Abstract
Background and Objectives: Periodontitis is a common chronic inflammatory disease and a leading cause of tooth loss worldwide. Traditional diagnostic methods, such as probing and radiographic assessment, are retrospective and fail to detect ongoing disease activity. In recent years, salivary biomarkers and oral microbiome profiling have emerged as promising tools for earlier detection and precision-based management. The aim of this review is to synthesize current evidence on salivary and microbiome-derived biomarkers in periodontitis and to evaluate their translational potential in diagnostics and therapy. Materials and Methods: A narrative review was performed using PubMed, Scopus, and Web of Science to identify studies published between 2020 and 2025. Search terms included periodontitis, salivary biomarkers, oral microbiome, dysbiosis, and precision therapy. Priority was given to systematic reviews, meta-analyses, and translational studies that addressed diagnostic or therapeutic applications. Eligible publications included English-language original studies and reviews reporting on the diagnostic or therapeutic relevance of salivary or microbiome biomarkers in periodontitis. Results: Salivary biomarkers such as cytokines, matrix metalloproteinases (MMPs), oxidative stress markers, microRNAs, and extracellular vesicles (EVs) show consistent associations with disease activity and treatment outcomes. Oral microbiome studies reveal that both classical pathogens and community-level dysbiosis contribute to disease risk. Translational advances include chairside immunoassays, biosensors, lab-on-a-chip devices, and artificial intelligence (AI)-driven analyses. Biomarker-guided therapies—such as microbiome modulation, natural bioactive compounds, host-response modulation, and smart biomaterials—are being evaluated with increasing frequency in translational studies. Conclusions: By integrating salivary and microbiome biomarkers with novel diagnostic technologies and emerging therapies, this review complements existing systematic evidence and offers a translational roadmap toward precision periodontology.
1. Introduction
Periodontitis is a chronic, multifactorial inflammatory disease characterized by the progressive destruction of the periodontal ligament, alveolar bone, and surrounding connective tissues, ultimately resulting in tooth mobility and, in advanced stages, tooth loss if untreated [1]. The disease originates from a dysbiotic interaction between the oral microbiota and host immune responses, where microbial biofilms trigger sustained inflammation and tissue breakdown. Globally, periodontitis is among the most prevalent oral disorders and represents a substantial burden on both oral and general health. According to the Global Burden of Disease Study, severe periodontitis ranks as the sixth most common disease worldwide, affecting over 700 million individuals [2,3]. Estimates suggest that up to 50% of adults present some degree of periodontal involvement, with severe forms impacting approximately 10–15% of the population [4].
Beyond tooth loss, periodontitis has been associated with systemic conditions such as cardiovascular disease, diabetes mellitus, chronic kidney disease, rheumatoid arthritis, respiratory infections, and adverse pregnancy outcomes [5,6]. This bidirectional relationship underscores the importance of early diagnosis and effective management, not only for preserving oral health but also to improve systemic health outcomes.
Despite therapeutic advances, conventional diagnostic methods remain retrospective and provide limited insight into current or future disease activity, making them unsuitable for early or subclinical detection [7]. These limitations highlight the need for diagnostic tools that are non-invasive, reproducible, and capable of identifying active disease processes before irreversible damage occurs. In this context, host- and microbe-derived biomarkers have gained increasing attention. Saliva is particularly promising because it can be collected non-invasively and reflects both local and systemic conditions [8,9]. Parallel to this, oral microbiome research has moved beyond the pathogen-centric “red complex” to the broader concept of dysbiosis, in which community-level imbalances drive inflammation and tissue destruction. The implications of dysbiosis are discussed further in Section 3 [10,11].
Several systematic reviews and meta-analyses have rigorously evaluated individual salivary biomarkers, such as Toll-like receptors and matrix metalloproteinases, or synthesized evidence for biomarker-based diagnostics [12,13]. While these provide valuable accuracy estimates, their scope remains narrow. This review differs by adopting a narrative and translational perspective, integrating salivary and microbiome-derived biomarkers with recent advances in diagnostic technologies and biomarker-guided therapies [14].
These discoveries are now being integrated into novel diagnostic platforms such as chairside tests, lab-on-a-chip devices, and biosensors [15,16]. AI-assisted analyses of salivary and microbiome datasets further enhance the ability to identify patterns predictive of disease progression [17]. Importantly, several molecular diagnostic kits for detecting major periodontal pathogens in gingival crevicular fluid (GCF) are already commercially available, bridging the gap between experimental research and clinical application. Recent exploratory work with SmartGel OV, a kit was employed to detect 11 key periodontal pathogens in GCF, complementing host-response biomarker analysis and providing a more comprehensive picture of periodontal status [9,11]. This illustrates how integrated diagnostic approaches can support both patient stratification and the development of personalized therapeutic strategies.
Knowledge Gap and Aim of the Review: Several systematic reviews and meta-analyses have examined isolated biomarkers, such as MMP-8 or Toll-like receptors, primarily focusing on their diagnostic accuracy. However, these studies remain fragmented, rarely integrating salivary and microbiome biomarkers within a translational framework or linking them to emerging diagnostic technologies and therapeutic innovations. This creates a gap in the literature regarding how biomarker discoveries can be translated into precision periodontology. Therefore, the aim of this narrative review is to synthesize recent evidence on salivary and microbiome biomarkers, highlight their integration with novel diagnostic platforms, and critically discuss their role in guiding personalized therapeutic strategies.
2. Materials and Methods
This review was conducted as a narrative synthesis of the literature. Although narrative reviews do not follow a standardized framework such as PRISMA, we sought to ensure transparency and reproducibility by clearly describing our search process and inclusion criteria. Relevant studies were identified through PubMed, Scopus, and Web of Science between January 2020 to July 2025 using combinations of the terms “periodontitis”, “salivary biomarkers”, “oral microbiome”, “dysbiosis”, and “precision therapy”. Priority was given to systematic reviews, meta-analyses, and translational studies that explicitly evaluated diagnostic or therapeutic applications of salivary and microbiome biomarkers.
The initial search in PubMed using “periodontitis” and “salivary biomarkers” yielded 160 results. After screening titles and abstracts, duplicate entries were removed and additional filters were applied (“oral microbiome”, “dysbiosis”). Similar searches of Scopus and Web of Science were performed. Following the removal of duplicates and application of inclusion/exclusion criteria, a total of 100 articles were selected for full-text review and analysis.
Eligible publications included English-language systematic reviews, meta-analyses, original clinical or translational studies, and narrative reviews reporting on the diagnostic or therapeutic relevance of salivary or microbiome biomarkers in periodontitis. Exclusion criteria were: (1) studies limited to in vitro or animal models without translational significance; (2) publications not directly focused on periodontal disease; and (3) papers without accessible full text.
Because narrative reviews inherently involve subjective interpretation, we attempted to minimize bias by having two authors independently screen and summarize evidence, with disagreements resolved through consensus. The emphasis was placed on reproducibility across populations, validation status of biomarkers, and clinical translation potential, rather than on exhaustive coverage.
3. Salivary Biomarkers in Periodontitis
3.1. Saliva as a Diagnostic Fluid
Saliva has emerged as one of the most promising biofluids for periodontal diagnostics due to its non-invasive, simple, and cost-effective collection. Unlike GCF, which requires site-specific sampling, saliva provides a composite reflection of the oral cavity and contains host- and microbe-derived molecules, extracellular vesicles, nucleic acids, and metabolites [18,19]. Its composition makes it highly suitable for detecting inflammatory mediators and microbial byproducts associated with periodontal disease. In addition, salivary sampling can be easily standardized for large-scale screenings, making it particularly relevant for both clinical and public health applications [20,21].
3.2. Inflammatory Cytokines
Pro-inflammatory cytokines represent one of the most extensively studied groups of salivary biomarkers in periodontitis. Interleukin-1β (IL-1β) is consistently elevated in patients with active periodontal disease and correlates strongly with pocket depth, attachment loss, and bleeding on probing [22]. Elevated salivary levels of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) have similarly been associated with disease severity and progression [23,24,25]. These cytokines are central mediators of host response, driving leukocyte recruitment, osteoclast activation, and connective tissue breakdown. Emerging studies also suggest that anti-inflammatory cytokines such as IL-10 and IL-4 may serve as potential biomarkers for monitoring treatment response and resolution of inflammation [26,27,28].
