Risk Factors for Treatment Failure of Drug-Susceptible Pulmonary Tuberculosis in Lithuania over 22 Years
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Operational Definitions
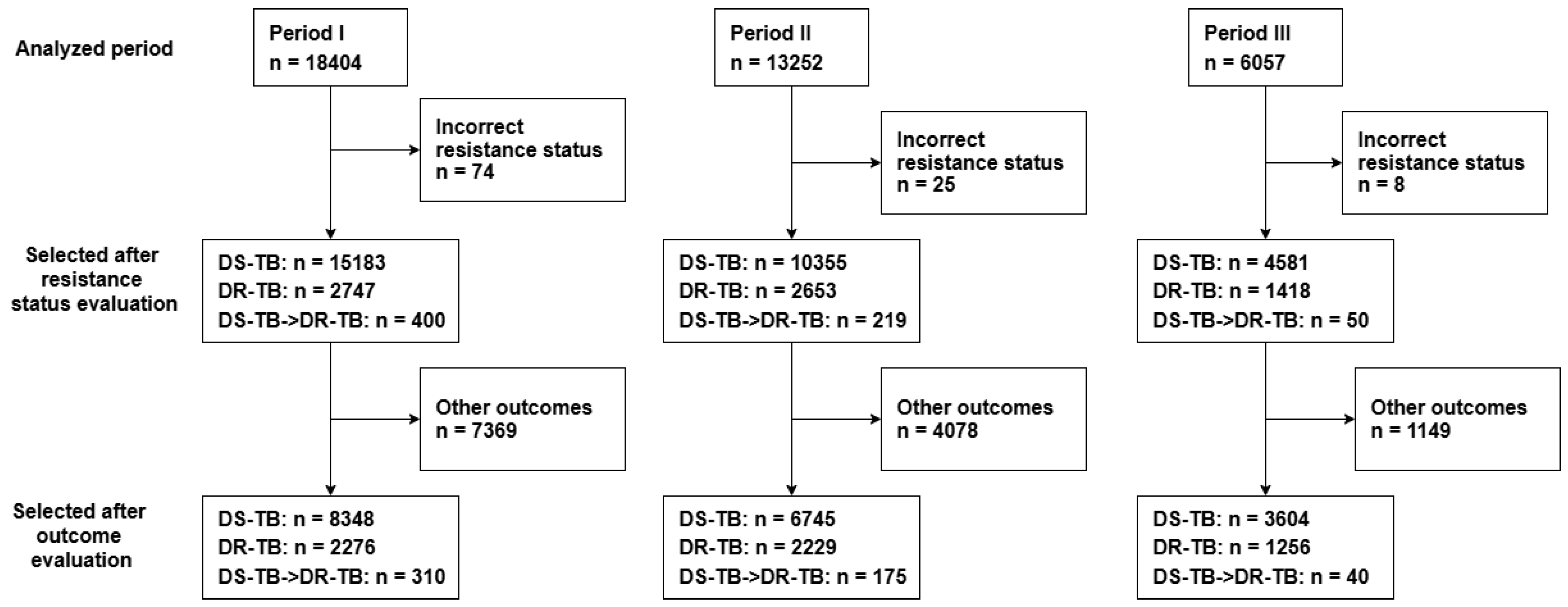
2.3. Study Population
2.4. Statistical Analysis
2.5. Ethical Statement
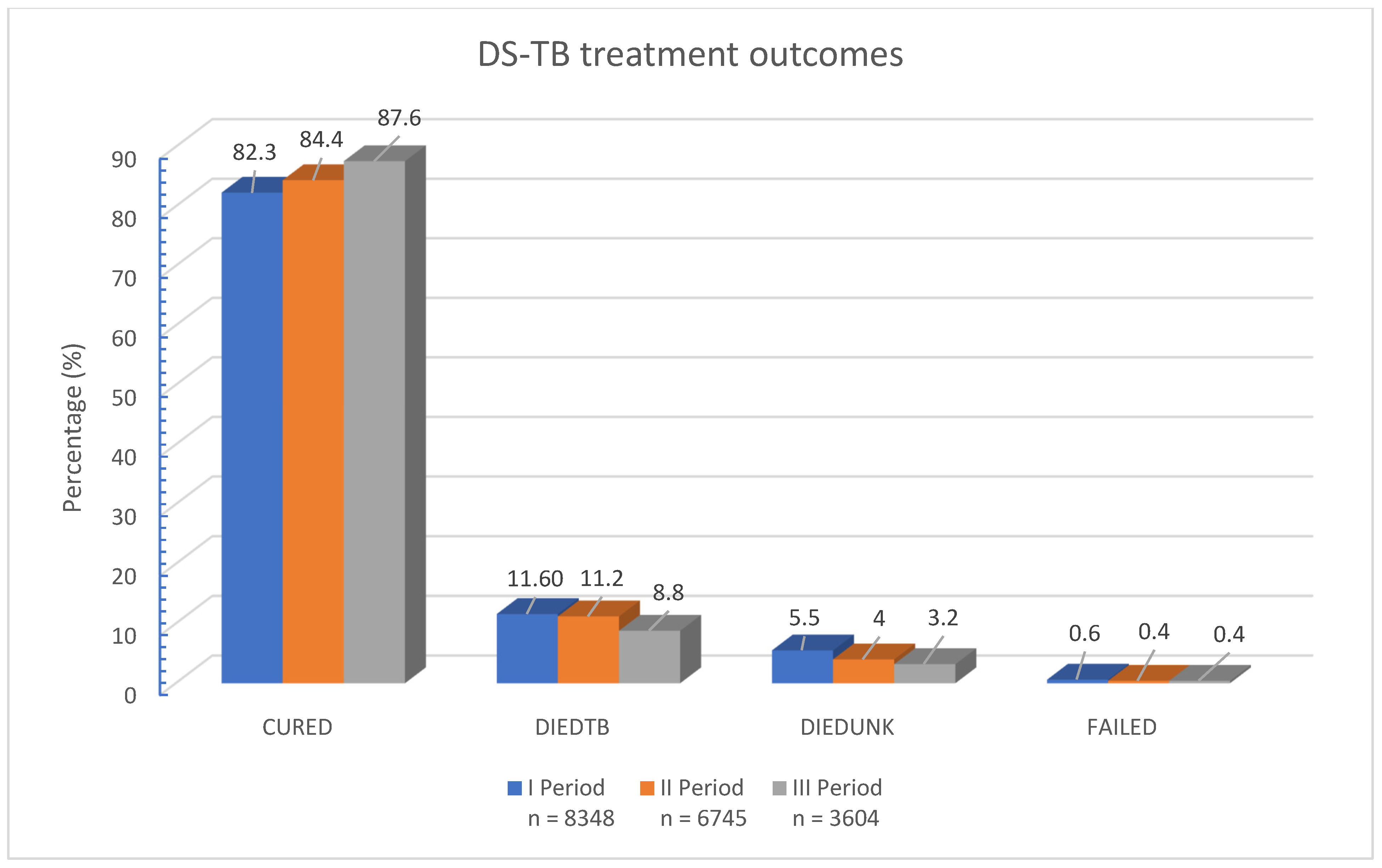
3. Results
3.1. Socio-Demographic and Clinical Attributes of Study Participants
3.2. A Comparative Analysis of Sociodemographic and Clinical Characteristics Between Patients with Unsuccessful TB Treatment and Treatment Success over Three Time Periods
3.2.1. Age and Gender Trends
3.2.2. Microbiological Indicators
3.2.3. Comorbidities
3.2.4. Social Factors
3.2.5. Harmful Habits
3.2.6. TB History and Relapse
3.3. Drug-Susceptible Pulmonary Tuberculosis Treatment Failure Risk Factors
3.3.1. Age
3.3.2. Sex
3.3.3. Body Mass Index
3.3.4. Comorbidities
3.3.5. Harmful Habits
3.3.6. Social Factors
3.3.7. TB History and Relapse
3.3.8. Factors Without a Significant Association
| Risk Factor | Patients (N = 18,697) | |||||
|---|---|---|---|---|---|---|
| Period I (N = 8348) | Period II (N = 6745) | Period III (N = 3604) | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Aged 50 and above | 1.04 (1.04–1.04) | <0.001 | 1.05 (1.04–1.05) | <0.001 | 1.05 (1.04–1.05) | <0.001 |
