The Association Between Psoriasis, Psoriatic Arthritis, and Fibromyalgia Syndrome: Effects on Treatment—A Population-Based Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Variables and Measures
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Characteristics of Patients with Psoriasis and Fibromyalgia
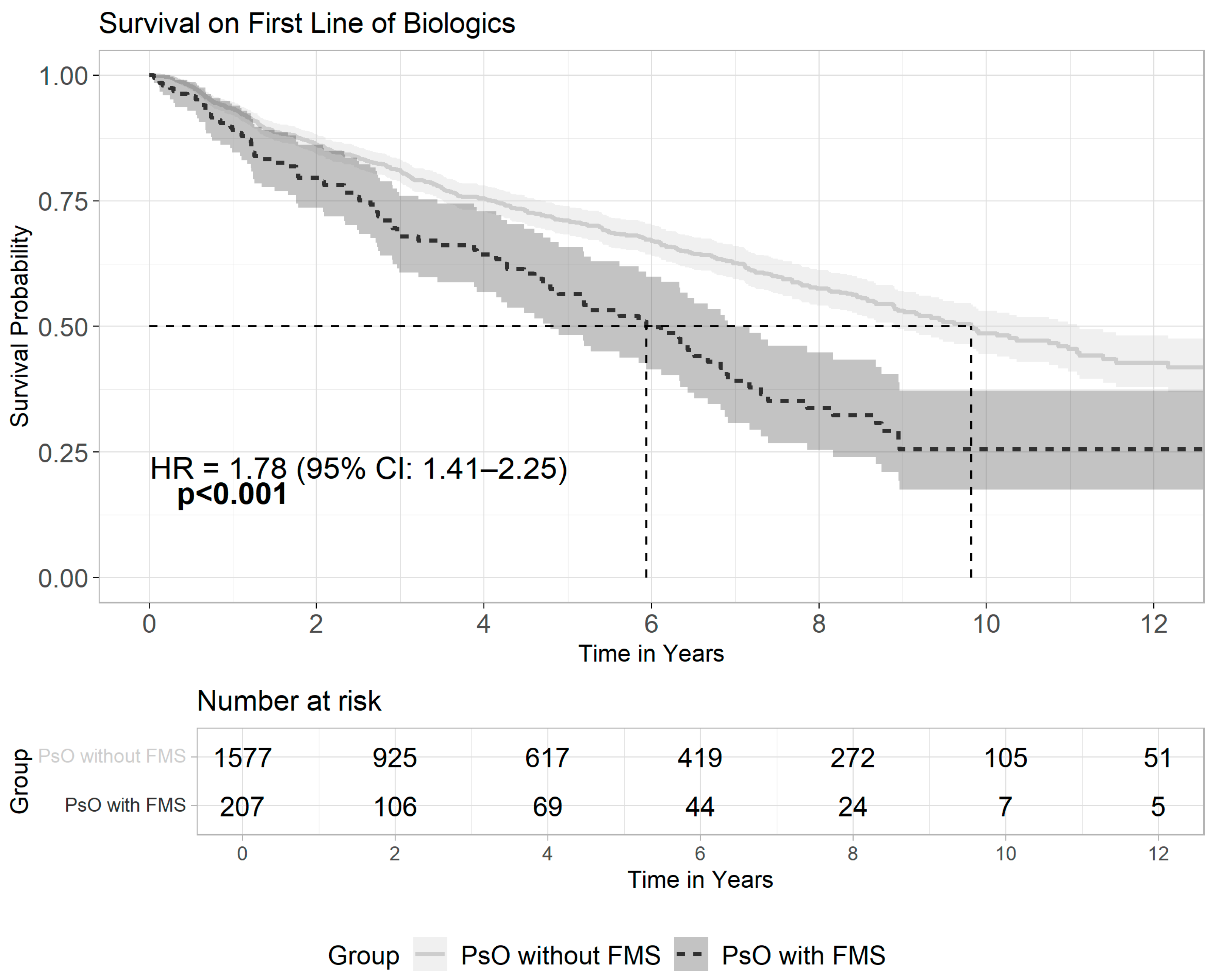
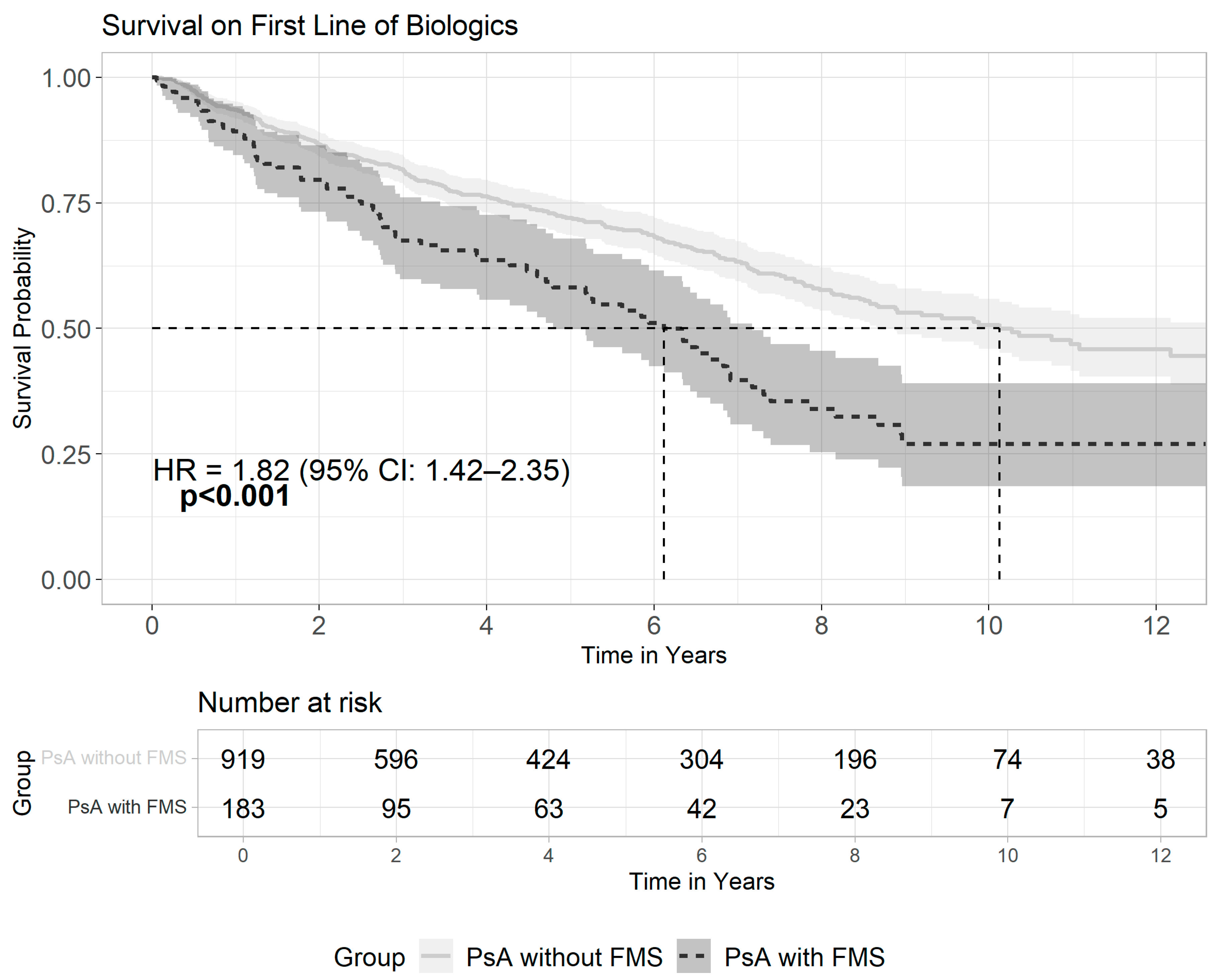
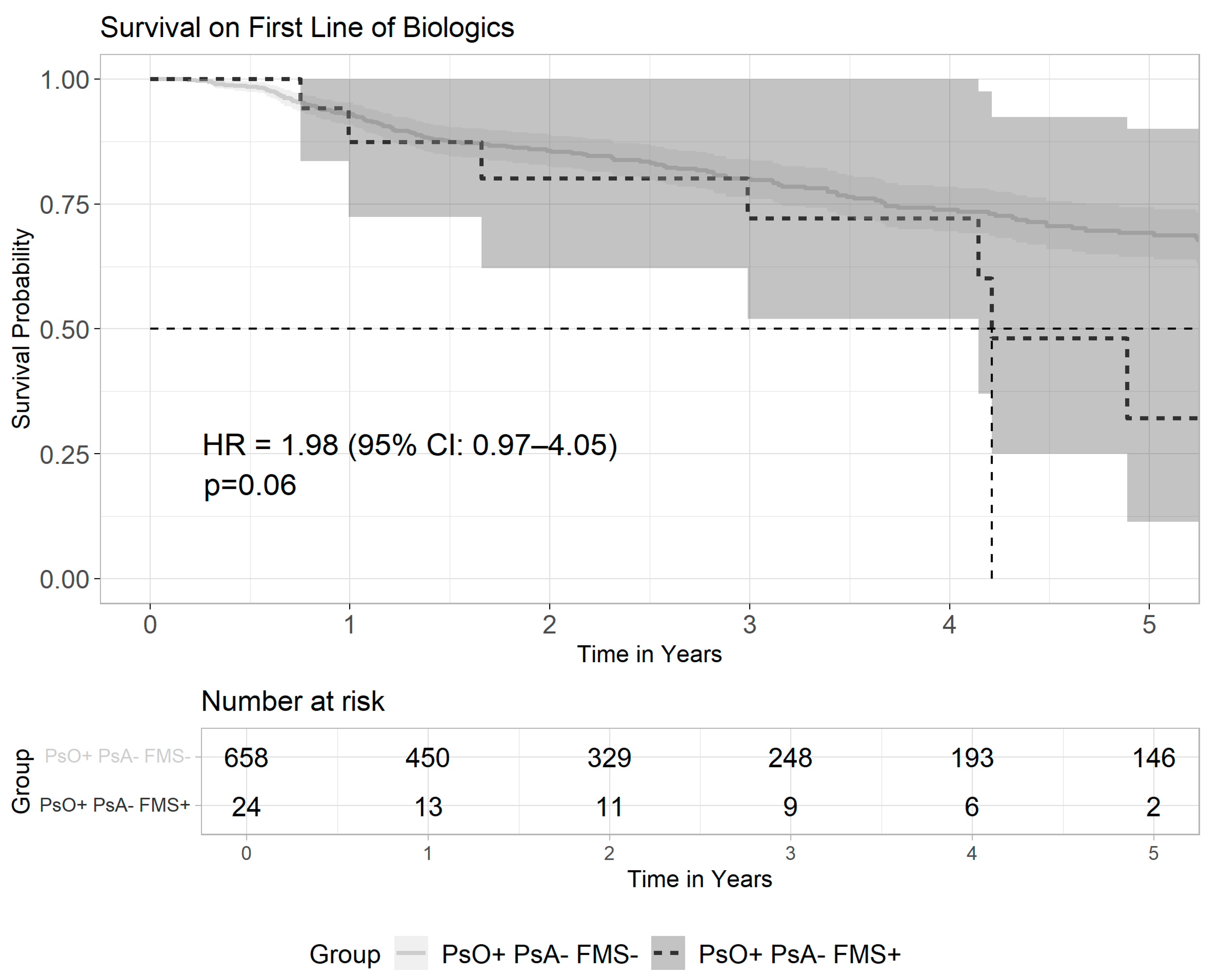
3.3. Patterns of Biologics Usage Among Patients with Psoriasis and Fibromyalgia
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Zachariae, H.; Zachariae, R.; Blomqvist, K.; Davidsson, S.; Molin, L.; Mørk, C.; Sigurgeirsson, B. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations. Data from the Nordic Quality of Life Study. Acta Derm. Venereol. 2002, 82, 108–113. [Google Scholar] [CrossRef]
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019, 80, 251–265.e19. [Google Scholar] [CrossRef]
- Perez-Chada, L.M.; Merola, J.F. Comorbidities associated with psoriatic arthritis: Review and update. Clin. Immunol. 2020, 214, 108397. [Google Scholar] [CrossRef]
- Polachek, A.; Touma, Z.; Anderson, M.; Eder, L. Risk of Cardiovascular Morbidity in Patients With Psoriatic Arthritis: A Meta-Analysis of Observational Studies. Arthritis Care Res. 2017, 69, 67–74. [Google Scholar] [CrossRef]
- Ulutatar, F.; Unal-Ulutatar, C.; Tuncay Duruoz, M. Fibromyalgia in patients with psoriatic arthritis: Relationship with enthesopathy, sleep, fatigue and quality of life. Int. J. Rheum. Dis. 2021, 24, 183–188. [Google Scholar] [CrossRef]
- Brikman, S.; Furer, V.; Wollman, J.; Borok, S.; Matz, H.; Polachek, A.; Elalouf, O.; Sharabi, A.; Kaufman, I.; Paran, D.; et al. The Effect of the Presence of Fibromyalgia on Common Clinical Disease Activity Indices in Patients with Psoriatic Arthritis: A Cross-sectional Study. J. Rheumatol. 