3.3. MMPs
MMPs, particularly MMP-8 (neutrophil collagenase) and MMP-9 (gelatinase B), are critical enzymes in the degradation of extracellular matrix components [9,29,30]. Elevated levels of salivary MMP-8 have been repeatedly linked with active periodontal tissue destruction. The clinical translation of MMP-8 into point-of-care assays is further discussed in Section 4 [31]. MMP-9, while less specific, is also commonly elevated in periodontitis and correlates with disease activity. In combination with cytokines, MMP levels can provide complementary information on the inflammatory and tissue-destructive phases of periodontal disease [32,33].
3.4. Oxidative Stress Markers
Periodontitis is characterized not only by inflammation but also by oxidative stress. Salivary biomarkers of oxidative damage, including 8-hydroxydeoxyguanosine (8-OHdG), malondialdehyde (MDA), and advanced oxidation protein products (AOPPs), have been associated with periodontal disease severity [34,35]. Total antioxidant capacity (TAC) is often reduced in periodontitis, reflecting disrupted oxidant–antioxidant balance [36]. These markers add an additional layer of diagnostic information by capturing the oxidative stress component of periodontal pathogenesis [37,38].
3.5. Emerging and Novel Biomarkers
Beyond cytokines and proteolytic enzymes, several emerging salivary biomarkers are gaining attention. MicroRNAs (miRNAs), such as miR-146a and miR-155, have been implicated in regulating immune and inflammatory pathways and may serve as sensitive molecular indicators of disease activity [39,40]. EVs, which carry proteins, nucleic acids, and lipids, are another promising frontier, with recent studies indicating that EV cargo can differentiate between health and disease [41,42,43,44]. Additionally, metabolites detected through salivary metabolomics—such as short-chain fatty acids (SCFAs) and amino acid derivatives—offer insights into host–microbe interactions and may support the development of metabolic biomarker panels [45,46].
3.6. Translation into Clinical Diagnostics
The transition from research to clinical practice is exemplified by the development of salivary diagnostic kits and biosensor technologies [12,47,48]. Multiplex platforms capable of simultaneously quantifying multiple cytokines and enzymes are under development, and lab-on-a-chip biosensors offer the potential for point-of-care, real-time diagnostics [8]. Importantly, integration of salivary biomarkers with microbiome profiling and AI-based analysis may enable comprehensive diagnostic platforms that surpass the predictive value of traditional clinical indices [49].
4. Oral Microbiome and Biomarkers in Periodontitis
4.1. Dysbiosis as a Central Concept
The oral microbiome is a highly diverse ecosystem, comprising more than 700 bacterial species, as well as fungi, viruses, and archaea [50,51]. Periodontal health is maintained by a symbiotic microbial community that coexists with host defenses. In contrast, periodontitis is associated with a shift toward dysbiosis, in which microbial diversity increases and pro-inflammatory species gain ecological advantage [52,53]. This dysbiotic state contributes to persistent inflammation, tissue destruction, and ultimately clinical disease progression. Importantly, dysbiosis is not driven by a single pathogen but results from complex ecological imbalances that alter microbial community structure and function [54].
4.2. Classical Pathogens and the “Red Complex”
Historically, periodontal pathogenesis has been attributed to a group of anaerobic Gram-negative bacteria collectively known as the “red complex”: Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola [55]. These species are strongly associated with clinical attachment loss and deep periodontal pockets and have been widely used as microbial markers of disease. Additional taxa such as Aggregatibacter actinomycetemcomitans (particularly in aggressive forms of periodontitis) have also been implicated [56,57]. While valuable, this pathogen-centric model has been increasingly recognized as overly simplistic, as it fails to explain disease heterogeneity and progression in the absence of these species.
4.3. Microbial Signatures Beyond the “Red Complex”
High-throughput sequencing has expanded the list of taxa associated with periodontitis, identifying new microbial signatures that distinguish health, gingivitis, and periodontitis. Species such as Filifactor alocis, Prevotella intermedia, Fusobacterium nucleatum, and Peptostreptococcus stomatis have been linked with disease activity [58,59]. Moreover, shifts in the relative abundance of commensal organisms—such as reductions in Streptococcus sanguinis and Streptococcus gordonii—also contribute to dysbiosis [60,61,62]. These findings support the concept of a “polymicrobial synergy and dysbiosis” model, where disease arises not from a single organism but from community-level interactions and host immune modulation [63].
4.4. Functional and Metagenomic Biomarkers
Recent advances in shotgun metagenomics and meta transcriptomics have revealed that functional changes in the microbiome may be more informative than the presence of specific taxa [14,64]. Periodontitis-associated communities are enriched in genes related to proteolysis, iron acquisition, lipopolysaccharide biosynthesis, and SCFA metabolism [65]. Similarly, transcriptomic analyses indicate that microbial virulence gene expression, rather than mere presence, correlates more closely with disease activity [46]. These functional biomarkers provide mechanistic insights into how microbial dysbiosis drives inflammation and tissue destruction, while also offering novel targets for diagnostic and therapeutic intervention [66].
4.5. Viral and Fungal Contributions
Although less studied than bacteria, viruses and fungi may also serve as relevant biomarkers of dysbiosis [64]. Periodontal pockets harbor diverse viral communities, including bacteriophages that modulate bacterial populations and host interactions. Candida species, particularly Candida albicans, have been identified more frequently in diseased sites and may synergize with bacteria to exacerbate inflammation [67]. Understanding the role of non-bacterial members of the oral microbiome could further refine biomarker-based approaches to periodontal diagnostics [68].
4.6. Translational Applications of Microbiome Biomarkers
Microbiome biomarkers are increasingly being incorporated into diagnostic platforms. Commercial test kits capable of detecting panels of periodontal pathogens in subgingival plaque or gingival crevicular fluid are already in use in research and select clinical contexts. These tests, including those applied in exploratory studies such as the SmartGel OV project, can detect up to 11 periodontal pathogens simultaneously, providing clinicians with a molecular profile of the microbial community [69]. Coupled with salivary biomarkers, these tools offer a more comprehensive assessment of disease risk and activity. Looking forward, AI-driven integration of microbial, host, and clinical data may allow real-time risk prediction and patient-specific treatment planning, marking a substantial step toward precision periodontology [70,71].
A summary of the principal salivary and microbiome biomarkers associated with periodontitis, their biological roles, and diagnostic relevance is presented in Table 1.

Table 1.
Key salivary and oral microbiome biomarkers in periodontitis and their clinical relevance.
A comparative overview of salivary and microbiome biomarkers in periodontitis is presented in Figure 1, highlighting the complementary insights provided by host- and microbe-derived indicators of disease activity.
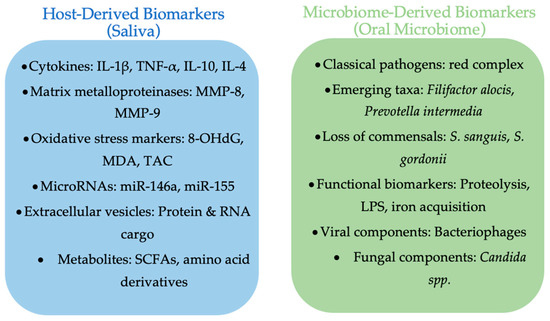
Figure 1.
Comparative overview of salivary and oral microbiome biomarkers in periodontitis. Salivary biomarkers include cytokines, proteolytic enzymes, oxidative stress markers, microRNAs, extracellular vesicles, and metabolites. Microbiome biomarkers encompass classical red complex species, emerging taxa, loss of commensals, functional gene shifts, as well as viral and fungal components. Together, these biomarker categories provide complementary insights into periodontal disease activity and progression (made with an AI-assisted design manager, accessed on 15 July 2025).