| Confirmed by culture | 0.68 (0.60–0.76) | <0.001 | 0.74 (0.65–0.85) | <0.001 | - | - |
| Low BMI (<18) | - | - | 11.5 (3.13–55.5) | <0.001 | 11.5 (3.13–55.5) | <0.001 |
| Coronary heart disease | 1.54 (0.98–2.34) | 0.049 | 7.83 (3.97–15.9) | <0.001 | 3.72 (2.48–5.50) | <0.001 |
| Chronic lung disease | 1.78 (1.36–2.31) | <0.001 | 2.77 (1.89–3.99) | <0.001 | 1.9 (1.11–3.12) | 0.015 |
| Diabetes mellitus | 2.28 (1.56–3.29) | <0.001 | - | 0.433 | - | 0.524 |
| Cancer disease | 4.66 (1.59–13.6) | 0.004 | 7.48 (4.50–12.6) | <0.001 | 7.8 (5.43–11.2) | <0.001 |
| Kidney disease | - | 0.318 | 5.41 (1.00–29.2) | 0.039 | 5.73 (2.18–14.6) | <0.001 |
| Liver disease | - | - | - | - | - | - |
| HIV | 5.71 (2.36–14.2) | <0.001 | 3.60 (2.09–6.08) | <0.001 | - | 0.057 |
| Smoking | 1.29 (1.15–1.46) | <0.001 | - | 0.779 | 0.79 (0.65–0.97) | 0.023 |
| Excessive alcohol consumption | 1.88 (1.62–2.17) | <0.001 | 1.45 (1.23–1.71) | <0.001 | 2.15 (1.70–2.72) | <0.001 |
| Moderate alcohol consumption | 0.66 (0.56–0.78) | <0.001 | 0.52 (0.43–0.63) | <0.001 | 0.67 (0.51–0.87) | 0.003 |
| Substance use | 2.33 (1.00–5.07) | 0.039 | - | 0.687 | 2.02 (0.98–3.83) | 0.042 |
| Low level of education * | 2.56–7.03 (1.71–11.1) | <0.001 | 1.63–5.03 (1.13–7.65) | <0.001 | 1.90–3.81 (1.16–7.17) | <0.001 |