2016, 43, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.; Tiosano, S.; Amital, H. The complexities of fibromyalgia and its comorbidities. Curr. Opin. Rheumatol. 2018, 30, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Puig, L.; Joshi, A.; Skup, M.; Williams, D.; Li, J.; Betts, K.A.; Augustin, M. Comparison of Biologics and Oral Treatments for Plaque Psoriasis: A Meta-analysis. JAMA Dermatol. 2020, 156, 258–269. [Google Scholar] [CrossRef]
- Mease, P.J. Fibromyalgia, a missed comorbidity in spondyloarthritis: Prevalence and impact on assessment and treatment. Curr. Opin. Rheumatol. 2017, 29, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Cazzola, M.; Benucci, M.; Di Franco, M.; Salaffi, F.; Sarzi-Puttini, P. Chronic widespread pain in the spectrum of rheumatological diseases. Best. Pract. Res. Clin. Rheumatol. 2011, 25, 165–171. [Google Scholar] [CrossRef]
- Marchesoni, A.; De Marco, G.; Merashli, M.; McKenna, F.; Tinazzi, I.; Marzo-Ortega, H.; McGonagle, D.G. The problem in differentiation between psoriatic-related polyenthesitis and fibromyalgia. Rheumatology 2018, 57, 32–40. [Google Scholar] [CrossRef]
- Akay, A.; Pekcanlar, A.; Bozdag, K.E.; Altintas, L.; Karaman, A. Assessment of depression in subjects with psoriasis vulgaris and lichen planus. J. Eur. Acad. Dermatol. Venereol. 2002, 16, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Ye, J.Y.; Cook, R.J.; Gladman, D.D.; Chandran, V. Depression and Anxiety Reduce the Probability of Achieving a State of Sustained Minimal Disease Activity in Patients With Psoriatic Arthritis. Arthritis Care Res. 2022, 74, 1430–1434. [Google Scholar] [CrossRef]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Patt, Y.S.; Ben-Shabat, N.; Sharif, K.; David, P.; Patt, C.; Elizur, Y.; Shani, U.; Zacay, G.; Watad, A.; Amital, H. Unraveling the connection: Uveitis prevalence and risk factors in psoriasis patients—A population-based study. J. Dermatol. 2024, 51, 558–566. [Google Scholar] [CrossRef]
