Ticagrelor Versus Clopidogrel in Patients with Acute Coronary Syndrome and Chronic Kidney Disease: A Real-World Analysis from a National Registry
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Patients
2.3. Covariate and Study Outcomes
2.4. Statistical Analysis
3. Results
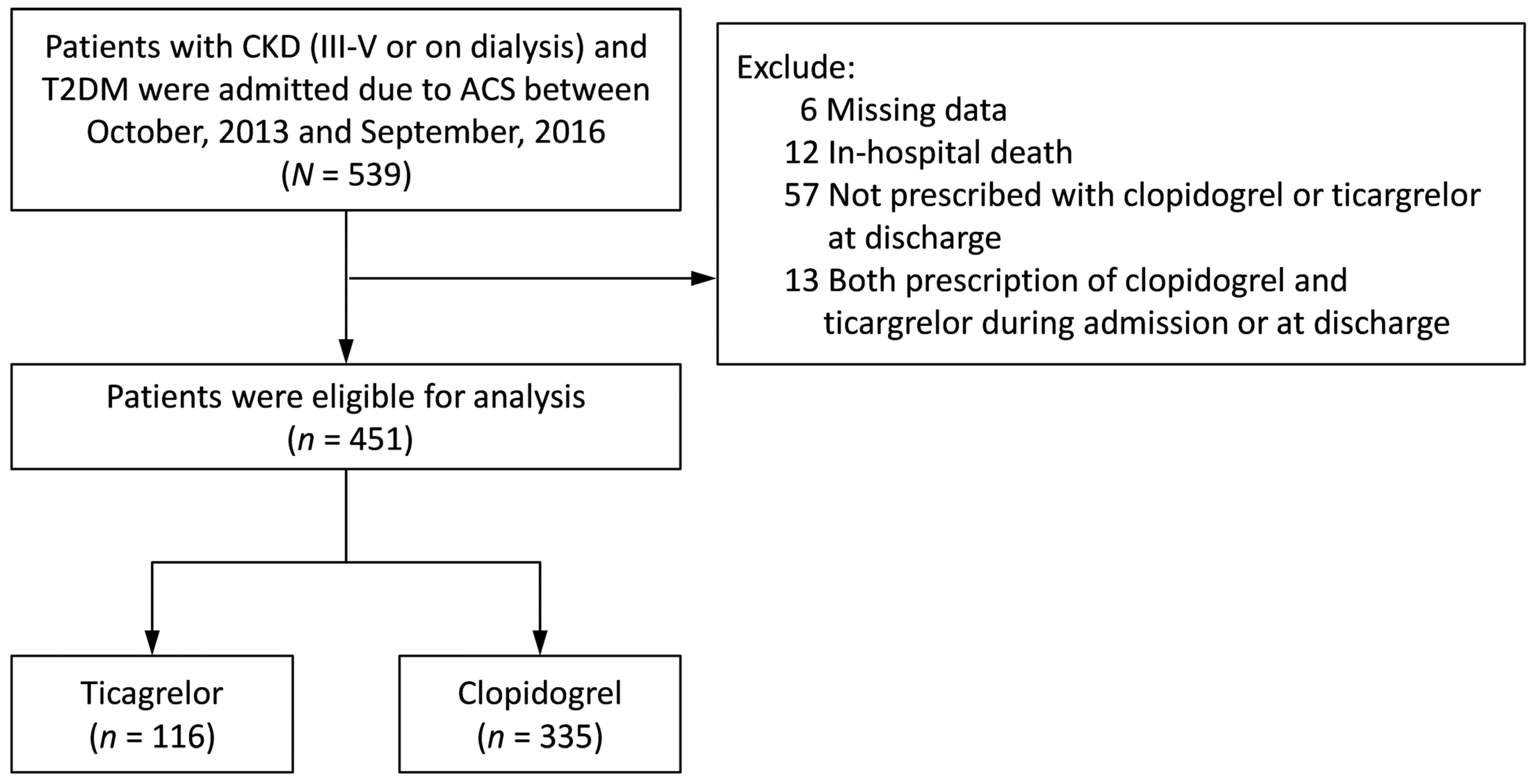
3.1. Study Population
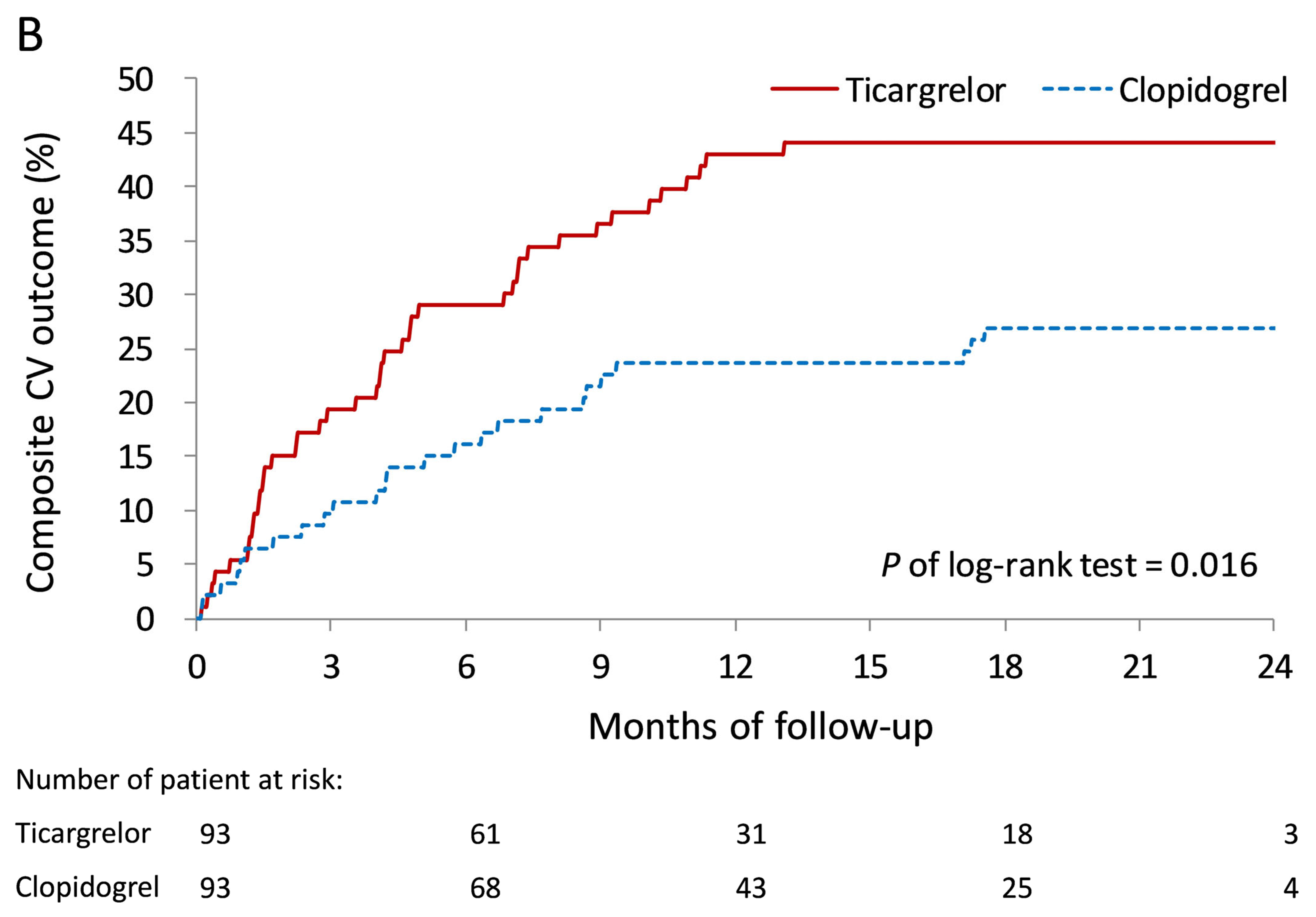
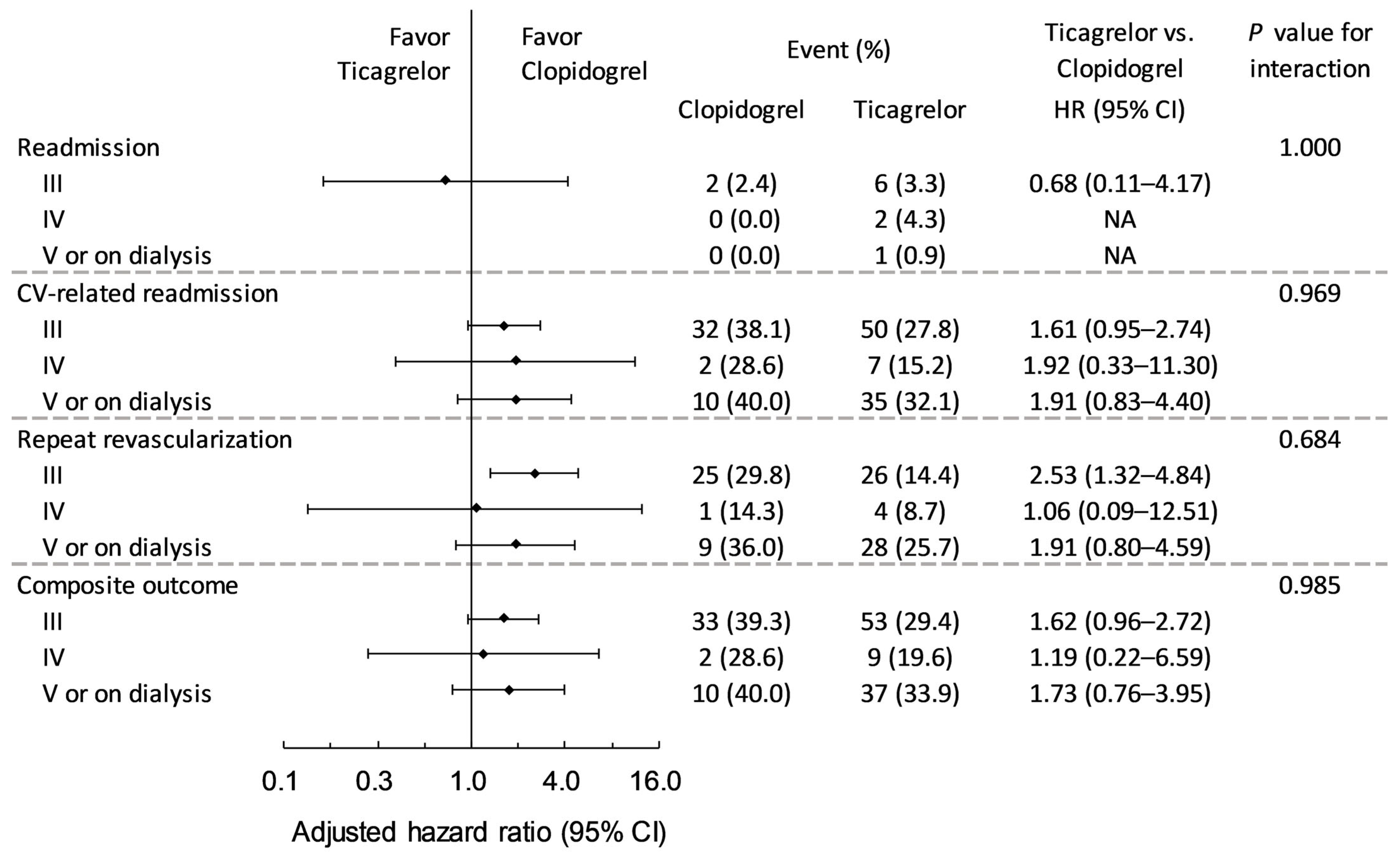
3.2. Primary Outcomes During Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jneid, H.; Bhatt, D.L.; Corti, R.; Badimon, J.J.; Fuster, V.; Francis, G.S. Aspirin and clopidogrel in acute coronary syndromes: Therapeutic insights from the cure study. Arch. Intern. Med. 2003, 163, 1145–1153. [Google Scholar] [CrossRef]
- James, S.; Akerblom, A.; Cannon, C.P.; Emanuelsson, H.; Husted, S.; Katus, H.; Skene, A.; Steg, P.G.; Storey, R.F.; Harrington, R.; et al. Comparison of ticagrelor, the first reversible oral p2y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the platelet inhibition and patient outcomes (plato) trial. Am. Heart J. 2009, 157, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Bonello, L.; Armero, S.; Ait Mokhtar, O.; Mancini, J.; Aldebert, P.; Saut, N.; Bonello, N.; Barragan, P.; Arques, S.; Giacomoni, M.P.; et al. Clopidogrel loading dose adjustment according to platelet reactivity monitoring in patients carrying the 2c19*2 loss of function polymorphism. J. Am. Coll. Cardiol. 2010, 56, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: A report of the american college of cardiology foundation/american heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation 2011, 124, e574–e651. [Google Scholar] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 aha/acc guideline for the management of patients with non-st-elevation acute coronary syndromes: A report of the american college of cardiology/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Storey, R.F.; Becker, R.C.; Harrington, R.A.; Husted, S.; James, S.K.; Cools, F.; Steg, P.G.; Khurmi, N.S.; Emanuelsson, H.; Cooper, A.; et al. Characterization of dyspnoea in plato study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur. Heart J. 2011, 32, 2945–2953. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Wojdyla, D.M.; Carroll, K.; Becker, R.C.; Storey, R.F.; Angiolillo, D.J.; Held, C.; Cannon, C.P.; James, S.; Pieper, K.S.; et al. Ticagrelor compared with clopidogrel by geographic region in the platelet inhibition and patient outcomes (plato) trial. Circulation 2011, 124, 544–554. [Google Scholar] [CrossRef]
- Turgeon, R.D.; Koshman, S.L.; Youngson, E.; Har, B.; Wilton, S.B.; James, M.T.; Graham, M.M. Association of ticagrelor vs clopidogrel with major adverse coronary events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA Intern. Med. 2020, 180, 420–428. [Google Scholar] [CrossRef]
- James, S.; Angiolillo, D.J.; Cornel, J.H.; Erlinge, D.; Husted, S.; Kontny, F.; Maya, J.; Nicolau, J.C.; Spinar, J.; Storey, R.F.; et al. Ticagrelor vs. Clopidogrel in patients with acute coronary syndromes and diabetes: A substudy from the platelet inhibition and patient outcomes (plato) trial. Eur. Heart J. 2010, 31, 3006–3016. [Google Scholar] [CrossRef]
- Goto, S.; Huang, C.H.; Park, S.J.; Emanuelsson, H.; Kimura, T. Ticagrelor vs. Clopidogrel in japanese, korean and taiwanese patients with acute coronary syndrome—Randomized, double-blind, phase iii philo study. Circ. J. 2015, 79, 2452–2460. [Google Scholar] [CrossRef]
- Park, D.W.; Kwon, O.; Jang, J.S.; Yun, S.C.; Park, H.; Kang, D.Y.; Ahn, J.M.; Lee, P.H.; Lee, S.W.; Park, S.W.; et al. Clinically significant bleeding with ticagrelor versus clopidogrel in korean patients with acute coronary syndromes intended for invasive management: A randomized clinical trial. Circulation 2019, 140, 1865–1877. [Google Scholar] [CrossRef]