| Unemployment | 7.32 (5.73–9.51) | <0.001 | 5.4 (4.13–7.21) | <0.001 | 5.36 (3.81–7.81) | <0.001 |
| Homelessness | 3.99 (2.77–5.72) | <0.001 | 2.30 (1.61–3.23) | <0.001 | 2.41 (1.34–4.13) | 0.002 |
| Living in a rural area | 1.30 (1.16–1.46) | <0.001 | - | 0.815 | - | 0.303 |
| TB relapse | 1.87 (1.62–2.15) | <0.001 | 1.31 (1.09–1.57) | 0.004 | - | 0.690 |
| Male sex | 1.59 (1.40–1.82) | <0.001 | 1.27 (1.09–1.48) | 0.002 | 1.16 (0.93–1.45) | 0.206 |
| Treatment failure, n (%) | 1479 (17.7) | 1055 (15.6) | 447 (12.4) |
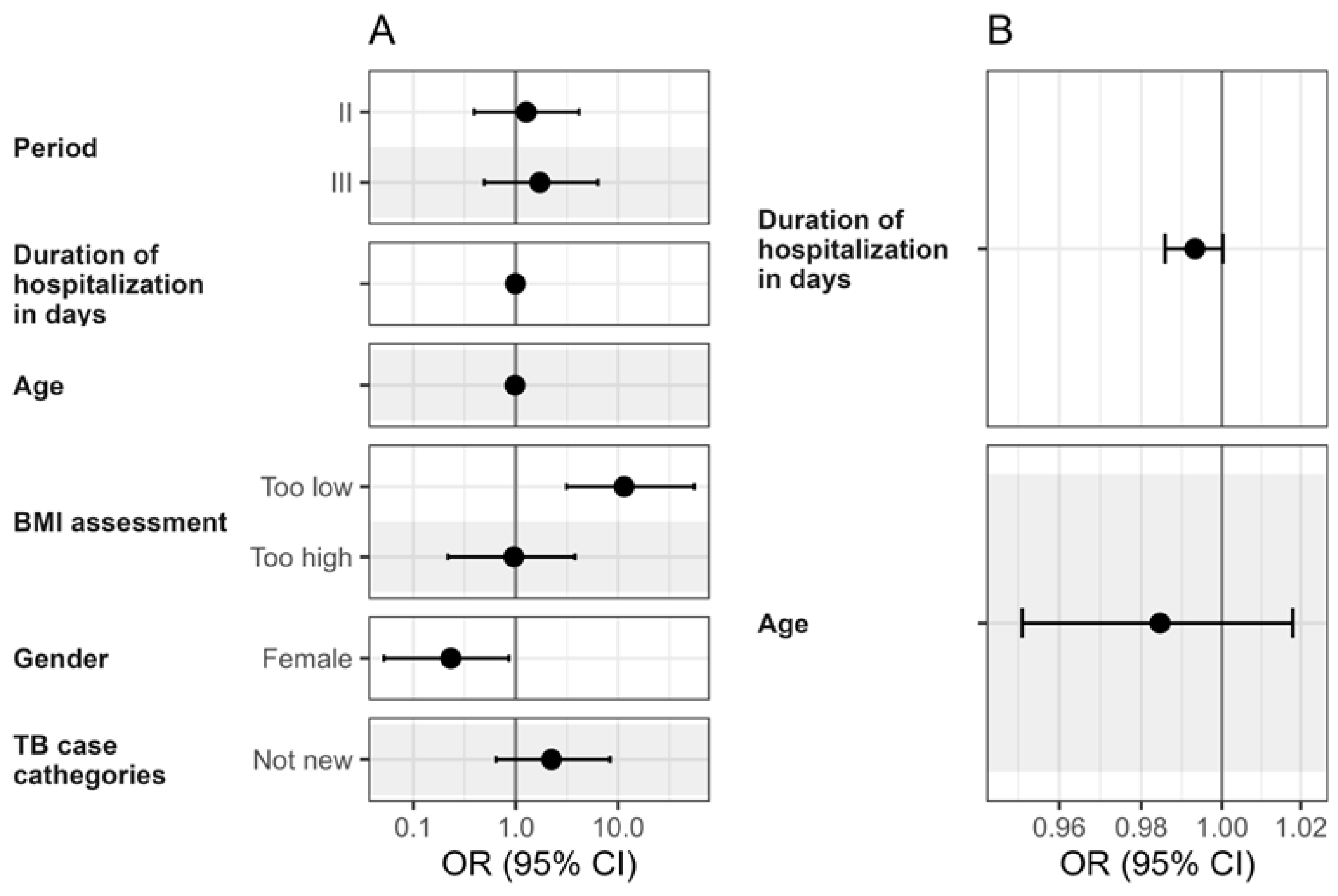
3.4. Risk Factors for Unsuccessful Pulmonary Drug-Susceptible Tuberculosis Treatment Outcomes: An Univariable Analysis Across Three Periods
3.5. Multivariable Logistic Regression Results for Pulmonary Drug-Susceptible Tuberculosis Treatment Outcome Predictors
4. Discussion
Future Directions
- Integration of TB services with social care programs focused on unemployment, housing, and substance use treatment;
- Community-based adherence support and outreach for vulnerable populations;
- Targeted nutritional assistance to address malnutrition and low BMI;
- Development of coordinated care models for patients with multiple risk factors and comorbidities.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef]
- Zaman, K. Tuberculosis: A Global Health Problem. J. Health Popul. Nutr. 2010, 28, 111–113. [Google Scholar] [CrossRef]
- World Health Organization. Definitions and Reporting Framework for Tuberculosis; World Health Organization: Geneva, Switzerland, 2013; revision (updated December 2014 and January 2020). [Google Scholar]
- World Health Organization (WHO). Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024; Available online: https://iris.who.int/bitstream/handle/10665/379339/9789240101531-eng.pdf?sequence=1 (accessed on 17 June 2025).
- World Health Organization. Tuberculosis [Internet]. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 17 July 2025).
- World Health Organization. The End TB Strategy [Internet]; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/teams/global-programme-on-tuberculosis-and-lung-health/the-end-tb-strategy (accessed on 17 July 2025).
- European Centre for Disease Prevention and Control; World Health Organization Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2025—2023 Data; ECDC/WHO Regional Office for Europe: Stockholm/Copenhagen, 2025; ISBN 978-92-890-6183-4. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/TB-2025-Surveillance-report.pdf (accessed on 17 July 2025).