- Shani, U.; Ben-Shabat, N.; Qassem, R.; Lahat, A.; Omar, M.; Savin, E.; Dotan, A.; Patt, Y.S.; Fisher, L.; Zacay, G.; et al. The association between psoriasis, psoriasis severity, and inflammatory bowel disease: A population-based analysis. Therap. Adv. Gastroenterol. 2024, 17, 17562848241227037. [Google Scholar] [CrossRef] [PubMed]
- Watad, A.; Zabotti, A.; Patt, Y.S.; Gendelman, O.; Dotan, A.; Ben-Shabat, N.; Fisher, L.; McGonagle, D.; Amital, H. From Psoriasis to Psoriatic Arthritis: Decoding the Impact of Treatment Modalities on the Prevention of Psoriatic Arthritis. Rheumatol. Ther. 2024, 11, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, Y.-H. Association of psoriasis with stroke and myocardial infarction: Meta-analysis of cohort studies. Br. J. Dermatol. 2012, 167, 1345–1350. [Google Scholar] [CrossRef]
- Yamazaki, F. Psoriasis: Comorbidities. J. Dermatol. 2021, 48, 732–740. [Google Scholar] [CrossRef]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and Comorbid Diseases Part I. Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Molon, B.; Viola, A.; Alaibac, M.; Angioni, R.; Piaserico, S. Psoriasis and Cardiovascular Diseases: An Immune-Mediated Cross Talk? Front. Immunol. 2022, 13, 868277. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhu, Y.-J.; Zou, S.; Zhou, P.; Hu, Y.-W.; Zhao, Q.-X.; Gu, L.-N.; Zhang, H.-Z.; Wang, Z.; Li, J. Metabolic Syndrome and Psoriasis: Mechanisms and Future Directions. Front. Immunol. 2021, 12, 711060. [Google Scholar] [CrossRef]
- Queiroz, L.P. Worldwide epidemiology of fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef]