- Tung, Y.C.; Chang, C.J.; Liu, J.R.; Chang, S.H.; Chan, Y.H.; Kuo, C.T.; See, L.C. Outcomes after ticagrelor versus clopidogrel treatment in end-stage renal disease patients with acute myocardial infarction: A nationwide cohort study. Sci. Rep. 2021, 11, 20826. [Google Scholar] [CrossRef]
- Li, Y.S.; Wang, S.H.; Hwang, S.J.; Yang, Y.H.; Hsieh, K.P. Comparison of effectiveness and safety between ticagrelor and clopidogrel in patients with acute coronary syndrome and on dialysis in taiwan. Br. J. Clin. Pharmacol. 2022, 88, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.L.; Lin, Y.; Chen, D.Y.; Lin, M.S.; Wang, C.Y.; Hsieh, I.C.; Yang, N.I.; Hung, M.J.; Chen, T.H. Ticagrelor versus adjusted-dose prasugrel in acute coronary syndrome with percutaneous coronary intervention. Clin. Pharmacol. Ther. 2024, 116, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Yin, W.H.; Wu, C.C.; Chan, S.H.; Wu, Y.W.; Yang Wang, K.; Chang, K.C.; Hwang, J.J.; Voon, W.C.; Hsieh, I.C.; et al. In-hospital implementation of evidence-based medications is associated with improved survival in diabetic patients with acute coronary syndrome—Data from tsoc acs-dm registry. Acta Cardiol. Sin. 2018, 34, 211–223. [Google Scholar] [PubMed]
- Li, Y.-H.; Yeh, H.-I.; Tsai, C.-T.; Liu, P.-Y.; Lin, T.-H.; Wu, T.-C.; Hung, K.-C.; Hsieh, Y.-C.; Mar, G.-Y.; Fang, C.-Y. 2012 guidelines of the Taiwan Society of Cardiology (TSOC) for the management of st-segment elevation myocardial infarction. Acta Cardiol. Sin. 2012, 28, 63–89. [Google Scholar]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013, 61, e78–e140. [Google Scholar] [CrossRef]
- James, S.; Budaj, A.; Aylward, P.; Buck, K.K.; Cannon, C.P.; Cornel, J.H.; Harrington, R.A.; Horrow, J.; Katus, H.; Keltai, M.; et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: Results from the platelet inhibition and patient outcomes (plato) trial. Circulation 2010, 122, 1056–1067. [Google Scholar] [CrossRef]
