4. Intervention Protocol
3.5. Intervention Duration
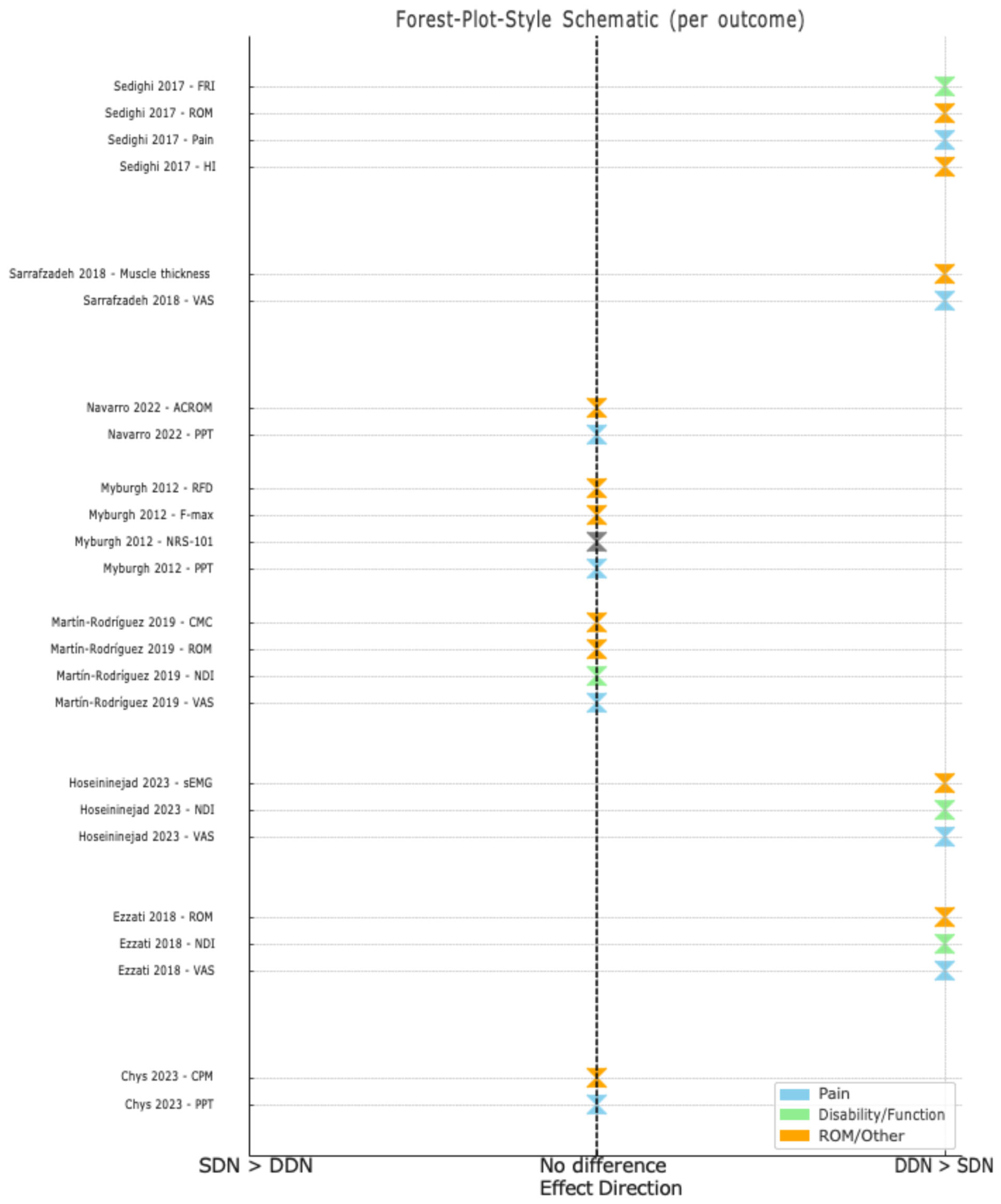
3.6. Effect on Pain Severity
3.7. Effect on Functional Disability
3.8. Effects on Other Outcome Measures
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPS | Myofascial pain syndrome |
| MTrPs | Myofascial trigger points |
| RCTs | Randomised controlled trials |
| MCIDs | Minimal clinically important differences |
Appendix A. Search Strategy
| Database | Date of Search | Search Strategy | Results |
| PubMed | 22 September 2024 | ((“Neck Pain”[Mesh] OR “Cervical Pain”[Mesh]) AND (“Dry Needling”[Mesh] OR “deep dry needling” OR “Superficial dry needling”)) AND (“Trigger Points”[Mesh] OR “Myofascial Pain Syndromes”[Mesh]) | 22 |
| Web of Science | 22 September 2024 | TS = (“Neck pain” OR “Cervical pain”) AND TS = (“deep dry needling” OR “Superficial dry needling”) AND TS = (“Trigger point*” OR “Myofascial pain syndrome”) | 20 |
| Scopus | 22 September 2024 | (TITLE-ABS-KEY (“Neck pain” OR “Cervical pain”)) AND (TITLE-ABS-KEY (“deep dry needling” OR “Superficial dry needling”)) AND (TITLE-ABS-KEY (“Trigger point*” OR “Myofascial pain syndrome”)) | 12 |
| Embase | 22 September 2024 | (‘neck pain’ OR ‘cervical pain’:ab,ti,kw) AND (‘deep dry needling’ OR ‘superficial dry needling’:ab,ti,kw) AND (‘trigger point*’ OR ‘myofascial pain syndrome’:ab,ti,kw) | 14 |
| Google Scholar | 22 September 2024 | intitle:(“Neck pain” OR “Cervical pain”) AND intitle:(“deep dry needling” OR “Superficial dry needling”) AND intitle:(“Trigger point*” OR “Myofascial pain syndrome”) | 51 |
| Dimensions | 22 September 2024 | (“Neck pain” OR “Cervical pain”) AND (“deep dry needling” OR “Superficial dry needling”) AND (“Trigger point*” OR “Myofascial pain syndrome”) | 10 |
| OpenAlex | 22 September 2024 | (“Neck pain” OR “Cervical pain”) AND (“deep dry needling” OR “Superficial dry needling”) AND (“Trigger point*” OR “Myofascial pain syndrome”) | 63 |
References
- Barone, R.; Szychlinska, M.A. Highlights in pathophysiology of the musculoskeletal system. Int. J. Mol. Sci. 2023, 24, 6412. [Google Scholar] [CrossRef]
- King, D.L.; Scola, R.M.; Hartvigsen, J.; Ferreira, M.L.; Blyth, F.M.; March, L.M. Disability burden due to musculoskeletal conditions and low back pain in Australia: Findings from the Global Burden of Disease Study 2019. Chiropr. Man. Therap. 2022, 30, 12. [Google Scholar]
- Baldry, P. Superficial versus deep dry needling. Acupunct. Med. 2002, 20, 78–81. [Google Scholar] [CrossRef]
- Ball, A.; Perreault, T.; Fernández-De-las-Peñas, C.; Agnone, M.; Spennato, J. Ultrasound Confirmation of the Multiple Loci Hypothesis of the Myofascial Trigger Point and the Diagnostic Importance of Specificity in the Elicitation of the Local Twitch Response. Diagnostics 2022, 12, 321. [Google Scholar] [CrossRef]
- Sabeh, A.M.; Bedaiwi, S.A.; Felemban, O.M.; Mawardi, H.H. Myofascial Pain Syndrome and Its Relation to Trigger Points, Facial Form, Muscular Hypertrophy, Deflection, Joint Loading, Body Mass Index, Age and Educational Status. J. Int. Soc. Prev. Community Dent. 2020, 10, 786–793. [Google Scholar] [CrossRef]