- Ablin, J.N.; Oren, A.; Cohen, S.; Aloush, V.; Buskila, D.; Elkayam, O.; Wollman, Y.; Berman, M. Prevalence of fibromyalgia in the Israeli population: A population-based study to estimate the prevalence of fibromyalgia in the Israeli population using the London Fibromyalgia Epidemiology Study Screening Questionnaire (LFESSQ). Clin. Exp. Rheumatol. 2012, 30, 39–43. [Google Scholar]
- D’Onghia, M.; Ursini, F.; Cinotti, E.; Calabrese, L.; Tognetti, L.; Cartocci, A.; Lazzeri, L.; Frediani, B.; Rubegni, P.; Trovato, E. Psoriasis and Fibromyalgia: A Systematic Review. J. Pers. Med. 2024, 14, 165. [Google Scholar] [CrossRef]
- Buskila, D.; Gladman, D.D.; Langevitz, P.; Urowitz, S.; Smythe, H.A. Fibromyalgia in human immunodeficiency virus infection. J. Rheumatol. 1990, 17, 1202–1206. [Google Scholar] [PubMed]
- Melikoglu, M.; Melikoglu, M.A. The prevalence of fibromyalgia in patients with Behçet’s disease and its relation with disease activity. Rheumatol. Int. 2013, 33, 1219–1222. [Google Scholar] [CrossRef]
- Dhir, V.; Lawrence, A.; Aggarwal, A.; Misra, R. Fibromyalgia is common and adversely affects pain and fatigue perception in North Indian patients with rheumatoid arthritis. J. Rheumatol. 2009, 36, 2443–2448. [Google Scholar] [CrossRef]
- Kridin, K.; Vanetik, S.; Damiani, G.; Cohen, A.D. Big data highlights the association between psoriasis and fibromyalgia: A population-based study. Immunol. Res. 2020, 68, 135–140. [Google Scholar] [CrossRef]
- Yunus, M.B. The role of gender in fibromyalgia syndrome. Curr. Rheumatol. Rep. 2001, 3, 128–134. [Google Scholar] [CrossRef]
- Thune, P.O. The prevalence of fibromyalgia among patients with psoriasis. Acta Derm. Venereol. 2005, 85, 33–37. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Mease, P.J.; Russell, A.S.; Russell, I.J.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Aguglia, A.; Salvi, V.; Maina, G.; Rossetto, I.; Aguglia, E. Fibromyalgia syndrome and depressive symptoms: Comorbidity and clinical correlates. J. Affect. Disord. 2011, 128, 262–266. [Google Scholar] [CrossRef]