5. Novel Diagnostic Tools for Periodontitis
5.1. Limitations of Conventional Methods
Traditional periodontal diagnostics rely on PD, CAL, BOP, and radiographic evaluation [81]. While these parameters remain the clinical gold standard, they are retrospective in nature and provide little insight into ongoing disease activity or future risk. This limitation has driven the development of novel diagnostic technologies that integrate host- and microbe-derived biomarkers with advanced analytical platforms [82].
5.2. Chairside Tests and Immunoassays
Point-of-care assays have emerged as practical tools for rapid detection of periodontal inflammation. Chairside kits such as PerioSafe® and ImplantSafe® (Dentognostics GmgH, Solingen, Germany), which detect active MMP-8 in saliva or GCF, allow clinicians to identify active tissue breakdown within minutes [83,84]. These tests are easy to use, cost-effective, and compatible with routine dental visits, making them among the most clinically ready biomarker-based diagnostic tools. However, their specificity and sensitivity still require validation in diverse populations and longitudinal studies [47,72,85].
5.3. Biosensors and Lab-on-a-Chip Devices
Advances in microfluidics and nanotechnology have enabled the development of biosensors and lab-on-a-chip devices capable of detecting multiple biomarkers simultaneously [8]. These platforms can integrate immunoassays, DNA hybridization, and electrochemical detection to measure cytokines, enzymes, and microbial DNA in small saliva or GCF samples. Their portability and rapid turnaround times make them highly suitable for chairside application and population-level screening. Recent prototypes have demonstrated high sensitivity and specificity for MMP-8, IL-6, and microbial DNA, suggesting strong potential for clinical translation [15,86].
5.4. High-Throughput Molecular Diagnostics
Molecular diagnostic assays based on polymerase chain reaction (PCR) and quantitative PCR (qPCR) remain widely used for detecting periodontal pathogens. Commercial kits capable of profiling multiple species simultaneously represent a bridge between microbiome research and practical application [40,87]. More recently, next-generation sequencing (NGS) approaches have been explored for comprehensive profiling of oral microbial communities. Although NGS remains expensive and time-consuming, its ability to identify microbial and functional signatures with high resolution offers unique potential for future diagnostic frameworks [88].
5.5. AI and Data Integration
AI is increasingly being applied to integrate complex datasets combining salivary biomarkers, microbiome profiles, and clinical parameters [89]. Machine learning models have shown promising results in predicting disease status, progression, and response to therapy. AI-assisted image analysis of radiographs and periodontal charts also offers enhanced diagnostic accuracy and reproducibility [90]. The integration of AI with biomarker-based diagnostics may ultimately enable personalized risk prediction and treatment planning, moving periodontal care closer to precision medicine [89,91,92,93].
5.6. Challenges for Clinical Translation
While the potential of novel diagnostic technologies is considerable, several barriers remain. Cost-effectiveness, ease of use, and standardization across laboratories are critical for adoption in daily practice. Regulatory approval and clinical validation in large, diverse populations will be necessary before widespread implementation. Furthermore, integration of these tools into existing dental workflows will require both clinician training and patient acceptance [72,94]. Addressing these challenges will be essential for moving from proof-of-concept prototypes to routine clinical use. The main categories of novel diagnostic platforms, their applications, advantages, and limitations are summarized in Table 2.

Table 2.
Novel diagnostic tools for periodontitis and their clinical applications.
6. Biomarker-Guided Therapeutic Innovations
6.1. From Conventional to Precision Periodontal Therapy
Traditional periodontal therapy has relied on mechanical debridement, adjunctive antimicrobials, and surgical approaches to control microbial biofilms and inflammation. While effective in many cases, these treatments are often applied uniformly across patients, without consideration of individual host–microbe profiles. Biomarker-driven strategies represent a paradigm shift, enabling clinicians to stratify patients according to their inflammatory and microbial status and to tailor therapy accordingly [95].
6.2. Microbiome Modulation: Probiotics, Prebiotics, and Postbiotics
Microbiome modulation is emerging as a complementary therapeutic strategy. Probiotics such as Lactobacillus reuteri have been shown to reduce gingival inflammation and modulate microbial balance in patients with periodontitis [96,97]. Prebiotics—substrates that selectively promote the growth of beneficial bacteria—offer another means of restoring microbial homeostasis. More recently, postbiotics (non-viable microbial products with bioactive properties) have gained attention for their ability to modulate immune responses and reduce inflammation [98]. The identification of specific microbial biomarkers enables targeted application of these therapies, maximizing their effectiveness [97].
6.3. Natural Bioactive Compounds and Essential Oils (EOs)
Natural compounds, particularly EOs, have demonstrated antimicrobial, anti-inflammatory, and antioxidant effects relevant to periodontitis [99,100,101]. Compounds such as carvacrol, thymol, and linalool have been shown to inhibit key periodontal pathogens and disrupt biofilms while modulating host immune responses [102]. Salivary and microbiome biomarkers can be used to monitor treatment efficacy, offering an objective framework for integrating natural bioactives as adjunctive therapies. Given the global challenge of antibiotic resistance, such biomarker-guided natural interventions represent a promising alternative in periodontal care [103,104].
6.4. Host-Response Modulation Therapies
In addition to targeting microbial dysbiosis, host-modulating therapies are increasingly being explored. Sub-antimicrobial dose doxycycline (SDD) is already used to inhibit MMP activity and reduce collagen breakdown [29,105]. Novel agents aimed at blocking pro-inflammatory cytokines or enhancing anti-inflammatory pathways are in development. The measurement of salivary cytokines and MMPs provides a means of tailoring host-modulation therapies to individual patients, optimizing their efficacy and safety [9].
6.5. Smart Biomaterials and Local Drug Delivery
Advances in biomaterials science have enabled the design of smart drug delivery systems capable of releasing therapeutic agents directly into periodontal pockets [89]. Hydrogels, nanofibers, and biodegradable microspheres have been engineered to provide controlled, sustained release of antimicrobials, anti-inflammatory drugs, or bioactive molecules [106,107]. By coupling biomarker detection (e.g., pathogen panels, cytokine levels) with localized release of antimicrobial or anti-inflammatory agents, such technologies exemplify the future of precision periodontal therapy [9,108].
6.6. Integration into Clinical Practice
Although many biomarker-guided therapies remain at the experimental or early clinical stage, their translational potential is substantial [48,109]. Personalized interventions guided by biomarker profiles could improve treatment outcomes, reduce unnecessary antibiotic use, and minimize disease recurrence [110,111,112]. However, clinical adoption will require robust validation through randomized controlled trials, regulatory approval, and demonstration of cost-effectiveness. Interdisciplinary collaboration between periodontists, microbiologists, biomaterials scientists, and data specialists will be crucial for bringing these innovations into routine practice [109,113].
Biomarker discovery informs the development of novel diagnostic platforms, which in turn guide biomarker-based therapeutic innovations. Together, these advances enable a transition from conventional symptom-based management toward precision diagnostics, personalized treatment, and improved clinical outcomes, as illustrated in Figure 2.
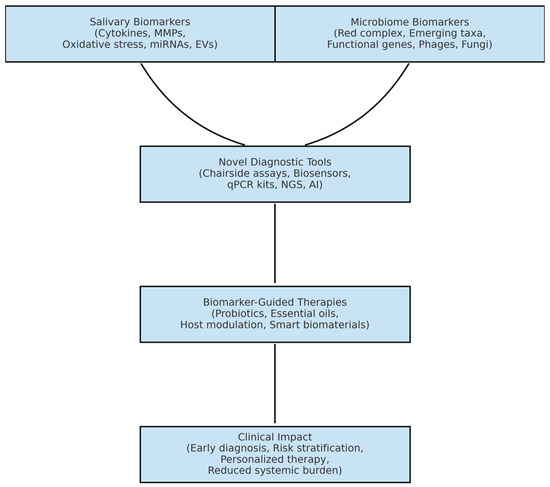
Figure 2.