- Barbero, M.; Schneebeli, A.; Koetsier, E.; Maino, P. Myofascial pain syndrome and trigger points: Evaluation and treatment in patients with musculoskeletal pain. Curr. Opin. Support. Palliat. Care 2019, 13, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Hooten, W.M. Advances in the diagnosis and management of neck pain. BMJ 2017, 358, j3221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, N.Y.; Chen, C.; Wang, T.; Wang, L.J.; Shi, X.L.; Li, S.M.; Guo, C.Q. Acupotomy Alleviates Energy Crisis at Rat Myofascial Trigger Points. Evid. Based Complement. Alternat. Med. 2020, 2020, 5129562. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Dommerholt, J. International consensus on diagnostic criteria and clinical considerations of myofascial trigger points: A delphi study. Pain Med. 2018, 19, 142–150. [Google Scholar] [CrossRef]
- Lew, J.; Kim, J. Comparison of dry needling and trigger point manual therapy in patients with neck and upper back myofascial pain syndrome: A systematic review and meta-analysis. J. Man. Manip. Ther. 2021, 29, 136–146. [Google Scholar] [CrossRef]
- Guzmán-Pavón, M.J.; Cavero-Redondo, I.; Martínez-Vizcaíno, V.; Fernández-Rodríguez, R.; Reina-Gutierrez, S.; Álvarez-Bueno, C. Effect of physical exercise programs on myofascial trigger points–related dysfunctions: A systematic review and meta-analysis. Pain Med. 2020, 21, 2986–2996. [Google Scholar] [CrossRef]
- Espejo-Antúnez, L.; Tejeda, J.F.H.; Albornoz-Cabello, M.; Rodríguez-Mansilla, J.; de la Cruz-Torres, B.; Ribeiro, F.; Silva, A.G. Dry needling in the management of myofascial trigger points: A systematic review of randomized controlled trials. Complement. Ther. Med. 2017, 33, 46–57. [Google Scholar] [CrossRef]
- Sánchez-Infante, J.; Navarro-Santana, M.J.; Bravo-Sánchez, A.; Jiménez-Diaz, F.; Abián-Vicén, J. Is dry needling applied by physical therapists effective for pain in musculoskeletal conditions? A systematic review and meta-analysis. Phys. Ther. 2021, 101, pzab070. [Google Scholar] [CrossRef] [PubMed]
- Legge, D. A history of dry needling. J. Musculoskelet. Pain 2014, 22, 301–307. [Google Scholar] [CrossRef]
- Griswold, D.; Wilhelm, M.; Donaldson, M.; Learman, K.; Cleland, J. The effectiveness of superficial versus deep dry needling or acupuncture for reducing pain and disability in individuals with spine-related painful conditions: A systematic review with meta-analysis. J. Man. Manip. Ther. 2019, 27, 128–140. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Nijs, J. Trigger point dry needling for the treatment of myofascial pain syndrome: Current perspectives within a pain neuroscience paradigm. J. Pain Res. 2019, 12, 1899–1911. [Google Scholar] [CrossRef]
- Ezzati, K.; Sarrafzadeh, J.; Ebrahimi Takamjani, I.; Khani, S. The Efficacy of Superficial and Deep Dry Needling Techniques on Functional Parameters in Subjects With Upper Trapezius Myofascial Pain Syndrome. Casp. J. Neurol. Sci. 2018, 4, 152–158. [Google Scholar] [CrossRef]
- Hoseininejad, Z.; Mohammadi, H.K.; Azadeh, H.; Taheri, N. Comparison of immediate and delayed effects of superficial and deep dry needling in patients with upper trapezius myofascial trigger points. J. Bodyw. Mov. Ther. 2023, 33, 106–111. [Google Scholar] [CrossRef]