- James, S.K.; Roe, M.T.; Cannon, C.P.; Cornel, J.H.; Horrow, J.; Husted, S.; Katus, H.; Morais, J.; Steg, P.G.; Storey, R.F.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: Substudy from prospective randomised platelet inhibition and patient outcomes (plato) trial. BMJ 2011, 342, d3527. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, J.; Li, Y.; Tang, Y.; Li, C.; Li, J.; Han, Y. Pharmacodynamics and pharmacokinetics of ticagrelor vs. Clopidogrel in patients with acute coronary syndromes and chronic kidney disease. Br. J. Clin. Pharmacol. 2018, 84, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, I.T.; Doundoulakis, I.; Zafeiropoulos, S.; Pagiantza, A.; Apostolidou-Kiouti, F.; Kourti, O.; Kassimis, G.; Haidich, A.-B.; Karvounis, H.; Giannakoulas, G. Comparative efficacy and safety of oral p2y12 inhibitors for patients with chronic kidney disease and acute coronary syndrome: A network meta-analysis. Hell. J. Cardiol. 2022, 63, 40–65. [Google Scholar] [CrossRef] [PubMed]
- Best, P.J.; Steinhubl, S.R.; Berger, P.B.; Dasgupta, A.; Brennan, D.M.; Szczech, L.A.; Califf, R.M.; Topol, E.J.; Investigators, C. The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: Results from the clopidogrel for the reduction of events during observation (credo) trial. Am. Heart J. 2008, 155, 687–693. [Google Scholar] [CrossRef]
- Edfors, R.; Sahlen, A.; Szummer, K.; Renlund, H.; Evans, M.; Carrero, J.J.; Spaak, J.; James, S.K.; Lagerqvist, B.; Varenhorst, C.; et al. Outcomes in patients treated with ticagrelor versus clopidogrel after acute myocardial infarction stratified by renal function. Heart 2018, 104, 1575–1582. [Google Scholar] [CrossRef]
- Jeong, Y.-H. “East asian paradox”: Challenge for the current antiplatelet strategy of “one-guideline-fits-all races” in acute coronary syndrome. Curr. Cardiol. Rep. 2014, 16, 485. [Google Scholar] [CrossRef]