- European Centre for Disease Prevention and Control; World Health Organization Regional Office for Europe. Tuberculosis Surveillance and Monitoring in Europe 2023—2021 Data; ECDC/WHO Regional Office for Europe: Stockholm, Sweden; Copenhagen, Denmark, 2023; Available online: https://www.ecdc.europa.eu/en/publications-data/tuberculosis-surveillance-and-monitoring-europe-2023-2021-data (accessed on 17 July 2025).
- European Centre for Disease Prevention and Control. Tuberculosis. In Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/publications-data/tuberculosis-annual-epidemiological-report-2022 (accessed on 17 July 2025).
- The State Information System of Tuberculosis. Tuberculosis treatment outcomes—2025 data on file.
- Lawrence, O.; Chengbo, Z.; Seung, K.J.; Mpinda, S.; Kunda, M.; Mitnick, C.; Kanu, M.; Tamirat, M.; Makaka, J.; Mofolo, M.; et al. Low Body Mass Index as a Predictor of Sputum Culture Conversion and Treatment Outcomes among Patients Receiving Treatment for Multidrug-Resistant Tuberculosis in Lesotho. Glob. Health Action 2024, 17, 2305930. [Google Scholar] [CrossRef]
- Koethe, J.R.; von Reyn, C.F. Protein-Calorie Malnutrition, Micronutrient Supplements, and Tuberculosis. Int. J. Tuberc. Lung Dis. 2016, 20, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Költringer, F.A.; Annerstedt, K.S.; Boccia, D.; Carter, D.J.; Rudgard, W.E. The Social Determinants of National Tuberculosis Incidence Rates in 116 Countries: A Longitudinal Ecological Study between 2005–2015. BMC Public Health 2023, 23, 337. [Google Scholar] [CrossRef]
- AlOsaimi, H.M.; Alshammari, M.K.; Almijlad, G.K.; Alotaibi, N.M.; Alqahtani, D.A.; Alshamrani, M.M.; Shutur, T.A.; Alhazmi, M.F.; Hurubi, M.A.; ALShammari, K.S.; et al. Prevalence, Clinical Characteristics and Determinants of Unsuccessful Treatment Outcomes Among Pulmonary Tuberculosis Patients: A 5-Year Registry-Based Retrospective Cohort Study. Patient Relat. Outcome Meas. 2024, 15, 187–198. [Google Scholar] [CrossRef]
- Matulyte, E.; Davidaviciene, E.; Kancauskiene, Z.; Diktanas, S.; Kausas, A.; Velyvyte, D.; Urboniene, J.; Lipnickiene, V.; Laurencikaite, M.; Danila, E.; et al. The Socio-Demographic, Clinical Characteristics and Outcomes of Tuberculosis among HIV-Infected Adults in Lithuania: A Thirteen-Year Analysis. PLoS ONE 2023, 18, e0282046. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ge, J.; Zhang, D.; Lin, X.; Wang, X.; Peng, L.; Chen, L. Clinical Characteristics and Outcomes in Chronic Kidney Disease Patients with Tuberculosis in China: A Retrospective Cohort Study. Int. J. Gen. Med. 2022, 15, 6661–6669. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, P.; Ugarte-Gil, C.; Golub, J.; Pearson, F.; Critchley, J. The Effects of Diabetes on Tuberculosis Treatment Outcomes: An Updated Systematic Review and Meta-Analysis. Int. J. Tuberc. Lung Dis. 2019, 23, 783–796. [Google Scholar] [CrossRef]
- Gautam, S.; Shrestha, N.; Mahato, S.; Nguyen, T.P.A.; Mishra, S.R.; Berg-Beckhoff, G. Diabetes among Tuberculosis Patients and Its Impact on Tuberculosis Treatment in South Asia: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 2113. [Google Scholar] [CrossRef]
- Nowiński, A.; Wesołowski, S.; Korzeniewska-Koseła, M. The Impact of Comorbidities on Tuberculosis Treatment Outcomes in Poland: A National Cohort Study. Front. Public Health 2023, 11, 1253615. [Google Scholar] [CrossRef]
- Cioboata, R.; Vasile, C.M.; Bălteanu, M.A.; Georgescu, D.E.; Toma, C.; Dracea, A.S.; Nicolosu, D. Evaluating Serum Calcium and Magnesium Levels as Predictive Biomarkers for Tuberculosis and COVID-19 Severity: A Romanian Prospective Study. Int. J. Mol. Sci. 2024, 25, 418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huangfu, P.; Laurence, Y.V.; Alisjahbana, B.; Ugarte-Gil, C.; Riza, A.-L.; Walzl, G.; Ruslami, R.; Moore, D.A.J.; Ioana, M.; McAllister, S.; et al. Point of care HbA1c level for diabetes mellitus management and its accuracy among tuberculosis patients: A study in four countries. Int. J. Tuberc. Lung Dis. 2019, 23, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.K.; Min, J.; Kim, H.W.; Lee, J.; Kim, J.S.; Park, J.S.; Lee, S.S. Prediction of Treatment Failure and Compliance in Patients with Tuberculosis. BMC Infect. Dis. 2020, 20, 622. [Google Scholar] [CrossRef]