- Sarrafzadeh, J.; Khani, S.; Ezzati, K.; Takamjani, I.E. Effects of Superficial and Deep Dry Needling on Pain and Muscle Thickness in Subject with Upper Trapezius Muscle Myofascial Pain Syndrome. J. Pain Relief 2018, 7, 4–9. [Google Scholar] [CrossRef]
- Sedighi, A.; Nakhostin Ansari, N.; Naghdi, S. Comparison of acute effects of superficial and deep dry needling into trigger points of suboccipital and upper trapezius muscles in patients with cervicogenic headache. J. Bodyw. Mov. Ther. 2017, 21, 810–814. [Google Scholar] [CrossRef]
- Hernández-Secorún, M.; Sánchez-Sánchez, J.L.; Muñoz-López, M.; López-López, D.; Pérez-Fernández, E.; Gil-Martínez, A. Effectiveness of dry needling in improving pain and function in comparison with other techniques in patients with chronic neck pain: A systematic review and meta-analysis. Pain Res. Manag. 2023, 2023, 1523834. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Huguet, M.; Barrot, L.; Fuster, A.; Casanova-Molla, J.; Montaner, J.; Fernández-de-las-Peñas, C. Dry needling in physical therapy treatment of chronic neck pain: Systematic review. J. Clin. Med. 2022, 11, 2370. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brown, D. A review of the PubMed PICO tool: Using evidence-based practice in health education. Health Promot. Pract. 2020, 21, 496–498. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Slavin, R.E. Best evidence synthesis: An intelligent alternative to meta-analysis. J. Clin. Epidemiol. 1995, 48, 9–18. [Google Scholar] [CrossRef]
- Chys, M.; Bontinck, J.; Voogt, L.; Sendarrubias, G.M.G.; Cagnie, B.; Meeus, M.; De Meulemeester, K. Immediate effects of dry needling on pain sensitivity and pain modulation in patients with chronic idiopathic neck pain: A single-blinded randomized clinical trial. Braz. J. Phys. Ther. 2023, 27, 100481. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.; Sáez-Olmo, E.; Pecos-Martín, D.; Calvo-Lobo, C. Effects of dry needling in the sternocleidomastoid muscle on cervical motor control in patients with neck pain: A randomised clinical trial. Acupunct. Med. 2019, 37, 151–163. [Google Scholar] [CrossRef]
- Myburgh, C.; Hartvigsen, J.; Aagaard, P.; Holsgaard-Larsen, A. Skeletal muscle contractility, self-reported pain and tissue sensitivity in females with neck/shoulder pain and upper Trapezius myofascial trigger points-a randomized intervention study. Chiropr. Man. Therap. 2012, 20, 36. [Google Scholar] [CrossRef]
- Navarro, S.M.; Medina, S.D.; Rico, J.M.B.; Ortiz, M.I.R.; Gracia, M.T.P. Analysis and comparison of pain pressure threshold and active cervical range of motion after superficial and deep dry needling techniques of the upper trapezius muscle. Acupunct. Med. 2022, 40, 13–23. [Google Scholar] [CrossRef]
- Ballyns, J.J.; Turo, D.; Otto, P.; Shah, J.P.; Hammond, J.; Gebreab, T.; Gerber, L.H.; Sikdar, S. Office-based elastographic technique for quantifying mechanical properties of skeletal muscle. J. Ultrasound Med. 2012, 31, 1209–1219. [Google Scholar] [CrossRef]