- Levine, G.N.; Jeong, Y.H.; Goto, S.; Anderson, J.L.; Huo, Y.; Mega, J.L.; Taubert, K.; Smith, S.C., Jr. Expert consensus document: World heart federation expert consensus statement on antiplatelet therapy in east asian patients with acs or undergoing pci. Nat. Rev. Cardiol. 2014, 11, 597–606. [Google Scholar] [CrossRef]
- Franchi, F.; Rollini, F.; Been, L.; Maaliki, N.; Abou Jaoude, P.; Rivas, A.; Zhou, X.; Jia, S.; Briceno, M.; Lee, C.H.; et al. Impact of chronic kidney disease on the pharmacodynamic and pharmacokinetic effects of ticagrelor in patients with diabetes mellitus and coronary artery disease. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 452–461. [Google Scholar] [CrossRef]
- Provenzano, M.; Rivoli, L.; Garofalo, C.; Faga, T.; Pelagi, E.; Perticone, M.; Serra, R.; Michael, A.; Comi, N.; Andreucci, M. Renal resistive index in chronic kidney disease patients: Possible determinants and risk profile. PLoS ONE 2020, 15, e0230020. [Google Scholar] [CrossRef]

| Variable | Missing Value (no.) | Ticagrelor (n = 116) | Clopidogrel (n = 335) | p Value | STD |
|---|---|---|---|---|---|
| Male sex, n (%) | 0 | 78 (67.2) | 223 (66.6) | 1.000 | 0.01 |
| Age (years) | 0 | 66.6 ± 11.2 | 68.7 ± 10.5 | 0.062 | −0.19 |
| Body mass index (kg/m2) | 56 | 25.9 ± 3.5 | 26.0 ± 4.2 | 0.712 | −0.03 |
| Waist circumference (cm) | 378 | 91.8 ± 6.4 | 93.1 ± 8.2 | 0.130 | −0.18 |
| Creatinine (mg/dL) | 8 | 3.0 ± 2.9 | 3.5 ± 3.1 | 0.108 | −0.17 |
| eGFR (mL/min/1.73 m2) | 8 | 36.9 ± 18.2 | 31.5 ± 18.5 | 0.006 | 0.29 |
| CKD stage, n (%) | 0 | 0.004 | |||
| III | 84 (72.4) | 180 (53.7) | 0.39 | ||
| IV | 7 (6.0) | 46 (13.7) | −0.26 | ||
| V | 7 (6.0) | 35 (10.4) | −0.16 | ||
| On dialysis | 18 (15.5) | 74 (22.1) | −0.17 | ||
| DM duration (years) | 138 | 11.2 ± 7.0 | 12.4 ± 7.3 | 0.115 | −0.17 |
| Etiology of ACS, n (%) | 0 | 0.001 | |||
| Atypical chest pain | 13 (11.2) | 56 (16.7) | −0.16 | ||
| Unstable angina | 11 (9.5) | 73 (21.8) | −0.34 | ||
| Myocardial Infarction | 92 (79.3) | 206 (61.5) | 0.40 | ||
| Culprit lesion | 0 | <0.001 | |||
| 50–70% | 6 (1.8) | 2 (1.7) | 0.01 | ||