Conceptual framework illustrating the role of salivary and oral microbiome biomarkers in periodontitis. Biomarker discovery informs the development of novel diagnostic platforms, which in turn guide biomarker-based therapeutic innovations, enabling precision diagnostics, personalized treatment, and improved clinical outcomes (made with an AI-assisted design manager, accessed on 15 July 2025).
7. Discussion
Recent advances in salivary and microbiome biomarkers highlight a shift from symptom-based diagnosis of periodontitis toward molecularly informed strategies. Saliva offers clear advantages as a diagnostic fluid: it is easily accessible, non-invasive, and reflects both local periodontal status and systemic conditions. Biomarkers such as cytokines (IL-1β, IL-6, TNF-α), MMPs (particularly MMP-8), and oxidative stress indicators have consistently demonstrated associations with disease severity and treatment response. Similarly, oral microbiome studies have moved beyond the “red complex” pathogens to reveal community-level dysbiosis as a driver of inflammation and tissue destruction.
Despite this progress, important challenges remain. Biomarker studies vary widely in their design, assay platforms, and populations, making cross-study comparisons difficult and limiting the definition of universal diagnostic thresholds. Many associations remain correlational, and the causal role of microbial shifts has not been fully established. In addition, reproducibility issues, regulatory approval, and the lack of cost-effectiveness data hinder large-scale clinical adoption.
Beyond the general challenges of biomarker validation, the study of oral microbiome biomarkers faces significant methodological variability. Differences in sampling sites (e.g., saliva, subgingival plaque, gingival crevicular fluid), sequencing methodologies (16S rRNA vs. shotgun metagenomics), and bioinformatic pipelines can lead to inconsistent findings and reduced reproducibility across studies. Recent research in oral potentially malignant disorders and oral squamous cell carcinoma has similarly highlighted these limitations, underscoring the need for methodological rigor and standardized protocols when translating microbiome data into clinical settings [114].
Translational progress, however, is evident. Chairside immunoassays, biosensors, and lab-on-a-chip devices now enable the rapid detection of host and microbial markers in real time. AI strengthens diagnostic accuracy by integrating complex datasets and identifying predictive patterns. The availability of commercial kits for detecting multiple periodontal pathogens in gingival crevicular fluid demonstrates how molecular diagnostics can complement clinical indices. Multiplex approaches that combine salivary and microbial markers may allow for improved patient stratification and more personalized interventions.
Looking forward, several priorities are clear. Standardization of assays and validation in large, diverse cohorts are essential to ensure reproducibility and clinical reliability. Longitudinal studies are needed to confirm whether biomarker profiles can truly predict progression and treatment response. Integration of biomarker data with imaging, clinical indices, and behavioral factors through AI-driven platforms holds promise for risk prediction models suitable for routine practice. On the therapeutic side, biomarker-guided strategies—such as microbiome modulation, natural bioactive compounds, host-response modulators, and smart biomaterials—require robust clinical trials to demonstrate safety, efficacy, and cost-effectiveness.
Current evidence suggests that not all biomarkers are equally validated. Host-derived markers such as IL-1β and active MMP-8 show the strongest reproducibility across diverse populations and are closest to clinical translation, as reflected in the availability of chairside assays. IL-6, TNF-α, and oxidative stress markers also demonstrate consistent associations, but assay variability and lack of standardized cut-off values remain barriers. In contrast, extracellular vesicles, microRNAs, and salivary metabolites remain exploratory, with evidence limited to small pilot studies and preclinical investigations. These emerging biomarkers highlight exciting directions but require large, multicenter studies before they can be considered for routine clinical use. While cytokines and MMPs (particularly IL-1β and active MMP-8) are supported by relatively robust validation and even chairside assays, other categories of salivary biomarkers—including extracellular vesicles, microRNAs, and salivary metabolites—remain largely exploratory. Most of the current evidence comes from single-center studies with small sample sizes, heterogeneous methodologies, and limited reproducibility. To avoid overinterpretation, it is essential to emphasize that these biomarkers require validation in large-scale, multicenter cohorts using standardized protocols before they can be translated into clinical diagnostics. Establishing such methodological and population-level robustness will be critical for distinguishing truly reliable markers from those that may reflect context-specific findings [25].
Current evidence shows that not all biomarkers are equally validated. IL-1β and active MMP-8 are the most reproducible and clinically advanced, supported by chairside assays, while IL-6, TNF-α, and oxidative stress markers remain promising but limited by assay variability. In contrast, EVs, microRNAs, and salivary metabolites are still exploratory and require validation in large, multicenter studies. The oral microbiome provides valuable insights into dysbiosis, yet challenges in standardization and causality persist.
Future research should prioritize large-scale validation, standardized assay platforms, and integration of biomarker data with imaging and clinical indices through AI-driven models. Robust clinical trials of biomarker-guided therapies—including microbiome modulation, natural bioactive compounds, host-response modulators, and smart biomaterials—are essential to establish clinical utility and cost-effectiveness.
By consolidating these perspectives, the present review delivers a translational synthesis that bridges biomarker discovery, diagnostic innovation, and therapeutic development, providing the foundation for the concluding roadmap toward precision periodontology. As this is a narrative review, selection and interpretation of evidence may reflect a degree of author subjectivity. Unlike systematic reviews, our methodology does not eliminate all risk of bias. Nevertheless, by prioritizing high-quality evidence such as systematic reviews, meta-analyses, and translational studies, we aimed to provide a balanced synthesis while acknowledging this limitation.
8. Conclusions
Periodontitis remains a major challenge for both oral and systemic health, and conventional diagnostic methods are limited by their retrospective nature. Advances in salivary and oral microbiome biomarkers provide new opportunities for earlier, non-invasive, and more precise disease management.
Among the available markers, IL-1β and active MMP-8 show the strongest reproducibility across populations and are closest to clinical translation, while microRNAs, EVs, and salivary metabolites remain exploratory and require further validation. Emerging diagnostic technologies—such as chairside immunoassays, biosensors, lab-on-a-chip platforms, and AI-driven analyses—are accelerating the translation of biomarker science into practice. Likewise, biomarker-guided therapies, including microbiome modulation, natural bioactive compounds, host-response modulation, and smart biomaterials, are expanding the horizon of precision periodontology.
This review complements systematic evidence by providing an integrated translational perspective that bridges biomarker discovery with diagnostic innovation and therapeutic development. Future progress will depend on assay standardization, validation across diverse populations, and demonstration of cost-effectiveness. Together, these advances provide a roadmap for transforming periodontal care from reactive treatment toward proactive, precision-based prevention.
The successful integration of biomarker-driven diagnostics and therapies into routine clinical workflows will require close interdisciplinary collaboration. Periodontists, microbiologists, biomaterials scientists, and data specialists must work together to translate laboratory discoveries into clinically applicable, cost-effective solutions. Such cross-disciplinary efforts will be essential to overcome current barriers, ensure methodological rigor, and accelerate the adoption of precision-based strategies in periodontology.
Author Contributions
Conceptualization, C.-M.R. and C.C.R.; methodology, C.-M.R.; software, C.C.R.; validation, C.-M.R.; formal analysis, C.C.R.; investigation, C.C.R.; resources, C.-M.R.; data curation, C.-M.R.; writing—original draft preparation, C.-M.R.; writing—review and editing, C.-M.R. and D.C.Z.; visualization, D.C.Z.; supervision, D.C.Z.; project administration, C.C.R. and D.C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The publication of this paper was supported by the University of Oradea, Oradea, Romania.