- Tander, B.; Cengiz, K.; Alayli, G.; Ilhanli, I.; Canbaz, S.; Canturk, F. A comparative evaluation of health related quality of life and depression in patients with fibromyalgia syndrome and rheumatoid arthritis. Rheumatol. Int. 2008, 28, 859–865. [Google Scholar] [CrossRef]
- Vishne, T.; Fostick, L.; Silberman, A.; Kupchick, M.; Rubinow, A.; Amital, H.; Amital, D. Fibromyalgia among major depression disorder females compared to males. Rheumatol. Int. 2008, 28, 831–836. [Google Scholar] [CrossRef]
- Magrey, M.N.; Antonelli, M.; James, N.; Khan, M.A. High Frequency of Fibromyalgia in Patients with Psoriatic Arthritis: A Pilot Study. Arthritis 2013, 2013, 762921. [Google Scholar] [CrossRef] [PubMed]
- Marchesoni, A.; Atzeni, F.; Spadaro, A.; Lubrano, E.; Provenzano, G.; Cauli, A.; Olivieri, I.; Melchiorre, D.; Salvarani, C.; Scarpa, R.; et al. Identification of the clinical features distinguishing psoriatic arthritis and fibromyalgia. J. Rheumatol. 2012, 39, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Iannone, F.; Nivuori, M.; Fornaro, M.; Venerito, V.; Cacciapaglia, F.; Lopalco, G. Comorbid fibromyalgia impairs the effectiveness of biologic drugs in patients with psoriatic arthritis. Rheumatology 2020, 59, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, P.L.; Sita, A.; Sewitch, M.J. Predictors of adherence to treatment in women with fibromyalgia. Clin. J. Pain 2006, 22, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami Shor, D.; Weitzman, D.; Dahan, S.; Gendelman, O.; Bar-On, Y.; Amital, D.; Shalev, V.; Chodick, G.; Amital, H. Adherence and Persistence with Drug Therapy among Fibromyalgia Patients: Data from a Large Health Maintenance Organization. J. Rheumatol. 2017, 44, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Prikhodkina, M.; Melnikov, S. Factors that influence medication adherence in women with fibromyalgia: A path analysis. J. Clin. Nurs. 2024, 33, 3943–3953. [Google Scholar] [CrossRef]
