The Effect of the Clinical-Pathological CPS+EG Staging System on Survival Outcomes in Patients with HER2-Positive Breast Cancer Receiving Neoadjuvant Treatment: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
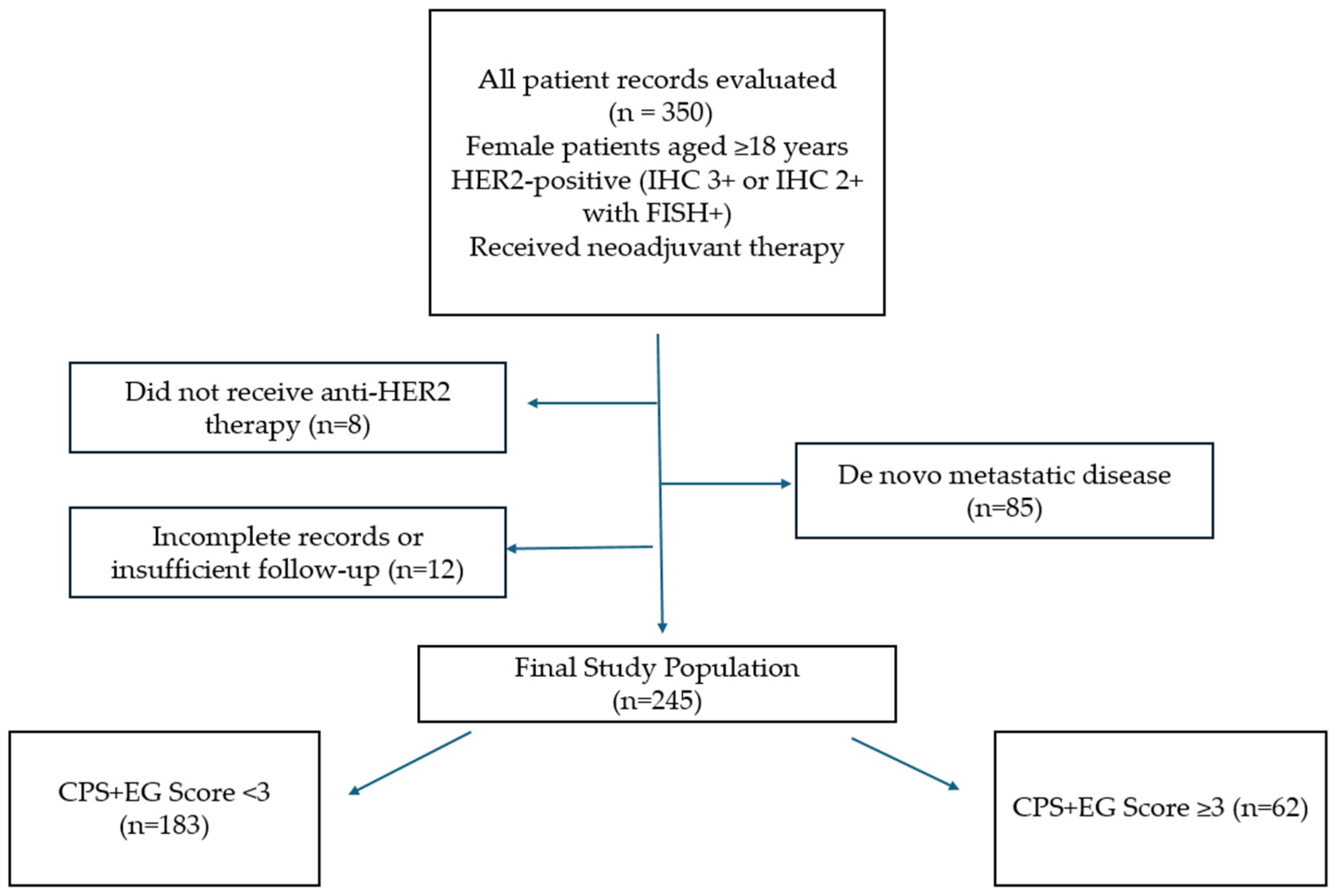
2.1. Patient Population
2.2. Data Collection and Work Termination Points
2.3. Treatment Protocols and Surgery
2.4. Scoring System
- -
- Low-risk group: CPS+EG < 3
- -
- High-risk group: CPS+EG ≥ 3
2.5. Statistical Analysis
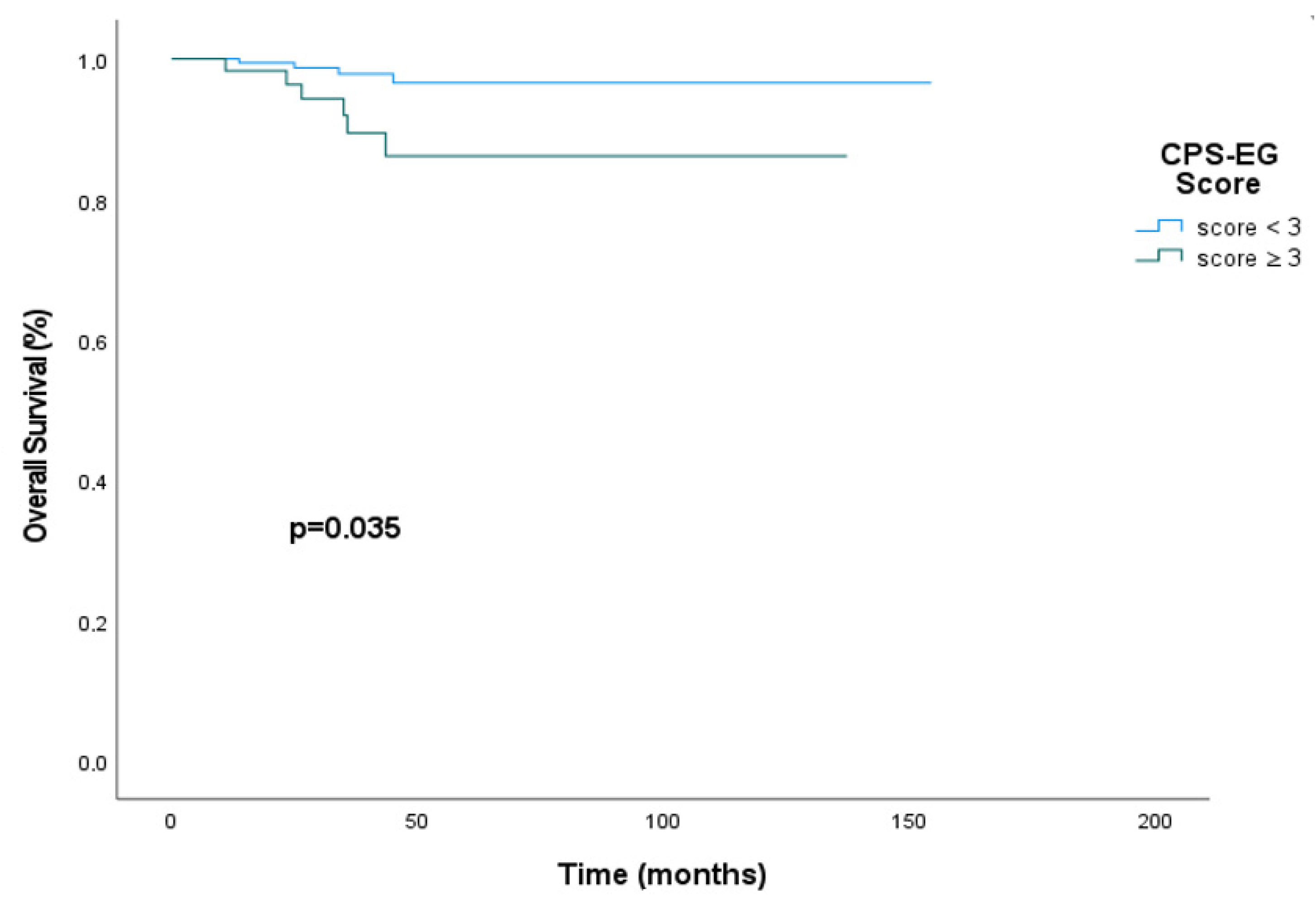
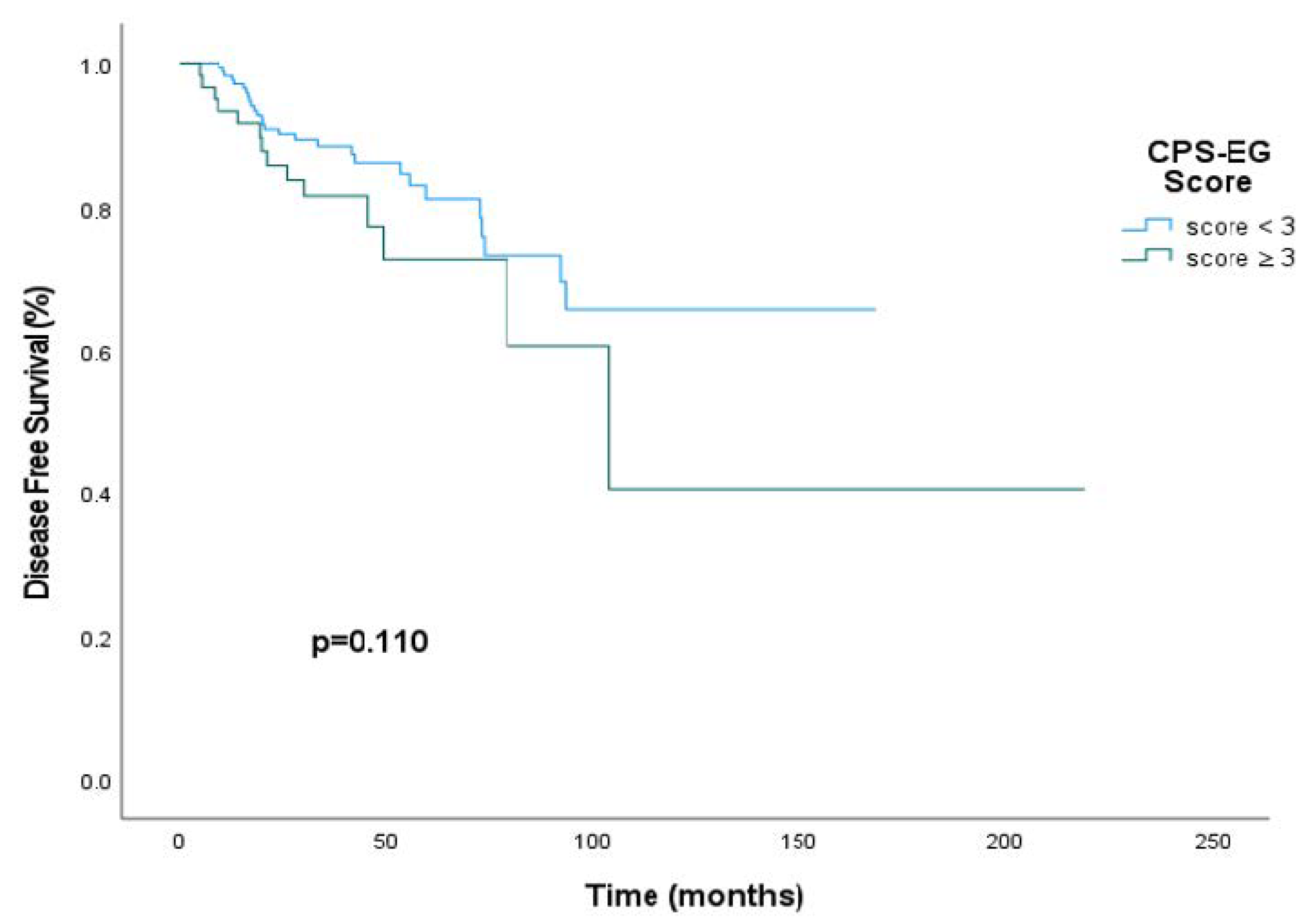
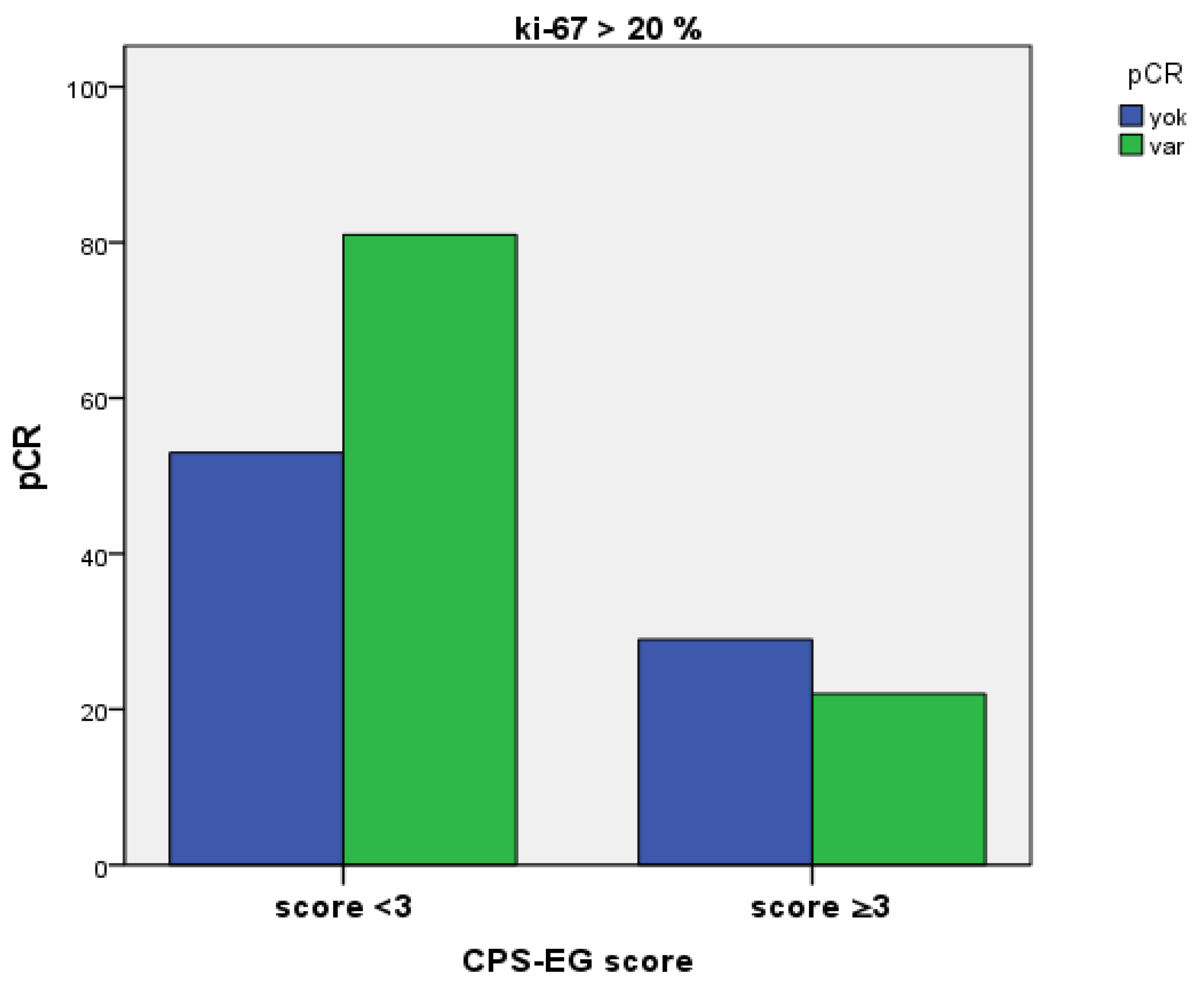
3. Results
4. Discussion
4.1. Limitations
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NACT | Neoadjuvant Chemotherapy |
| HER2 | Human epidermal growth factor receptor 2 |
| CPS+EG | Clinical and pathologic stage, estrogen receptor status, and histologic grade |
| FISH | Fluorescence in situ hybridisation |
| DCIS | Ductal carcinoma in situ |
| pCR | Pathological complete response |
| ITC | Isolated tumour cells |
| ER | Estrogen receptor |
| ctDNA | circulating tumour DNA |
| TILs | tumour-infiltrating lymphocytes |
| IHC | Immunohistochemistry |
| HP | Trastuzumab + Pertuzumab |
| ASCO | American Society of Clinical Oncology |
| CAP | College of American Pathologists |
| AC | Doxorubicin and Cyclophosphamide |
| OS | Overall survival |
| DFS | Disease-free survival |
| HR | Hazard ratio |
| CI | Confidence interval |
| RCB | Residual Cancer Burden |
| T-DM1 | Trastuzumab emtansine |
| AJCC | American Joint Committee on Cancer |
References
- Mercogliano, M.F.; Bruni, S.; Mauro, F.L.; Schillaci, R. Emerging targeted therapies for HER2-positive breast cancer. Cancers 2023, 15, 1987. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Toi, M. Neoadjuvant treatment for HER2-positive breast cancer. Chin. Clin. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, S.; Wu, C.; Fei, X.; Shen, K.; Chen, X. HER2-low status improves prognosis prediction in breast cancer patients receiving neoadjuvant treatment: A comparison of pathological stage, modified CPS+ EG scoring system, and Neo-Bioscore. Chin. J. Cancer Res. 2024, 36, 729. [Google Scholar] [CrossRef] [PubMed]