Data Availability Statement
Information provided in this research are supported by the inserted references.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Murray, C.J.L. The Global Burden of Disease Study at 30 Years. Nat. Med. 2022, 28, 2019–2026. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990–2010: A Systematic Review and Meta-Regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Fischer, R.G.; Lira Junior, R.; Retamal-Valdes, B.; de Figueiredo, L.C.; Malheiros, Z.; Stewart, B.; Feres, M. Periodontal Disease and Its Impact on General Health in Latin America. Section V: Treatment of Periodontitis. Braz. Oral Res. 2020, 34, e026. [Google Scholar] [CrossRef] [PubMed]
- Sudhakara, P.; Gupta, A.; Bhardwaj, A.; Wilson, A. Oral Dysbiotic Communities and Their Implications in Systemic Diseases. Dent. J. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.M.; Yekta-Michael, S.S.; Schittenhelm, F.; Reichert, S.; Kupietz, D.; Dommisch, H.; Kasaj, A.; Wied, S.; Vela, O.C.; Stratul, S.I. Comparison of Three Full-Mouth Concepts for the Non-Surgical Treatment of Stage III and IV Periodontitis: A Randomized Controlled Trial. J. Clin. Periodontol. 2021, 48, 1516–1527. [Google Scholar] [CrossRef]
- Cafiero, C.; Spagnuolo, G.; Marenzi, G.; Martuscelli, R.; Colamaio, M.; Leuci, S. Predictive Periodontitis: The Most Promising Salivary Biomarkers for Early Diagnosis of Periodontitis. J. Clin. Med. 2021, 10, 1488. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.; Martin, L.; Patel, M.; Hu, W.; Bittinger, K.; Kallan, M.J.; Chandrasekaran, G.; Cucchiara, A.J.; Giannobile, W.V.; Stephens, D.; et al. Gingival Crevicular Fluid Biomarkers During Periodontitis Progression and After Periodontal Treatment. J. Clin. Periodontol. 2025, 52, 40–55. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, Fallacies and the Future. Periodontology 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Radu, C.-M.; Radu, C.C.; Zaha, D.C. SmartGel OV: A Natural Origanum Vulgare-Based Adjunct for Periodontitis with Clinical and Microbiological Evaluation. Medicina 2025, 61, 1423. [Google Scholar] [CrossRef]
- Boynes, S.G.; Sofiyeva, N.; Saw, T.; Nieto, V.; Palomo, L. Assessment of Salivary Matrix Metalloproteinase (MMP8) and Activated Salivary Matrix Metalloproteinase (AMMP8) in Periodontitis Patients: A Systematic Review and Meta-Analysis. Front. Oral Health 2025, 6, 1444399. [Google Scholar] [CrossRef]
- Alarcón-Sánchez, M.A.; Rodríguez-Montaño, R.; Lomelí-Martínez, S.M.; Martínez-Bugarin, C.H.; Mosaddad, S.A.; Heboyan, A. Are Salivary and Plasma Levels of Toll-Like Receptors 2 and 4 Elevated in Subjects With Chronic Periodontitis?: A Systematic Review and Meta-Analysis. Int. J. Inflam. 2025, 2025, 7405066. [Google Scholar] [CrossRef]
- Mira, A. Oral Microbiome Studies: Potential Diagnostic and Therapeutic Implications. Adv. Dent. Res. 2018, 29, 71–77. [Google Scholar] [CrossRef]
- Umeizudike, K.A.; Lähteenmäki, H.; Räisänen, I.T.; Taylor, J.J.; Preshaw, P.M.; Bissett, S.M.; Tervahartiala, T.; O Nwhator, S.; Pärnänen, P.; Sorsa, T. Ability of Matrix Metalloproteinase-8 Biosensor, IFMA, and ELISA Immunoassays to Differentiate between Periodontal Health, Gingivitis, and Periodontitis. J. Periodontal Res. 2022, 57, 558–567. [Google Scholar] [CrossRef]
- Bornes, R.; Montero, J.; Correia, A.; Marques, T.; Rosa, N. Peri-Implant Diseases Diagnosis, Prognosis and Dental Implant Monitoring: A Narrative Review of Novel Strategies and Clinical Impact. BMC Oral Health 2023, 23, 183. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Urbanová, W.; Novák, B.; Czako, L.; Siebert, T.; Stano, P.; Mareková, S.; Fountoulaki, G.; Kosnáčová, H.; Varga, I. Where Is the Artificial Intelligence Applied in Dentistry? Systematic Review and Literature Analysis. Healthcare 2022, 10, 1269. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Ma, S.; Yadlapati, R. Salivary Biomarkers and Esophageal Disorders. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2022, 35, doac018. [Google Scholar] [CrossRef]
- Teles, F.R.F.; Chandrasekaran, G.; Martin, L.; Patel, M.; Kallan, M.J.; Furquim, C.; Hamza, T.; Cucchiara, A.J.; Kantarci, A.; Urquhart, O.; et al. Salivary and Serum Inflammatory Biomarkers during Periodontitis Progression and after Treatment. J. Clin. Periodontol. 2024, 51, 1619–1631. [Google Scholar] [CrossRef]
- Corana, M.; Baima, G.; Iaderosa, G.; Franco, F.; Zhang, J.; Berta, G.N.; Romano, F.; Aimetti, M. Salivary Proteomics for Detecting Novel Biomarkers of Periodontitis: A Systematic Review. J. Periodontal Res. 2025, 60, 633–655. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Kirakodu, S.S.; Zhang, X.; Dawson, D., 3rd; Miller, C.S. Salivary Microbiome and Biomarker Characteristics of Diabetics with Periodontitis. Mol. Oral Microbiol. 2025, 40, 37–49. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, H.-N. Changes in Inflammatory Cytokines in Saliva after Non-Surgical Periodontal Therapy: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 18, 194. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Kirakodu, S.S.; Zhang, X.D.; Dawson, D., 3rd; Miller, C.S. Salivary Features of Periodontitis and Gingivitis in Type 2 Diabetes Mellitus. Sci. Rep. 2024, 14, 30649. [Google Scholar] [CrossRef]
- Pérez-Pacheco, C.G.; Fernandes, N.A.R.; Primo, F.L.; Tedesco, A.C.; Bellile, E.; Retamal-Valdes, B.; Feres, M.; Guimarães-Stabili, M.R.; Rossa, C. Local Application of Curcumin-Loaded Nanoparticles as an Adjunct to Scaling and Root Planing in Periodontitis: Randomized, Placebo-Controlled, Double-Blind Split-Mouth Clinical Trial. Clin. Oral Investig. 2021, 25, 3217–3227. [Google Scholar] [CrossRef]
- Alavi, S.E.; Sharma, L.A.; Sharma, A.; Ebrahimi Shahmabadi, H. Salivary Biomarkers in Periodontal Disease: Revolutionizing Early Detection and Precision Dentistry. Mol. Diagn. Ther. 2025. [Google Scholar] [CrossRef]
- Relvas, M.; Mendes-Frias, A.; Gonçalves, M.; Salazar, F.; López-Jarana, P.; Silvestre, R.; Viana da Costa, A. Salivary IL-1β, IL-6, and IL-10 Are Key Biomarkers of Periodontitis Severity. Int. J. Mol. Sci. 2024, 25, 8401. [Google Scholar] [CrossRef] [PubMed]
- Al-Kubaisi, A.A.; Ghazi, M.A.; Majeed, N.S.; Aldelaimi, E.R.; Enezei, H.H. Soluble Urokinase Plasminogen Activator Receptor (SuPAR) Is a Potential Biomarker of Stage III-IV, Grade C Periodontitis through the Impact of Post-Radiotherapy on Head and Neck Cancer Patients. BMC Oral Health 2024, 24, 1144. [Google Scholar] [CrossRef] [PubMed]
- Neurath, N.; Kesting, M. Cytokines in Gingivitis and Periodontitis: From Pathogenesis to Therapeutic Targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Balsa-Castro, C.; Nibali, L.; Donos, N.; Tomás, I. Accuracy of Single Molecular Biomarkers in Saliva for the Diagnosis of Periodontitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2020, 47, 2–18. [Google Scholar] [CrossRef]