- Stolwijk, C.; van Onna, M.; Boonen, A.; van Tubergen, A. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis Care Res. 2016, 68, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Psoriasis (n = 61,003) | Controls (n = 244,012) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SD | 36.1 ± 19.3 | 36.1 ± 19.3 | matched |
| Female gender, n (%) | 31,008 (50.8) | 124,003 (50.8) | matched |
| Arab ethnicity, n (%) | 6996 (11.5) | 27,984 (11.5) | matched |
| Chronic Comorbidities † | |||
| Obesity, n (%) | 14,540 (26.3) | 49,526 (22.4) | <0.001 |
| Diabetes, n (%) | 9937 (16.3) | 34,065 (14.0) | <0.001 |
| HTN, n (%) | 18,213 (29.9) | 66,802 (27.4) | <0.001 |
| IHD, n (%) | 6196 (10.2) | 22,072 (9.0) | <0.001 |
| Stroke, n (%) | 3623 (5.9) | 13,236 (5.4) | <0.001 |
| COPD, n (%) | 5808 (9.5%) | 19,040 (7.8) | <0.001 |
| CHF, n (%) | 1822 (3.0) | 6372 (2.6) | <0.001 |
| Dyslipidemia, n (%) | 27,460 (45.0) | 100,389 (41.1) | <0.001 |
| Malignancy, n (%) | 7250 (11.9) | 25,663 (10.5) | <0.001 |
| Dementia, n (%) | 1051 (1.7) | 4757 (1.9) | <0.001 |
| Depression, n (%) | 9008 (14.8) | 32,156 (13.2) | <0.001 |
| Fibromyalgia, n (%) | 2037 (3.3) | 5678 (2.3) | <0.001 |
| Smoking, n (%) | 3402 (19.9) | 12,869 (20) | 0.6 |
| SpA-related Comorbidities † | |||
| Psoriatic arthritis ‡, n (%) | 5223 (8.6) | 5637 (2.3) | <0.001 |
| Peripheral arthritis, n (%) | 3697 (6.1) | 108 (0.0) | <0.001 |
| Axial spondyloarthritis, n (%) | 1958 (3.2) | 5539 (2.3) | <0.001 |
| Inflammatory Bowel Disease, n (%) | 1495 (2.5) | 3834 (1.6) | <0.001 |
| Treatment | |||
| Phototherapy, n (%) | 5039 (8.3) | - | - |
| Methotrexate, n (%) | 2289 (3.8) | - | - |
| Anti-TNF, n (%) | 1385 | - | - |
| Anti-IL-12/23, n (%) | 247 | - | - |
| Anti-IL-17, n (%) | 128 | - | - |
| JAKi, n (%) | 24 | - | - |