- Marmé, F.; Solbach, C.; Michel, L.; Schneeweiss, A.; Blohmer, J.-U.; Huober, J.; Fasching, P.A.; Jackisch, C.; Nekljudova, V.; Link, T. Utility of the CPS+ EG scoring system in triple-negative breast cancer treated with neoadjuvant chemotherapy. Eur. J. Cancer 2021, 153, 203–212. [Google Scholar] [CrossRef]
- Öner, İ.; Türkel, A.; İnci, B.K.; Tolunay, P.K.; Ateş, Ö.; Karaçin, C. Impact of the CPS-EG score as a new prognostic biomarker in triple-negative breast cancer patients who received neoadjuvant chemotherapy. BMC Cancer 2024, 24, 1338. [Google Scholar] [CrossRef]
- Roussot, N.; Constantin, G.; Desmoulins, I.; Bergeron, A.; Arnould, L.; Beltjens, F.; Mayeur, D.; Kaderbhai, C.; Hennequin, A.; Jankowski, C. Prognostic stratification ability of the CPS+EG scoring system in HER2-low and HER2-zero early breast cancer treated with neoadjuvant chemotherapy. Eur. J. Cancer 2024, 202, 114037. [Google Scholar] [CrossRef]
- Carey, L.A.; Metzger, R.; Dees, E.C.; Collichio, F.; Sartor, C.I.; Ollila, D.W.; Klauber-DeMore, N.; Halle, J.; Sawyer, L.; Moore, D.T. American joint committee on cancer tumor–node–metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J. Natl. Cancer Inst. 2005, 97, 1137–1142. [Google Scholar] [CrossRef]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Buzdar, A.U.; Ibrahim, N.K.; Francis, D.; Booser, D.J.; Thomas, E.S.; Theriault, R.L.; Pusztai, L.; Green, M.C.; Arun, B.K.; Giordano, S.H. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2–positive operable breast cancer. J. Clin. Oncol. 2005, 23, 3676–3685. [Google Scholar] [CrossRef]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Manikhas, A.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Vazquez, F.; Byakhow, M.; Lichinitser, M. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010, 375, 377–384. [Google Scholar]