- Zalewska, E.A.; Ławicka, R.; Grygorczuk, P.; Nowosielska, M.; Kicman, A.; Ławicki, S. Importance of Metalloproteinase 8 (MMP-8) in the Diagnosis of Periodontitis. Int. J. Mol. Sci. 2024, 25, 2721. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S.; Yang, W.-S.; Kim, C.-H. Physiological Properties, Functions, and Trends in the Matrix Metalloproteinase Inhibitors in Inflammation-Mediated Human Diseases. Curr. Med. Chem. 2023, 30, 2075–2112. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Sandu, D. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Ding, X.; Dawson, D.R., 3rd; Ebersole, J.L. Salivary Biomarkers for Discriminating Periodontitis in the Presence of Diabetes. J. Clin. Periodontol. 2021, 48, 216–225. [Google Scholar] [CrossRef]
- Alrashdan, M.S.; Al-Shorman, H.; Al-Dwairi, A.; Qutieshat, A.; Al-Omiri, M.K. Salivary Oxidative Stress Biomarkers in Periodontitis-Free Smokers: A Cross Sectional Study. Minerva Dent. Oral Sci. 2024, 73, 209–216. [Google Scholar] [CrossRef]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative Stress-Related Biomarkers in Saliva and Gingival Crevicular Fluid Associated with Chronic Periodontitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef]
- Sawutdeechaikul, P.; Hwang, S.; Klangprapan, J.; Phan, T.V.; Lam, C.B.; Yoon, Y.-J.; Seo, S.; Hong, S.; Lim, J.-Y.; Ferreira, J.N. Mechanisms Tackling Salivary Gland Diseases with Extracellular Vesicle Therapies. J. Dent. Res. 2025, 104, 704–714. [Google Scholar] [CrossRef]
- Viglianisi, G.; Tartaglia, G.M.; Santonocito, S.; Amato, M.; Polizzi, A.; Mascitti, M.; Isola, G. The Emerging Role of Salivary Oxidative Stress Biomarkers as Prognostic Markers of Periodontitis: New Insights for a Personalized Approach in Dentistry. J. Pers. Med. 2023, 13, 166. [Google Scholar] [CrossRef]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis Is an Inflammatory Disease of Oxidative Stress: We Should Treat It That Way. Periodontology 2000 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Fumimoto, C.; Yamauchi, N.; Minagawa, E.; Umeda, M. MiR-146a Is Mutually Regulated by High Glucose-Induced Oxidative Stress in Human Periodontal Ligament Cells. Int. J. Mol. Sci. 2024, 25, 10702. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Mu, C.; Feng, J.; Sun, X. MiR-146a Participates in Regulating the Progression of Periodontitis through the Wnt/β-Catenin Signaling Pathway. PLoS ONE 2025, 20, e0330739. [Google Scholar] [CrossRef]
- Cai, R.; Wang, L.; Zhang, W.; Liu, B.; Wu, Y.; Pang, J.; Ma, C. The Role of Extracellular Vesicles in Periodontitis: Pathogenesis, Diagnosis, and Therapy. Front. Immunol. 2023, 14, 1151322. [Google Scholar] [CrossRef]
- Ma, X.; Shin, Y.-J.; Yoo, J.-W.; Park, H.-S.; Kim, D.-H. Extracellular Vesicles Derived from Porphyromonas gingivalis Induce Trigeminal Nerve-Mediated Cognitive Impairment. J. Adv. Res. 2023, 54, 293–303. [Google Scholar] [CrossRef]
- Ha, J.Y.; Seok, J.; Kim, S.-J.; Jung, H.-J.; Ryu, K.-Y.; Nakamura, M.; Jang, I.-S.; Hong, S.-H.; Lee, Y.; Lee, H.-J. Periodontitis Promotes Bacterial Extracellular Vesicle-Induced Neuroinflammation in the Brain and Trigeminal Ganglion. PLoS Pathog. 2023, 19, e1011743. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Cutler, J.; Ivanovski, S.; Lee, R.S.; Han, P. Microbial- and Host Immune Cell-Derived Extracellular Vesicles in the Pathogenesis and Therapy of Periodontitis: A Narrative Review. J. Periodontal Res. 2024, 59, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Condor, A.-M.; Kui, A.I.; Buduru, S.D.; Negucioiu, M.; Condor, D.C.; Lucaciu, P.-O. Metabolomics Analysis as a Tool in Periodontitis Diagnosis: A Systematic Review. Clin. Exp. Dent. Res. 2025, 11, e70095. [Google Scholar] [CrossRef]
- Dekaboruah, E.; Suryavanshi, M.V.; Chettri, D.; Verma, A.K. Human Microbiome: An Academic Update on Human Body Site Specific Surveillance and Its Possible Role. Arch. Microbiol. 2020, 202, 2147–2167. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Sahni, V.; Buduneli, N.; Gupta, S.; Räisänen, I.T.; Golub, L.M.; Lee, H.-M.; Pätilä, T.; Bostanci, N.; Meurman, J.; et al. Active Matrix Metalloproteinase-8 (AMMP-8) Point-of-Care Test (POCT) in the COVID-19 Pandemic. Expert Rev. Proteom. 2021, 18, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Abdulhadi, Z.T.; Mahdee, A.F.; Gul, S.S. Accuracy of Gingival Crevicular Fluid Biomarkers of MMP8, TIMP1, RANK, RANKL, and OPG in Differentiating Symptomatic and Asymptomatic Apical Periodontitis. Diagnostics 2024, 14, 1872. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the Human Oral Microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef]
- Krishnan, K.; Chen, T.; Paster, B.J. A Practical Guide to the Oral Microbiome and Its Relation to Health and Disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and The Unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Deandra, F.A.; Ketherin, K.; Rachmasari, R.; Sulijaya, B.; Takahashi, N. Probiotics and Metabolites Regulate the Oral and Gut Microbiome Composition as Host Modulation Agents in Periodontitis: A Narrative Review. Heliyon 2023, 9, e13475. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, R.; Asopa, S.J.; Joseph, M.D.; Singh, B.; Rajguru, J.P.; Saidath, K.; Sharma, U. Red Complex: Polymicrobial Conglomerate in Oral Flora: A Review. J. Fam. Med. Prim. Care 2019, 8, 3480–3486. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Song, B.; Brandt, B.W.; Cheng, L.; Zhou, X.; Exterkate, R.A.M.; Crielaard, W.; Deng, D.M. Comparison of Red-Complex Bacteria Between Saliva and Subgingival Plaque of Periodontitis Patients: A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2021, 11, 727732. [Google Scholar] [CrossRef]
- Chigasaki, O.; Aoyama, N.; Sasaki, Y.; Takeuchi, Y.; Mizutani, K.; Ikeda, Y.; Gokyu, M.; Umeda, M.; Izumi, Y.; Iwata, T.; et al. Porphyromonas Gingivalis, the Most Influential Pathogen in Red-Complex Bacteria: A Cross-Sectional Study on the Relationship between Bacterial Count and Clinical Periodontal Status in Japan. J. Periodontol. 2021, 92, 1719–1729. [Google Scholar] [CrossRef]
- Colombo, A.P.V.; Bennet, S.; Cotton, S.L.; Goodson, J.M.; Kent, R.; Haffajee, A.D.; Socransky, S.S.; Hasturk, H.; Van Dyke, T.E.; Dewhirst, F.E.; et al. Impact of Periodontal Therapy on the Subgingival Microbiota of Severe Periodontitis: Comparison between Good Responders and Individuals with Refractory Periodontitis Using the Human Oral Microbe Identification Microarray. J. Periodontol. 2012, 83, 1279–1287. [Google Scholar] [CrossRef]