- Shah, J.P.; Danoff, J.V.; Desai, M.J.; Parikh, S.; Nakamura, L.Y.; Phillips, T.M.; Gerber, L.H. Biochemicals Associated With Pain and Inflammation are Elevated in Sites Near to and Remote From Active Myofascial Trigger Points. Arch. Phys. Med. Rehabil. 2008, 89, 16–23. [Google Scholar] [CrossRef]
- Dunning, J.; Butts, R.; Mourad, F.; Young, I.; Flannagan, S.; Perreault, T. Dry needling: A literature review with implications for clinical practice guidelines. Phys. Ther. Rev. 2014, 19, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Hsieh, L.F.; Kuan, T.S.; Kao, M.J.; Chou, L.W.; Hong, C.Z. Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am. J. Phys. Med. Rehabil. 2010, 89, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Sachiko, F.; Motohiro, I.; Miwa, N.; Megumi, I. Difference between therapeutic effects of deep and superficial acupuncture needle insertion for low back pain: A randomized controlled clinical trial. J. Acupunct. Meridian Stud. 2011, 7, 37–45. [Google Scholar]
- Edwards, J.; Knowles, N. Superficial dry needling and active stretching in the treatment of myofascial pain--a randomised controlled trial. Acupunct. Med. 2003, 21, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Romero, E.A.; Lim, T.; Villafañe, J.H.; Boutin, G.; Riquelme Aguado, V.; Martin Pintado-Zugasti, A.; Alonso Pérez, J.L.; Fernández Carnero, J. The influence of verbal suggestion on post-needling soreness and pain processing after dry needling treatment: An experimental study. Int. J. Environ. Res. Public Health 2021, 18, 4206. [Google Scholar] [CrossRef]
- Ceccherelli, F.; Rigoni, M.T.; Gagliardi, G.; Ruzzante, L. Comparison of superficial and deep acupuncture in the treatment of lumbar myofascial pain: A double-blind randomized controlled study. Clin. J. Pain 2002, 18, 149–153. [Google Scholar] [CrossRef]
- Näslund, J.; Näslund, U.B.; Odenbring, S.; Lundeberg, T. Sensory stimulation (acupuncture) for the treatment of idiopathic anterior knee pain. J. Rehabil. Med. 2002, 34, 231–238. [Google Scholar] [CrossRef]
- Ja, A.C.; Jd, C.; Psycho, W.J.M. Psychometric Properties of the Neck Disability Index and Numeric Pain Rating Scale in Patients With Mechanical. Arch. Phys. Med. Rehabil. 2008, 89, 69–74. [Google Scholar] [CrossRef]
- Lauche, R.; Langhorst, J.; Dobos, G.J.; Cramer, H. Clinically meaningful differences in pain, disability and quality of life for chronic nonspecific neck pain—A reanalysis of 4 randomized controlled trials of cupping therapy. Evid. Based Complement. Alternat. Med. 2013, 2013, 342–347. [Google Scholar] [CrossRef]
- Ailliet, L.; Rubinstein, S.M.; De Vet, H.C.; Van Tulder, M.W.; Terwee, C.B. Reliability, responsiveness and interpretability of the neck disability index-Dutch version in primary care. Eur. Spine J. 2015, 24, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Pool, J.J.M.; Ostelo, R.W.J.G.; Hoving, J.L.; Bouter, L.M.; De Vet, H.C. Minimal clinically important change of the Neck Disability Index and the Numerical Rating Scale for patients with neck pain. Spine 2007, 32, 3047–3051. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.N.; Komesh, S.; Naghdi, S.; Fakhari, Z.; Alaei, P. Responsiveness of minimal clinically important change for the Persian functional rating index in patients with chronic low back pain. Asian Spine J. 2018, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Chansirinukor, W. Thai version of the Functional Rating Index for patients with back and neck pain: Part II responsiveness and head-to-head comparisons. Physiother. Res. Int. 2018, 98, S97–S105. [Google Scholar] [CrossRef]