- Kenya, Uganda, Zambia, and Zimbabwe TB Disability Study Group; Adakun, S.A.; Banda, F.M.; Bloom, A.; Bochnowicz, M.; Chakaya, J.; Chansa, A.; Chiguvare, H.; Chimzizi, R.; Colvin, C.; et al. Disability, Comorbidities and Risk Determinants at End of TB Treatment in Kenya, Uganda, Zambia and Zimbabwe. IJTLD Open 2024, 1, 197–205. [Google Scholar] [CrossRef]
- Kaliner, E.; Bornstein, S.; Kabha, D.; Lidji, M.; Sheffer, R.; Mor, Z. A Retrospective Cohort Analysis of Treatment Outcomes of Patients with Tuberculosis Who Used Substances in Tel Aviv, Israel. Alcohol. Alcohol. 2024, 59, agad073. [Google Scholar] [CrossRef]
- Yerezhepov, D.; Gabdulkayum, A.; Akhmetova, A.; Kozhamkulov, U.; Rakhimova, S.; Kairov, U.; Zhunussova, G.; Kalendar, R.; Akilzhanova, A. Pulmonary Tuberculosis Epidemiology and Genetics in Kazakhstan. Front. Public Health 2024, 12, 1340673. [Google Scholar] [CrossRef]
- Sánchez-Pérez, H.J.; Gordillo-Marroquín, C.; Vázquez-Marcelín, J.; Martín-Mateo, M.; Gómez-Velasco, A. Sociodemographic Factors Associated with the Success or Failure of Antituberculosis Treatment in the Chiapas Highlands, Mexico, 2019–2022. PLoS ONE 2024, 19, e0296924. [Google Scholar] [CrossRef]
- Alavi-Naini, R.; Sharifi-Mood, B.; Metanat, M. Association between Tuberculosis and Smoking. Int. J. High Risk Behav. Addict. 2012, 1, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Bishwakarma, R.; Kinney, W.H.; Honda, J.R.; Mya, J.; Strand, M.J.; Gangavelli, A.; Bai, X.; Ordway, D.J.; Iseman, M.D.; Chan, E.D. Epidemiologic Link between Tuberculosis and Cigarette/Biomass Smoke Exposure: Limitations Despite the Vast Literature. Respirology 2015, 20, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.S.; Van Den Eeden, S.K.; Baxter, R.; Shan, J.; Van Rie, A.; Herring, A.H.; Richardson, D.B.; Emch, M.; Gammon, M.D. Cigarette Smoking and Pulmonary Tuberculosis in Northern California. J. Epidemiol. Community Health 2015, 69, 568–573. [Google Scholar] [CrossRef]
- Coutinho, S.E.; Lima de Braga, R.S.; Santos, A.K.; Velho, J.S.; Rossato Silva, D. Smoking Cessation among Tuberculosis Patients during the Coronavirus Disease-2019 Pandemic. Monaldi Arch. Chest Dis. 2024, 95, 2970. [Google Scholar] [CrossRef]
- Falzon, D.; Schünemann, H.J.; Harausz, E.; González-Angulo, L.; Lienhardt, C.; Jaramillo, E.; Weyer, K. World Health Organization Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update. Eur. Respir. J. 2017, 49, 1602308. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, T.; Jaramillo, E.; Weil, D.; Raviglione, M.; Lönnroth, K. Financial Burden for Tuberculosis Patients in Low- and Middle-Income Countries: A Systematic Review. Eur. Respir. J. 2014, 43, 1763–1775. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Treatment of Tuberculosis: Guidelines, 4th ed.; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- World Health Organization. Rapid Communication: Key Changes to the Treatment of Drug-Susceptible Tuberculosis; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Conradie, F.; Diacon, A.H.; Ngubane, N.; Howell, P.; Everitt, D.; Crook, A.M.; Mendel, C.M.; Egizi, E.; Moreira, J.; Timm, J.; et al. Treatment of Highly Drug-Resistant Pulmonary Tuberculosis. N. Engl. J. Med. 2020, 382, 893–902. [Google Scholar] [CrossRef]
- Government of the Republic of Lithuania. Resolution No. 1482 of 28 November 2012 on the Approval of the National Progress Programme for 2014–2020; Document No. 142-7294; Government of the Republic of Lithuania: Vilnius, Lithuania, 2012. [Google Scholar]
- Minister of Health of the Republic of Lithuania. Order No. V-815 of 16 July 2014 on the Approval of the Action Plan for Reducing Health Inequalities in Lithuania for 2014–2023. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/40be0b700df611e48595a3375cdcc8a3/vnzrzALKZv (accessed on 17 July 2025).