- Mittendorf, E.A.; Wu, Y.; Scaltriti, M.; Meric-Bernstam, F.; Hunt, K.K.; Dawood, S.; Esteva, F.J.; Buzdar, A.U.; Chen, H.; Eksambi, S. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin. Cancer Res. 2009, 15, 7381–7388. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Jeruss, J.S.; Tucker, S.L.; Kolli, A.; Newman, L.A.; Gonzalez-Angulo, A.M.; Buchholz, T.A.; Sahin, A.A.; Cormier, J.N.; Buzdar, A.U. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J. Clin. Oncol. 2011, 29, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Jeruss, J.S.; Mittendorf, E.A.; Tucker, S.L.; Gonzalez-Angulo, A.M.; Buchholz, T.A.; Sahin, A.A.; Cormier, J.N.; Buzdar, A.U.; Hortobagyi, G.N.; Hunt, K.K. Staging of breast cancer in the neoadjuvant setting. Cancer Res. 2008, 68, 6477–6481. [Google Scholar] [CrossRef] [PubMed]
- Marmé, F.; Lederer, B.; Blohmer, J.-U.; Costa, S.D.; Denkert, C.; Eidtmann, H.; Gerber, B.; Hanusch, C.; Hilfrich, J.; Huober, J. Utility of the CPS+ EG staging system in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with neoadjuvant chemotherapy. Eur. J. Cancer 2016, 53, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Jeruss, J.S.; Mittendorf, E.A.; Tucker, S.L.; Gonzalez-Angulo, A.M.; Buchholz, T.A.; Sahin, A.A.; Cormier, J.N.; Buzdar, A.U.; Hortobagyi, G.N.; Hunt, K.K. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J. Clin. Oncol. 2008, 26, 246–252. [Google Scholar] [CrossRef]
- Choi, M.; Park, Y.H.; Ahn, J.S.; Im, Y.-H.; Nam, S.J.; Cho, S.Y.; Cho, E.Y. Assessment of pathologic response and long-term outcome in locally advanced breast cancers after neoadjuvant chemotherapy: Comparison of pathologic classification systems. Breast Cancer Res. Treat. 2016, 160, 475–489. [Google Scholar] [CrossRef]