- Zieliński, M. Physiotherapy-specific effect size thresholds and sample size considerations for clinical trials. Arch. Phys. Med. Rehabil. 2025, 106, e65–e73. [Google Scholar] [CrossRef]
| PICO Element | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population (P) | Adults with neck pain and active myofascial trigger points (MTrPs) in the upper trapezius |
|
| Intervention (I) | Deep dry needling | — |
| Comparator (C) | Superficial dry needling | — |
| Outcomes (O) | Pain intensity and functional disability | — |
| Other criteria |
|
|
| Measure | Value |
|---|---|
| Total number of articles screened | 127 |
| Articles included by both reviewers | 55 (43.3%) |
| Articles excluded by both reviewers | 72 (56.7%) |
| Articles with disagreements | 0 (0%) |
| Percentage of agreement | 100% |
| Cohen’s kappa (κ) | 1.00 |
| 95% confidence interval for kappa | 1.00–1.00 |
| Statistical significance (p-value) | <0.001 |
| Authors | Sample Size | Diagnostic Criteria | Interventions | No. of Sessions | Follow-Ups | Outcome Measures | Main Results |
|---|---|---|---|---|---|---|---|
| Chys et al., 2023 [28] | 54 (DDN: 26, SDN: 28) | Palpable tight band, local pain on pressure and referred pain | DDN vs. SDN in the upper trapezius | 1 session | Immediately post-treatment | PPT CPM | There were no significant differences between DDN and SDN for PPT at local or distant sites. DDN significantly improved the relative CPM efficiency. |
| Ezzati et al., 2018 [17] | 50 (DDN: 25, SDN: 25) | Palpable tight band, local pain on pressure and recognised pain | DDN vs. SDN in the upper trapezius | 3 sessions | 15 days | VAS NDI ROM | Both groups improved, but DDN showed greater gains in ROM and NDI over follow-up. |
| Hoseininejad et al., 2023 [18] | 50 (DDN: 25, SDN: 25) | Neck/shoulder pain with at least one active trigger point in the upper trapezius persisting for 3 months | DDN vs. SDN in the upper trapezius | 1 session | 1 week | VAS NDI sEMG | Both groups improved in VAS and NDI, but only DDN significantly increased sEMG. |
| Martín-Rodríguez et al., 2019 [29] | 34 (DDN: 17, control: 17) | Palpable tight band with local and familiar pain, and restricted ROM during full extension | Trigger point DDN vs. sham dry needling | 1 session | 1 month | CMC VAS ROM NDI | DDN improved pain, ROM and motor control, but there were no significant differences compared to sham DDN. |
| Myburgh et al., 2012 [30] | 77 (symptomatic/asymptomatic) | Symptomatic group: significant MTrP and self-reported pain ≥ 3 on NRS-101. Asymptomatic group: no MTrP or pain (0). | DDN vs. SDN in the upper trapezius | 1 session | 28 h post-treatment | PPT NRS-101 F-max RFD | Both groups reduced pain, but PPT decreased across all participants. There were no significant differences in F-max or RFD. |