- Teughels, W.; Durukan, A.; Ozcelik, O.; Pauwels, M.; Quirynen, M.; Haytac, M.C. Clinical and Microbiological Effects of Lactobacillus Reuteri Probiotics in the Treatment of Chronic Periodontitis: A Randomized Placebo-Controlled Study. J. Clin. Periodontol. 2013, 40, 1025–1035. [Google Scholar] [CrossRef]
- Tambur, Z.; Miljković-Selimović, B.; Opačić, D.; Vuković, B.; Malešević, A.; Ivančajić, L.; Aleksić, E. Inhibitory Effects of Propolis and Essential Oils on Oral Bacteria. J. Infect. Dev. Ctries. 2021, 15, 1027–1031. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L.J. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.T.; Kreth, J.; Soundarajan, M.; Sivuilu, L.S. Influence of a Model Human Defensive Peroxidase System on Oral Streptococcal Antagonism. Microbiology 2009, 155, 3691–3700. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Beyond the Red Complex and into More Complexity: The Polymicrobial Synergy and Dysbiosis (PSD) Model of Periodontal Disease Etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef]
- Kumar, P.S. Microbiomics: Were We All Wrong Before? Periodontology 2000 2021, 85, 8–11. [Google Scholar] [CrossRef]
- Cafferata, E.A.; Alvarez, C.; Diaz, K.T.; Maureira, M.; Monasterio, G.; González, F.E.; Covarrubias, C.; Vernal, R. Multifunctional Nanocarriers for the Treatment of Periodontitis: Immunomodulatory, Antimicrobial, and Regenerative Strategies. Oral Dis. 2019, 25, 1866–1878. [Google Scholar] [CrossRef]
- Tacheau, C.; Weisgerber, F.; Fagot, D.; Bastien, P.; Verdier, M.P.; Liboutet, M.; Sore, G.; Bernard, B.A. Vichy Thermal Spring Water (VTSW), a Cosmetic Ingredient of Potential Interest in the Frame of Skin Ageing Exposome: An in Vitro Study. Int. J. Cosmet. Sci. 2018, 40, 377–387. [Google Scholar] [CrossRef]
- Hurtado, R.; Peltroche, N.; Mauricio, F.; Gallo, W.; Alvítez-Temoche, D.; Vilchez, L.; Mayta-Tovalino, F. Antifungal Efficacy of Four Different Concentrations of the Essential Oil of Cinnamomum zeylanicum (Canela) against Candida albicans: An In Vitro Study. J. Int. Soc. Prev. Community Dent. 2020, 10, 724–730. [Google Scholar] [CrossRef]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Chávez-González, M.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Chapter 11—Essential Oils: A Natural Alternative to Combat Antibiotics Resistance; Kon, K., Rai, M.B.T.-A.R., Eds.; Academic Press: New York, NY, USA, 2016; pp. 227–237. ISBN 978-0-12-803642-6. [Google Scholar]
- Patil, S.; Albogami, S.; Hosmani, J.; Mujoo, S.; Kamil, M.A.; Mansour, M.A.; Abdul, H.N.; Bhandi, S.; Ahmed, S.S.S.J. Artificial Intelligence in the Diagnosis of Oral Diseases: Applications and Pitfalls. Diagnostics 2022, 12, 1029. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, X.; Liu, M.; Deng, K.; Tullini, A.; Zhang, X.; Shi, J.; Lai, H.; Tonetti, M.S. Enhanced Control of Periodontitis by an Artificial Intelligence-Enabled Multimodal-Sensing Toothbrush and Targeted MHealth Micromessages: A Randomized Trial. J. Clin. Periodontol. 2024, 51, 1632–1643. [Google Scholar] [CrossRef]
- Aji, N.R.A.S.; Sahni, V.; Penttala, M.T.; Sakellari, D.; Grigoriadis, A.; Pätilä, T.; Pärnänen, P.; Neefs, D.; Pfützner, A.; Gupta, S.; et al. Oral Medicine and Oral Clinical Chemistry Game Changers for Future Plaque Control and Maintenance: PerioSafe(®) AMMP-8 POCT, Lumoral(®) 2× PDT- and Lingora(®) Fermented Lingonberry Oral Rinse-Treatments. Dent. J. 2025, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, P.; Jin, Y.; Ghuman, M.; Shoaie, S.; Spratt, D.; Troiano, G.; Nibali, L. Microbiological and Molecular Profile of Furcation Defects in a Population with Untreated Periodontitis. J. Clin. Periodontol. 2024, 51, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zheng, J.; Lu, Y.; Lin, P.; Lin, Y.; Zheng, Y.; Xu, R.; Mai, Z.; Guo, B.; Zhao, X. New Frontiers in Salivary Extracellular Vesicles: Transforming Diagnostics, Monitoring, and Therapeutics in Oral and Systemic Diseases. J. Nanobiotechnol. 2024, 22, 171. [Google Scholar] [CrossRef]
- Morrison, A.G.; Sarkar, S.; Umar, S.; Lee, S.T.M.; Thomas, S.M. The Contribution of the Human Oral Microbiome to Oral Disease: A Review. Microorganisms 2023, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.J.; Silva, W.O.; Romeiro, K.; Gominho, L.F.; Alves, F.R.F.; Rôças, I.N. Apical Root Canal Microbiome Associated with Primary and Posttreatment Apical Periodontitis: A Systematic Review. Int. Endod. J. 2024, 57, 1043–1058. [Google Scholar] [CrossRef]
- Helliwell, E.; Choi, D.; Merritt, J.; Kreth, J. Environmental Influences on Streptococcus sanguinis Membrane Vesicle Biogenesis. ISME J. 2023, 17, 1430–1444. [Google Scholar] [CrossRef]
- Tran, P.L.; Luth, K.; Wang, J.; Ray, C.; de Souza, A.; Mehta, D.; Moeller, K.W.; Moeller, C.D.; Reid, T.W. Efficacy of a Silver Colloidal Gel against Selected Oral Bacteria in Vitro [Version 1; Peer Review: 2 Approved]. F1000Research 2019, 8, 267. [Google Scholar] [CrossRef]
- Ramos Meyers, G.; Samouda, H.; Bohn, T. Short Chain Fatty Acid Metabolism in Relation to Gut Microbiota and Genetic Variability. Nutrients 2022, 14, 5361. [Google Scholar] [CrossRef]
- Bhavikatti, S.K.; Zainuddin, S.L.A.; Ramli, R.B.; Nadaf, S.J.; Dandge, P.B.; Khalate, M.; Karobari, M.I. Insights into the Antioxidant, Anti-Inflammatory and Anti-Microbial Potential of Nigella Sativa Essential Oil against Oral Pathogens. Sci. Rep. 2024, 14, 11878. [Google Scholar] [CrossRef]
- Mailoa, J.; Lin, G.-H.; Khoshkam, V.; MacEachern, M.; Chan, H.-L.; Wang, H.-L. Long-Term Effect of Four Surgical Periodontal Therapies and One Non-Surgical Therapy: A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 1150–1158. [Google Scholar] [CrossRef]
- Zaura, E.; Nicu, E.A.; Krom, B.P.; Keijser, B.J.F. Acquiring and Maintaining a Normal Oral Microbiome: Current Perspective. Front. Cell. Infect. Microbiol. 2014, 4, 85. [Google Scholar] [CrossRef]
- Aji, N.R.A.S.; Räisänen, I.T.; Rathnayake, N.; Lundy, F.T.; Mc Crudden, M.T.C.; Goyal, L.; Sorsa, T.; Gupta, S. AMMP-8 POCT vs. Other Potential Biomarkers in Chair-Side Diagnostics and Treatment Monitoring of Severe Periodontitis. Int. J. Mol. Sci. 2024, 25, 9421. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Ding, X.; Nagarajan, R.; Dawson, D.R., 3rd; Ebersole, J.L. Biomarker Panel Discriminates Diabetics with and without Periodontitis Pre- and Post-Therapy. J. Periodontal Res. 2023, 58, 493–502. [Google Scholar] [CrossRef]
- Brandt, E.; Keskin, M.; Tervahartiala, T.; Yılmaz, M.; Harmankaya, İ.; Karaçetin, D.; İpek, T.; Gürsoy, U.K.; Rautava, J.; Gupta, S.; et al. Radiotherapy Increases AMMP-8-Levels and Neutrophil/Lymphocyte Ratio Rapidly in Head and Neck Cancer Patients: A Pilot Study. Cancer Control 2023, 30, 10732748231163652. [Google Scholar] [CrossRef]