- Symmans, W.F.; Wei, C.; Gould, R.; Yu, X.; Zhang, Y.; Liu, M.; Walls, A.; Bousamra, A.; Ramineni, M.; Sinn, B. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J. Clin. Oncol. 2017, 35, 1049–1060. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Vila, J.; Tucker, S.L.; Chavez-MacGregor, M.; Smith, B.D.; Symmans, W.F.; Sahin, A.A.; Hortobagyi, G.N.; Hunt, K.K. The neo-bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: Incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol. 2016, 2, 929–936. [Google Scholar] [CrossRef]
- Geyer, C., Jr.; Garber, J.; Gelber, R.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G. OlympiA Clinical Trial Steering Committee and Investigators. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Marmé, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.-B.; Bear, H.; McCarthy, N.; Melé Olivé, M.; Gelmon, K. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—The penelope-B trial. J. Clin. Oncol. 2021, 39, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Marmé, F.; Hanusch, C.; Furlanetto, J.; Morris, P.; Link, T.; Denkert, C.; Fasching, P.A.; Jackisch, C.; Antolín, S.; Solbach, C. 58O Safety interim analysis (SIA) of the phase III postneoadjuvant SASCIA study evaluating sacituzumab govitecan (SG) in patients with primary HER2-negative breast cancer (BC) at high relapse risk after neoadjuvant treatment. Ann. Oncol. 2022, 33, S148–S149. [Google Scholar] [CrossRef]
- Xu, L.; Duan, X.; Zhou, B.; Liu, Y.; Ye, J.; Liu, Z.; Ma, C.; Zhang, H.; Zhang, S.; Zhang, L. Validation of the CPS+ EG and Neo-Bioscore staging systems after preoperative systemic therapy for breast cancer in a single center in China. Breast 2018, 40, 29–37. [Google Scholar] [CrossRef]
- van den Ende, N.S.; Nguyen, A.H.; Jager, A.; Kok, M.; Debets, R.; van Deurzen, C.H. Triple-negative breast cancer and predictive markers of response to neoadjuvant chemotherapy: A systematic review. Int. J. Mol. Sci. 2023, 24, 2969. [Google Scholar] [CrossRef]
- Turkoglu, E.; Akdag Topal, G.; Yildirim, S.; Kinikoglu, O.; Sariyar Busery, N.; Aydogan, M.; Yildiz, H.S.; Orman, S.; Bayramgil, A.; Gunes, T.K. Comparison of paclitaxel and docetaxel in dual HER2 blockade: Efficacy and safety in neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res. Treat. 2025, 211, 743–752. [Google Scholar] [CrossRef]