| Multiple biologics lines, n (%) | 525 (0.9) | - | - |
| Characteristics (N = 61,003) | FMS (n = 2037) | Without FMS (n = 58,966) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age at psoriasis diagnosis, mean ± SD † | 46.0 ± 14.5 | 36.3 ± 19.4 | <0.001 |
| Female gender, n (%) ‡ | 1653 (81.1) | 29,355 (49.8) | <0.001 |
| Arab ethnicity, n (%) ‡ | 267 (13.1) | 6729 (11.4) | <0.001 |
| Chronic Comorbidities ‡ | |||
| Obesity, n (%) | 767 (38.1) | 13,773 (25.8) | <0.001 |
| Diabetes, n (%) | 524 (25.7) | 9413 (16.0) | <0.001 |
| HTN, n (%) | 916 (45.0) | 17,297 (29.3) | <0.001 |
| IHD, n (%) | 298 (14.6) | 5898 (10.0) | <0.001 |
| Stroke, n (%) | 266 (13.1) | 3357 (5.7) | <0.001 |
| COPD, n (%) | 363 (17.8) | 5445 (9.2) | <0.001 |
| CHF, n (%) | 80 (3.9) | 1742 (3.0) | 0.01 |
| Dyslipidemia, n (%) | 1356 (66.6) | 26,104 (44.3) | <0.001 |
| Malignancy, n (%) | 379 (18.9) | 6871 (11.7) | <0.001 |
| Depression, n (%) | 881 (43.2) | 8127 (13.8) | <0.001 |
| Dementia, n (%) | 46 (2.3) | 1005 (1.7) | 0.06 |
| Smoking, n (%) | 173 (22.2) | 3229 (19.7) | 0.09 |
| SpA-related Comorbidities ‡ | |||
| Psoriatic arthritis, n (%): | 685 (33.6) | 4538 (7.7) | <0.001 |
| Peripheral arthritis, n (%) | 507 (24.9) | 3190 (5.4) | <0.001 |
| Axial spondyloarthritis, n (%) | 284 (13.9) | 1674 (2.8) | <0.001 |
| Inflammatory bowel disease, n (%) | 119 (5.8) | 1376 (2.3) | <0.001 |
| Treatment ‡ | |||
| Phototherapy, n (%) | 192 (9.4) | 4847 (8.2) | <0.001 |
| Methotrexate, n (%) | 304 (14.9) | 1985 (3.4) | <0.001 |
| Anti-TNF, n (%) | 184 (9) | 1226 (2.1) | <0.001 |
| Anti-IL-12/23, n (%) | 38 (1.9) | 477 (0.8) | <0.001 |
| Anti-IL-17, n (%) | 66 (3.2) | 343 (0.6) | <0.001 |
| JAKi, n (%) | 30 (1.5) | 82 (0.1) | <0.001 |
| Any biologics | 207 (10.2) | 1577 (2.7) | <0.001 |
| Multiple biologics lines, n (%) | 85 (4.2) | 440 (0.7) | <0.001 |