| Navarro et al., 2022 [31] | 180 (DDN: 60, SDN: 60, placebo: 60) | Presence of latent MTrPs in the upper trapezius | DDN vs. SDN vs. placebo | 1 session | 1 week | PPT ACROM | Both DDN and SDN improved PPT and ROM over time, but DDN showed better ipsilateral rotation improvement at 7 days. |
| Sarrafzadeh et al., 2018 [19] | 50 (DDN: 25, SDN: 25) | Palpable tight band, local pain on pressure and recognition of pain by the participants | DDN vs. SDN in the upper trapezius | 3 sessions | 15 days | VAS Ultrasonic evaluation | Both DDN and SDN reduced pain and increased muscle thickness, but DDN was superior in pain reduction. |
| Sedighi et al., 2017 [20] | 30 (DDN: 15, SDN: 15) | Unilateral neck pain spreading to the frontotemporal area, worsened by movement, restricted ROM and C1–C3 tenderness | DDN vs. SDN in the suboccipital/upper trapezius | 1 session | 1 week | HI Pain intensity TrP tenderness ROM FRI | Both groups reduced HI and tenderness, but DDN showed superior improvements in ROM and FRI. |
| Domain | Percentage of Agreement (%) | Cohen’s Kappa (κ) | 95% Confidence Interval (CI) | p-Value |
|---|---|---|---|---|
| Domain 1 | 87.5% | 0.73 | 0.28–1.00 | 0.009 |
| Domain 2 | 100% | 1.00 | 1.00–1.00 | 0.005 |
| Domain 3 | 100% | 1.00 | 1.00–1.00 | 0.005 |
| Domain 4 | 100% | 1.00 | 1.00–1.00 | 0.000 |
| Domain 5 | 87.5% | 0.75 | 0.30–1.00 | 0.028 |
| Overall | 87.5% | 0.78 | 0.646–1.00 | 0.002 |
| Study | Bias Due to the Randomisation Process | Bias Due to Deviation from the Intended Interventions | Bias Due to Missing Outcome Data | Bias in Outcome Measurement | Bias in the Selection of the Reported Result | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| Chys et al., 2023 [28] | Low | Low | Low | Low | Low | Low |
| Ezzati et al., 2018 [17] | High | Low | Low | High | Some concerns | High |
| Hoseininejad et al., 2023 [18] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Martín-Rodríguez et al., 2019 [29] | Low | Low | Low | Low | Low | Low |
| Navarro et al., 2022 [31] | Some concerns | Low | Low | Low | Low | Some concerns |
| Sarrafzadeh et al., 2018 [19] | Some concerns | Low | Low | Low | Low | Some concerns |
| Sedighi et al., 2017 [20] | Some concerns | High | Low | Some concerns | Some concerns | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhakami, A.M.; Sahely, A.; Alshami, A.M.Y. Deep Versus Superficial Dry Needling for Neck Pain: A Systematic Review of Randomised Clinical Trials. Medicina 2025, 61, 1832. https://doi.org/10.3390/medicina61101832
Alhakami AM, Sahely A, Alshami AMY. Deep Versus Superficial Dry Needling for Neck Pain: A Systematic Review of Randomised Clinical Trials. Medicina. 2025; 61(10):1832. https://doi.org/10.3390/medicina61101832
Chicago/Turabian StyleAlhakami, Anas M., Ahmad Sahely, and Ali M. Y. Alshami. 2025. "Deep Versus Superficial Dry Needling for Neck Pain: A Systematic Review of Randomised Clinical Trials" Medicina 61, no. 10: 1832. https://doi.org/10.3390/medicina61101832
APA StyleAlhakami, A. M., Sahely, A., & Alshami, A. M. Y. (2025). Deep Versus Superficial Dry Needling for Neck Pain: A Systematic Review of Randomised Clinical Trials. Medicina, 61(10), 1832. https://doi.org/10.3390/medicina61101832