- Geyer, C.E., Jr.; Untch, M.; Huang, C.-S.; Mano, M.S.; Mamounas, E.P.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; Fischer, H.H. Survival with trastuzumab emtansine in residual HER2-positive breast cancer. N. Engl. J. Med. 2025, 392, 249–257. [Google Scholar] [CrossRef]
- Allred, D.C.; Wu, Y.; Mao, S.; Nagtegaal, I.D.; Lee, S.; Perou, C.M.; Mohsin, S.K.; O’COnnell, P.; Tsimelzon, A.; Medina, D. Ductal carcinoma in situ and the emergence of diversity during breast cancer evolution. Clin. Cancer Res. 2008, 14, 370–378. [Google Scholar] [CrossRef]
- Lesurf, R.; Aure, M.R.; Mørk, H.H.; Vitelli, V.; Lundgren, S.; Børresen-Dale, A.-L.; Kristensen, V.; Wärnberg, F.; Hallett, M.; Sørlie, T.; et al. Molecular features of subtype-specific progression from ductal carcinoma in situ to invasive breast cancer. Cell Rep. 2016, 16, 1166–1179. [Google Scholar] [CrossRef]
- Kerlikowske, K.; Molinaro, A.M.; Gauthier, M.L.; Berman, H.K.; Waldman, F.; Bennington, J.; Sanchez, H.; Jimenez, C.; Stewart, K.; Chew, K.; et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J. Natl. Cancer Inst. 2010, 102, 627–637. [Google Scholar] [CrossRef]
- Marmé, F.; Aigner, J.; Lorenzo Bermejo, J.; Sinn, P.; Sohn, C.; Jäger, D.; Schneeweiss, A. Neoadjuvant epirubicin, gemcitabine and docetaxel for primary breast cancer: Long-term survival data and major prognostic factors based on two consecutive neoadjuvant phase I/II trials. Int. J. Cancer 2013, 133, 1006–1015. [Google Scholar] [CrossRef]
- Petrelli, F.; Viale, G.; Cabiddu, M.; Barni, S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: A systematic review and meta-analysis of 64,196 patients. Breast Cancer Res. Treat. 2015, 153, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Calhoun, B.C.; Portier, B.; Wang, Z.; Minca, E.C.; Budd, G.T.; Lanigan, C.; Tubbs, R.R.; Morrison, L.E. MET and PTEN gene copy numbers and Ki-67 protein expression associate with pathologic complete response in ERBB2-positive breast carcinoma patients treated with neoadjuvant trastuzumab-based therapy. BMC Cancer 2016, 16, 695. [Google Scholar] [CrossRef][Green Version]