| Variable | Group | Mean ± SD | B | Beta | 95% CI | Pv |
|---|---|---|---|---|---|---|
| Fibromyalgia | No | 4.2 ± 3.6 | Ref. | −1.53 to −0.4 | 0.001 | |
| Yes | 3.0 ± 3.1 | −0.97 | −0.102 | |||
| Ethnicity | Jewish | 4.2 ± 3.6 | Ref. | −1.98 to −0.6 | <0.001 | |
| Arab | 2.9 ±2.8 | −1.29 | −0.11 | |||
| Sex | Male | 4.4 ± 3.5 | Ref. | −0.94 to −0.10 | 0.016 | |
| Female | 3.7 ± 3.5 | −0.51 | −0.073 | |||
| Age at 1st Biologic Treatment | −0.02 | −0.094 | −0.04 to 0.01 | 0.002 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elizur, Y.; Amital, M.; Ben-Shabat, N.; Patt, C.; Zacay, G.; Lassman, S.; McGonagle, D.; Watad, A.; Gendelman, O.; Amital, H. The Association Between Psoriasis, Psoriatic Arthritis, and Fibromyalgia Syndrome: Effects on Treatment—A Population-Based Study. Medicina 2025, 61, 1809. https://doi.org/10.3390/medicina61101809
Elizur Y, Amital M, Ben-Shabat N, Patt C, Zacay G, Lassman S, McGonagle D, Watad A, Gendelman O, Amital H. The Association Between Psoriasis, Psoriatic Arthritis, and Fibromyalgia Syndrome: Effects on Treatment—A Population-Based Study. Medicina. 2025; 61(10):1809. https://doi.org/10.3390/medicina61101809
Chicago/Turabian StyleElizur, Yoav, Mor Amital, Niv Ben-Shabat, Chen Patt, Galia Zacay, Simon Lassman, Dennis McGonagle, Abdulla Watad, Omer Gendelman, and Howard Amital. 2025. "The Association Between Psoriasis, Psoriatic Arthritis, and Fibromyalgia Syndrome: Effects on Treatment—A Population-Based Study" Medicina 61, no. 10: 1809. https://doi.org/10.3390/medicina61101809
APA StyleElizur, Y., Amital, M., Ben-Shabat, N., Patt, C., Zacay, G., Lassman, S., McGonagle, D., Watad, A., Gendelman, O., & Amital, H. (2025). The Association Between Psoriasis, Psoriatic Arthritis, and Fibromyalgia Syndrome: Effects on Treatment—A Population-Based Study. Medicina, 61(10), 1809. https://doi.org/10.3390/medicina61101809