- Pozhitkov, A.E.; Beikler, T.; Flemmig, T.; Noble, P.A. High-Throughput Methods for Analysis of the Human Oral Microbiome. Periodontology 2000 2011, 55, 70–86. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, J.; Song, Y.; Raka, R.N.; Xiang, J.; Wu, H.; Xiao, J.; Jin, J.; Hui, X. Antibacterial Activity of Oregano Essential Oils against Streptococcus Mutans in Vitro and Analysis of Active Components. BMC Complement. Med. Ther. 2023, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Bonny, T.; Al Nassan, W.; Obaideen, K.; Al Mallahi, M.N.; Mohammad, Y.; El-Damanhoury, H.M. Contemporary Role and Applications of Artificial Intelligence in Dentistry. F1000Research 2023, 12, 1179. [Google Scholar] [CrossRef]
- Ahmed, N.; Abbasi, M.S.; Zuberi, F.; Qamar, W.; Bin Halim, M.S.; Maqsood, A.; Alam, M.K. Artificial Intelligence Techniques: Analysis, Application, and Outcome in Dentistry—A Systematic Review. BioMed Res. Int. 2021, 2021, 9751564. [Google Scholar] [CrossRef]
- Kourounis, G.; Elmahmudi, A.A.; Thomson, B.; Hunter, J.; Ugail, H.; Wilson, C. Computer Image Analysis with Artificial Intelligence: A Practical Introduction to Convolutional Neural Networks for Medical Professionals. Postgrad. Med. J. 2023, 99, 1287–1294. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, S.; Zou, T.; Jin, Z.; Jiang, S. Artificial Intelligence Models for Periodontitis Classification: A Systematic Review. J. Dent. 2025, 156, 105690. [Google Scholar] [CrossRef]
- Polizzi, A.; Quinzi, V.; Lo Giudice, A.; Marzo, G.; Leonardi, R.; Isola, G. Accuracy of Artificial Intelligence Models in the Prediction of Periodontitis: A Systematic Review. JDR Clin. Transl. Res. 2024, 9, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Dhopte, A.; Bagde, H. Smart Smile: Revolutionizing Dentistry with Artificial Intelligence. Cureus 2023, 15, e41227. [Google Scholar] [CrossRef]
- Hu, H.; Leung, W.K. Mass Spectrometry-Based Proteomics for Discovering Salivary Biomarkers in Periodontitis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 14599. [Google Scholar] [CrossRef]
- Nie, Q.; Wan, X.; Tao, H.; Yang, Q.; Zhao, X.; Liu, H.; Hu, J.; Luo, Y.; Shu, T.; Geng, R.; et al. Multi-Function Screening of Probiotics to Improve Oral Health and Evaluating Their Efficacy in a Rat Periodontitis Model. Front. Cell. Infect. Microbiol. 2023, 13, 1261189. [Google Scholar] [CrossRef]
- Woolery-Lloyd, H.; Andriessen, A.; Day, D.; Gonzalez, N.; Green, L.; Grice, E.; Henry, M. Review of the Microbiome in Skin Aging and the Effect of a Topical Prebiotic Containing Thermal Spring Water. J. Cosmet. Dermatol. 2023, 22, 96–102. [Google Scholar] [CrossRef]
- Baddouri, L.; Hannig, M. Probiotics as an Adjunctive Therapy in Periodontitis Treatment-Reality or Illusion-a Clinical Perspective. npj Biofilms Microbiomes 2024, 10, 148. [Google Scholar] [CrossRef]
- Radu, C.-M.; Radu, C.C.; Bochiș, S.-A.; Arbănași, E.M.; Lucan, A.I.; Murvai, V.R.; Zaha, D.C. Revisiting the Therapeutic Effects of Essential Oils on the Oral Microbiome. Pharmacy 2023, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Beresescu, G.; Bereczki-Temistocle, D.L.; Beresescu, L.; Ormenisan, A.; Monea, A.; Razvan-Marius, I. Effectiveness of an Essential Oil Mouthwash on Halitosis in Obese Patients with Periodontitis: A Short-Term Clinical Evaluation. J. Clin. Med. 2025, 14, 5225. [Google Scholar] [CrossRef] [PubMed]
- Potra Cicalău, G.I.; Vicaș, L.G.; Ciavoi, G.; Ghitea, T.C.; Csaba, N.; Cristea, R.A.; Miere Groza, F.; Ganea, M. A Natural Approach to the Prevention and Treatment of Gingivitis and Periodontitis: A Review of Pomegranate’s Bioactive Properties. Life 2024, 14, 1298. [Google Scholar] [CrossRef]
- Radu, C.-M.; Radu, C.C.; Arbănaşi, E.-M.; Hogea, T.; Murvai, V.R.; Chiș, I.-A.; Zaha, D.C. Exploring the Efficacy of Novel Therapeutic Strategies for Periodontitis: A Literature Review. Life 2024, 14, 468. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; Calvo-Irabien, L.M.; Dzul-Beh, A.D.; Dzib-Baak, H.E.; Grijalva-Arango, R.; Molina-Salinas, G.M. Potential Anti-Infectious Activity of Essential Oil Chemotypes of Lippia Origanoides Kunth on Antibiotic-Resistant Staphylococcus aureus Strains. Plants 2024, 13, 1172. [Google Scholar] [CrossRef]
- Yuan, Y.; Hui, X.; Liu, Z.; Sun, J.; Raka, R.N.; Xiao, J.; Zhang, Z.; Wu, H. Investigation of Differential Multi-Mode Antibacterial Mechanisms of Essential Oils of Satureja montana L. and Leptospermum scoparium J.R.Forst. & G.Forst. Against Porphyromonas Gingivalis. BMC Complement. Med. Ther. 2025, 25, 283. [Google Scholar] [CrossRef]
- Hashim, N.T.; Babiker, R.; Priya, S.P.; Mohammed, R.; Chaitanya, N.C.; Padmanabhan, V.; El Bahra, S.; Rahman, M.M.; Gismalla, B.G. Microbial Dynamics in Periodontal Regeneration: Understanding Microbiome Shifts and the Role of Antifouling and Bactericidal Materials: A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 12196–12213. [Google Scholar] [CrossRef] [PubMed]
- Muresan, S.M.C.; Dreanca, A.; Repciuc, C.; Dejescu, C.; Rotar, O.; Pop, R.A.; Pantea, S.; Pall, E.; Ciotlaus, I.; Sarosi, C.; et al. Dental Hydrogels with Essential Oils with Potential Activity in Periodontitis. Appl. Sci. 2023, 13, 1787. [Google Scholar] [CrossRef]
- Salem, S.S.; Elsayed, H.E.; Shabana, S.; Khazaal, M.T.; Moharram, F.A. Phytochemical Profile and Antimicrobial Activity of Essential Oils from Two Syzygium Species against Selected Oral Pathogens. BMC Complement. Med. Ther. 2023, 23, 448. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The Pervasive Effects of an Antibiotic on the Human Gut Microbiota, as Revealed by Deep 16S RRNA Sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Lin, P.; Liu, A.; Tsuchiya, Y.; Noritake, K.; Ohsugi, Y.; Toyoshima, K.; Tsukahara, Y.; Shiba, T.; Nitta, H.; Aoki, A.; et al. Association between Periodontal Disease and Chronic Obstructive Pulmonary Disease. Jpn. Dent. Sci. Rev. 2023, 59, 389–402. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and Surgical Treatment of Periodontitis: How Many Options for One Disease? Periodontology 2000 2017, 75, 152–188. [Google Scholar] [CrossRef]
- Aimetti, M. Nonsurgical Periodontal Treatment. Int. J. Esthet. Dent. 2014, 9, 251–267. [Google Scholar]
- Gad, M.M.; Fouda, S.M. Current Perspectives and the Future of Candida Albicans-Associated Denture Stomatitis Treatment. Dent. Med. Probl. 2020, 57, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tao, C.; Goh, C.; Shrestha, A. Innovative Biomaterials for the Treatment of Periodontal Disease. Front. Dent. Med. 2023, 4, 1163562. [Google Scholar] [CrossRef] [PubMed]
- Špiljak, B.; Ozretić, P.; Andabak Rogulj, A.; Lončar Brzak, B.; Brailo, V.; Škerlj, M.; Vidović Juras, D. Oral Microbiome Research in Biopsy Samples of Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma and Its Challenges. Appl. Sci. 2024, 14, 11405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).