- Jones, R.L.; Salter, J.; A’Hern, R.; Nerurkar, A.; Parton, M.; Reis-Filho, J.S.; Smith, I.E.; Dowsett, M. Relationship between oestrogen receptor status and proliferation in predicting response and long-term outcome to neoadjuvant chemotherapy for breast cancer. Breast Cancer Res. Treat. 2010, 119, 315–323. [Google Scholar] [CrossRef]
- Tchabashvili, L.; Leivaditis, V.; Kitsou, K.-S.; Papadaki, H.; Argentou, M.-I. The association between chemerin expression in breast cancer cells and aggressiveness. Menopausal Rev. 2025, 24, 152474. [Google Scholar] [CrossRef]
| Variable | Category | Points |
|---|---|---|
| Clinical stage (cStage) | cI | 0 |
| cII | 1 | |
| cIII | 2 | |
| Pathological stage (pStage) | p0–I | 0 |
| pII | 1 | |
| pIII | 2 | |
| Estrogen receptor (ER) status | Positive | 0 |
| Negative | 1 | |
| Nuclear grade | Grade 1–2 | 0 |
| Grade 3 | 1 |
| Variable | Total (n = 245) | CPS+EG Score < 3 (183) | CPS+EG Score ≥ 3 (62) | p-Value |
|---|---|---|---|---|
| Median age (years) | 0.86 | |||
| ≤50 | 117 (47.8%) | 88 (48.1%) | 29 (46.8%) | |
| >50 | 128 (52.2%) | 95 (51.9%) | 33 (53.2%) | |
| Menopausal Status | 0.68 | |||
| Pre/perimenopause | 109 (44.5%) | 80 (43.7%) | 29 (46.8%) | |
| Postmenopause | 136 (55.5%) | 103 (56.3%) | 33 (53.2) | |
| ER status | <0.001 | |||
| Positive | 138 (43.7%) | 124 (67.8%) | 14 (22.6%) | |
| Negative | 107 (56.3%) | 59 (32.2%) | 48 (77.4%) | |
| PR status | <0.001 | |||
| Positive | 113 (46.1%) | 98 (53.6%) | 15 (24.2%) | |
| Negative | 132 (53.9%) | 85 (46.4%) | 47 (75.8%) | |
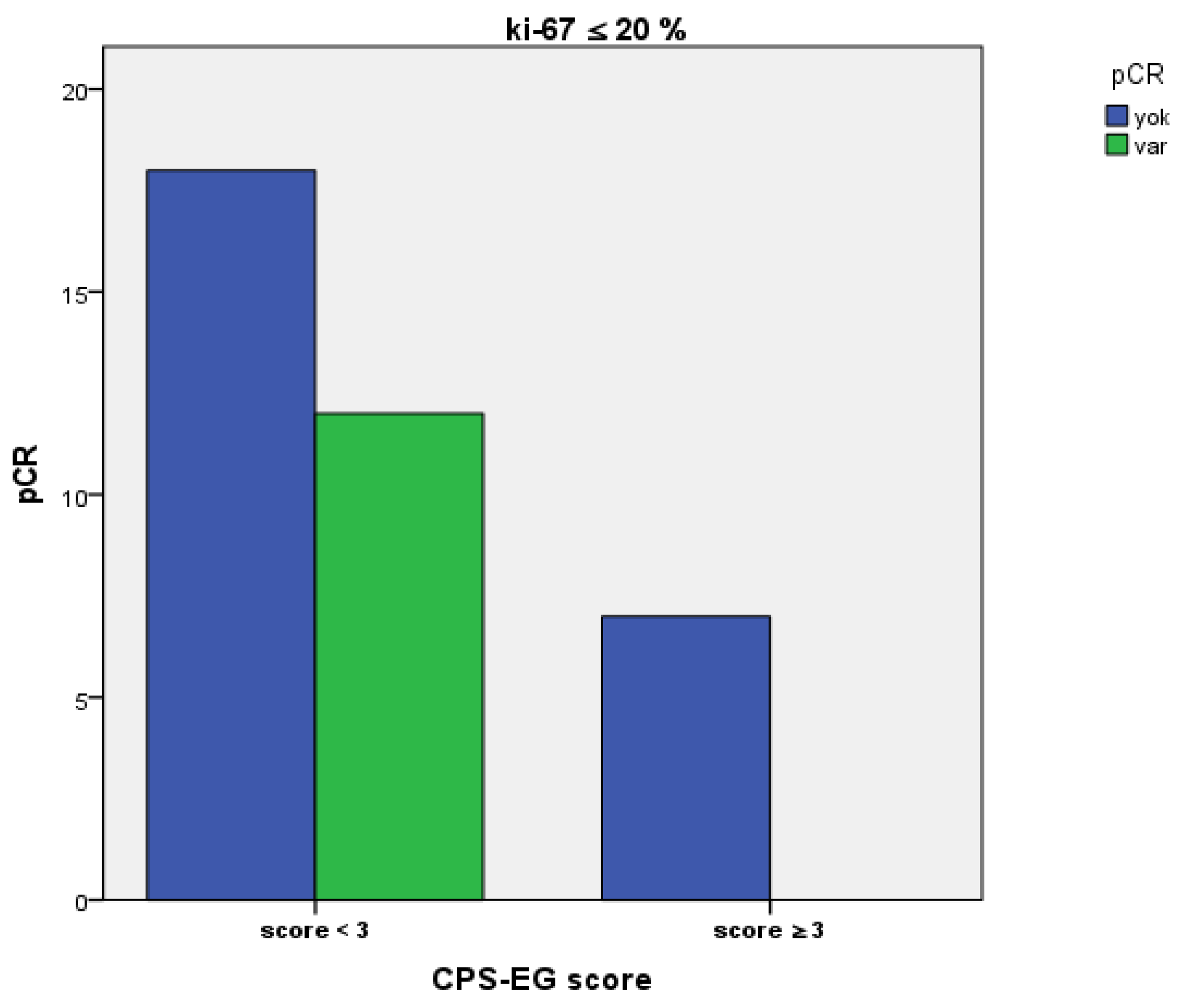
| Pretreatment Ki-67 Status | 0.312 | |||
| ≤20 | 37 (16.7%) | 30 (18.3%) | 7 (12.1%) | |
| >20 | 185 (83.3%) | 134 (81.7%) | 51 (87.9%) | |
| Clinical Stage | <0.01 | |||
| Stage 1 | 17 (7%) | 17 (9.3%) | 0 (0%) | |