- da Silva, T.C.; Pinto, M.L.; Orlandi, G.M.; Monteiro de Figueiredo, T.M.R.; de Siqueira França, F.O.; Bertolozzi, M. Tuberculosis from the perspective of men and women. Rev. Esc. Enferm. USP 2022, 56, e20220137. [Google Scholar] [CrossRef]
- Peer, V.; Schwartz, N.; Green, M.S. Gender differences in tuberculosis incidence rates—A pooled analysis of data from seven high-income countries by age group and time period. Front. Public Health 2022, 10, 997025. [Google Scholar] [CrossRef]
- Rickman, H.M.; Phiri, M.D.; Feasey, H.R.A.; Krutikov, M.; Shao, H.; Horton, K.C.; Dowdy, D.W.; Nightingale, E.S.; Dodd, P.J.; Corbett, E.L.; et al. Sex differences in the risk of Mycobacterium tuberculosis infection: A systematic review and meta-analysis of population-based immunoreactivity surveys. Lancet Public Health 2025, 10, e588–e598. [Google Scholar] [CrossRef]
| Term | Definition |
|---|---|
| Bacteriologically confirmed TB | TB case confirmed by positive smear microscopy, culture, or WHO-recommended rapid diagnostic tests (e.g., Xpert MTB/RIF). |
| Clinically diagnosed TB | TB case diagnosed based on clinical, radiological, or histological findings in the absence of bacteriological confirmation. |
| New patient | A person who has never received TB treatment or has received it for less than one month. |
| Relapse patient | A person previously treated for TB who was cured or completed treatment and is now diagnosed again, due to either relapse or reinfection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kėvelaitienė, K.; Puronaitė, R.; Davidavičienė, V.E.; Nakčerienė, B.; Danila, E. Risk Factors for Treatment Failure of Drug-Susceptible Pulmonary Tuberculosis in Lithuania over 22 Years. Medicina 2025, 61, 1805. https://doi.org/10.3390/medicina61101805
Kėvelaitienė K, Puronaitė R, Davidavičienė VE, Nakčerienė B, Danila E. Risk Factors for Treatment Failure of Drug-Susceptible Pulmonary Tuberculosis in Lithuania over 22 Years. Medicina. 2025; 61(10):1805. https://doi.org/10.3390/medicina61101805
Chicago/Turabian StyleKėvelaitienė, Karolina, Roma Puronaitė, Valerija Edita Davidavičienė, Birutė Nakčerienė, and Edvardas Danila. 2025. "Risk Factors for Treatment Failure of Drug-Susceptible Pulmonary Tuberculosis in Lithuania over 22 Years" Medicina 61, no. 10: 1805. https://doi.org/10.3390/medicina61101805
APA StyleKėvelaitienė, K., Puronaitė, R., Davidavičienė, V. E., Nakčerienė, B., & Danila, E. (2025). Risk Factors for Treatment Failure of Drug-Susceptible Pulmonary Tuberculosis in Lithuania over 22 Years. Medicina, 61(10), 1805. https://doi.org/10.3390/medicina61101805