| Stage 2 | 170 (69.7%) | 133 (73.1%) | 37 (59.7%) | |
| Stage 3 | 57 (23.4%) | 32 (17.6%) | 25 (40.3) | |
| pCR | 0.017 | |||
| Yes | 127 (51.8%) | 103 (56.3%) | 24 (38.7%) | |
| No | 118 (48.2%) | 80 (43.7%) | 38 (61.3%) | |
| HER2 Status | 0.80 | |||
| Score 2 FISH-positive | 42 (17.1%) | 32 (17.5%) | 10 (16.1%) | |
| 3+ | 203 (82.9%) | 151 (82.5%) | 52 (83.9%) | |
| Clinical N | <0.01 | |||
| 0 | 42 (17.1%) | 41 (22.4%) | 1 (1.6%) | |
| 1 | 156 (63.7%) | 116 (63.4%) | 40 (64.5%) | |
| 2 | 39 (15.9%) | 24 (13.1%) | 15 (24.2%) | |
| 3 | 8 (3.3%) | 2 (1.1%) | 6 (9.7%) | |
| Grade | <0.01 | |||
| 2 | 124 (54.9%) | 114 (68.3%) | 10 (16.9%) | |
| 3 | 102 (45.1%) | 53 (31.7%) | 49 (83.1%) | |
| NACT Type | 0.65 | |||
| AC+HP+Taxan | 150 (61.2%) | 110 (60.1%) | 40 (64.5%) | |
| AC+H+Taxan | 95 (38.8%) | 73 (39.9%) | 22 (35.5%) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR, 95% CI | p Value | HR, 95% CI | p Value | |
Median age (years)
| 1.901 (0.475–7.605) | 0.364 | ||
Menopausal status (%)
| 1.120 (0.300–4.174 | 0.866 | ||
pCR
| 0.333 (0.069–1.607) | 0.171 | 0.558 (0.102–3.056) | 0.501 |
| ER positivity | 0.338 (0.084–1.353) | 0.125 | ||
| PR positivity | 0.335 (0.070–1.612) | 0.172 | ||
Pretreatment ki-67
| 0.445 (0.111–1.782) | 0.253 | 0.349 (0.057–2.136) | 0.255 |
Grade
| 2.150 (0.514–9.001) | 0.295 | 0.566 (0.084–3.830) | 0.559 |
cT stage
| 0.579 (0.192–1.743) | 0.331 | 0.191 (0.036–1.005) | 0.051 |
cN stage
| 0.887 (0.463–1.699) | 0.717 | 1.279 (0.444–3.686) | 0.649 |
Clinical stage
| 0.961 (0.560–1.650) | 0.886 | ||
CPS+EG score
| 4.376 (1.234–15.517) | 0.022 | 0.048 (0.004–0.577) | 0.017 |
| NACT type Dual Anti-HER2 vs. mono Anti-HER2 | 0.650 (0.167–2.528) | 0.534 | ||
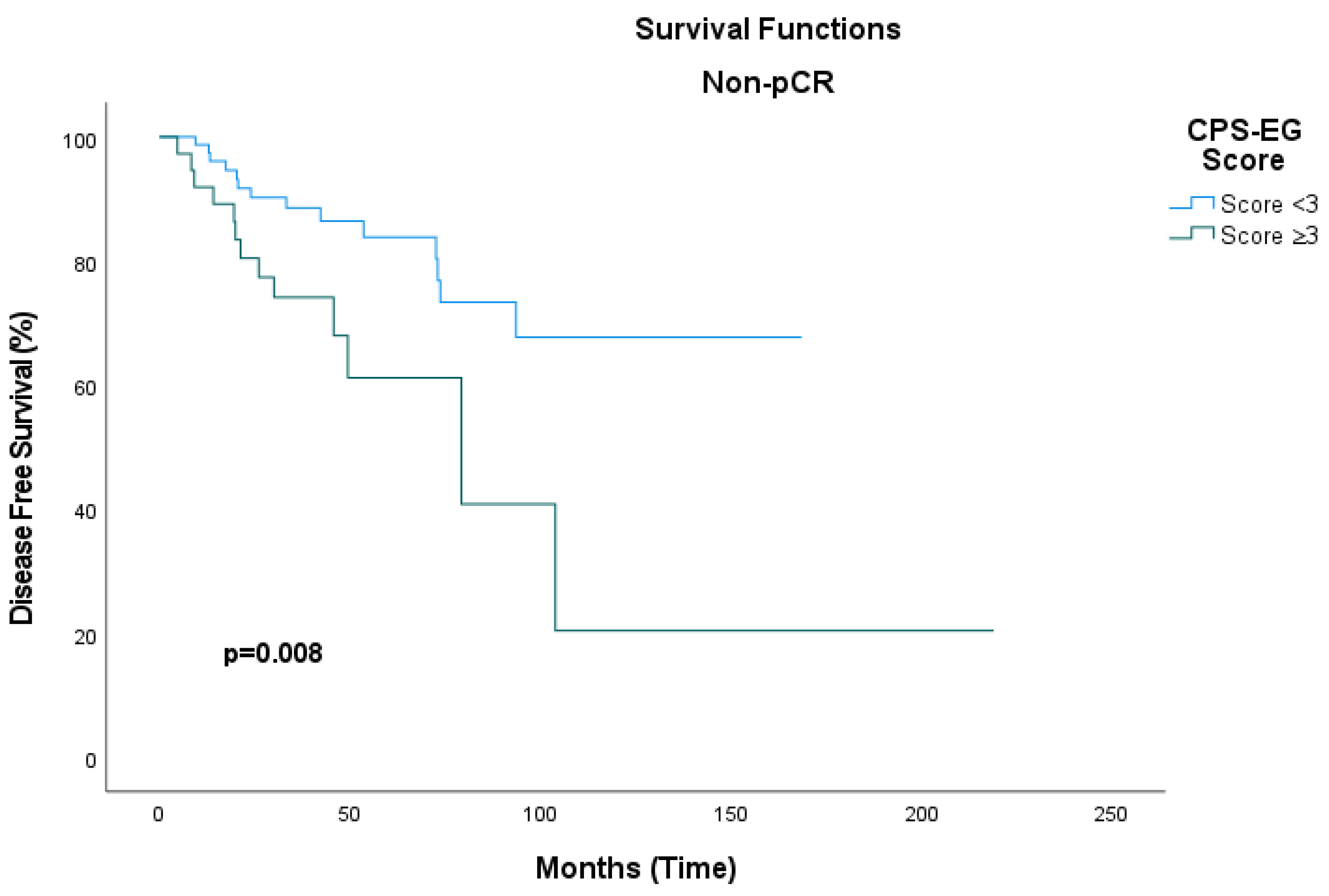
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| CPS+EG Score | 0.60 (0.31–1.13) | 0.114 | 0.35 (0.14–0.86) | 0.023 |
| pCR | 0.66 (0.35–1.25) | 0.198 | 0.30 (0.03–2.67) | 0.281 |
| ER positivity | 0.642 (0.350–1.177) | 0.152 | ||
| PR positivity | 0.576 (0.306–1.087) | 0.089 | ||
Pretreatment ki-67
| 1.50 (0.45–4.95) | 0.508 | 1.14 (0.46–2.80) | 0.783 |
| Grade | 1.16 (0.71–1.92) | 0.554 | 1.33 (0.70–2.51) | 0.382 |
| cT stage | 1.16 (0.78–1.74) | 0.464 | 1.49 (0.81–2.73) | 0.195 |
| cN stage | 0.96 (0.73–1.25) | 0.753 | 1.17 (0.77–1.80) | 0.884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orman, S.; Aydoğan, M.; Kınıkoğlu, O.; Yıldırım, S.; Sarıyar Busery, N.; Yıldız, H.Ş.; Türkoğlu, E.; Kaya, T.; Işık, D.; Ay Ersoy, S.; et al. The Effect of the Clinical-Pathological CPS+EG Staging System on Survival Outcomes in Patients with HER2-Positive Breast Cancer Receiving Neoadjuvant Treatment: A Retrospective Study. Medicina 2025, 61, 1813. https://doi.org/10.3390/medicina61101813
Orman S, Aydoğan M, Kınıkoğlu O, Yıldırım S, Sarıyar Busery N, Yıldız HŞ, Türkoğlu E, Kaya T, Işık D, Ay Ersoy S, et al. The Effect of the Clinical-Pathological CPS+EG Staging System on Survival Outcomes in Patients with HER2-Positive Breast Cancer Receiving Neoadjuvant Treatment: A Retrospective Study. Medicina. 2025; 61(10):1813. https://doi.org/10.3390/medicina61101813
Chicago/Turabian StyleOrman, Seval, Miray Aydoğan, Oğuzcan Kınıkoğlu, Sedat Yıldırım, Nisanur Sarıyar Busery, Hacer Şahika Yıldız, Ezgi Türkoğlu, Tuğba Kaya, Deniz Işık, Seval Ay Ersoy, and et al. 2025. "The Effect of the Clinical-Pathological CPS+EG Staging System on Survival Outcomes in Patients with HER2-Positive Breast Cancer Receiving Neoadjuvant Treatment: A Retrospective Study" Medicina 61, no. 10: 1813. https://doi.org/10.3390/medicina61101813
APA StyleOrman, S., Aydoğan, M., Kınıkoğlu, O., Yıldırım, S., Sarıyar Busery, N., Yıldız, H. Ş., Türkoğlu, E., Kaya, T., Işık, D., Ay Ersoy, S., Odabaş, H., & Turan, N. (2025). The Effect of the Clinical-Pathological CPS+EG Staging System on Survival Outcomes in Patients with HER2-Positive Breast Cancer Receiving Neoadjuvant Treatment: A Retrospective Study. Medicina, 61(10), 1813. https://doi.org/10.3390/medicina61101813