| 70–90% | 124 (37.0) | 11 (9.5) | 0.69 | ||
| ≥90% | 205 (61.2) | 103 (88.8) | −0.67 | ||
| TIMI flow | 0 | 0.684 | |||
| Occluded (TIMI 0/1) | 163 (48.7) | 59 (50.9) | −0.04 | ||
| Slow (TIMI 2) | 36 (10.7) | 8 (6.9) | 0.13 | ||
| Normal (TIMI 3) | 76 (22.7) | 26 (22.4) | 0.01 | ||
| Unknown or Missing | 60 (17.9) | 23 (19.8) | −0.05 | ||
| LM stenosis | 0 | 40 (11.9) | 11 (9.5) | 0.610 | |
| Number of vessels | 0 | 0.105 | |||
| 0 (including normal) | (0.0) | 1 (0.9) | |||
| I | 100 (29.9) | 51 (44.0) | −0.30 | ||
| II | 91 (27.2) | 23 (19.8) | 0.18 | ||
| III | 104 (31.0) | 30 (25.9) | 0.11 | ||
| LM | 1 (0.3) | (0.0) | 0.08 | ||
| LM + I | 4 (1.2) | 1 (0.9) | 0.03 | ||
| LM + II | 8 (2.4) | 3 (2.6) | −0.01 | ||
| LM + III | 27 (8.1) | 7 (6.0) | 0.08 | ||
| Culprit artery territory | 0 | 0.095 | |||
| LM | 9 (2.7) | 3 (2.6) | 0.01 | ||
| LAD | 147 (43.9) | 37 (31.9) | 0.25 | ||
| LCx | 56 (16.7) | 25 (21.6) | −0.12 | ||
| RCA | 113 (33.7) | 50 (43.1) | −0.19 | ||
| None or missing | 10 (3.0) | 1 (0.9) | 0.15 | ||
| LVEF | 0 | 0.594 | |||
| Normal | 134 (40.0) | 49 (42.2) | −0.04 | ||
| 40–50% | 77 (23.0) | 21 (18.1) | 0.12 | ||
| 30–40% | 33 (9.9) | 16 (13.8) | −0.12 | ||
| <30% | 22 (6.6) | 9 (7.8) | −0.05 | ||
| Missing | 69 (20.6) | 21 (18.1) | 0.06 | ||
| Treatment during the admission | |||||
| Coronary intervention | 0 | 110 (94.8) | 298 (89.0) | 0.068 | 0.21 |
| Coronary stenting | 0 | 107 (92.2) | 297 (88.7) | 0.378 | 0.12 |
| Concomitant disease, n (%) | |||||
| Hypertension | 0 | 99 (85.3) | 303 (90.4) | 0.165 | −0.16 |
| Dyslipidemia | 0 | 58 (50.0) | 189 (56.4) | 0.236 | −0.13 |
| History of event, n (%) | |||||
| Known CAD | 0 | 48 (41.4) | 159 (47.5) | 0.281 | −0.12 |
| Old myocardial infarction | 0 | 20 (17.2) | 77 (23.0) | 0.238 | −0.15 |
| Previous PCI | 0 | 33 (28.4) | 118 (35.2) | 0.210 | −0.15 |
| Previous CABG | 0 | 2 (1.7) | 31 (9.3) | 0.006 | −0.34 |
| Atrial fibrillation | 0 | 2 (1.7) | 18 (5.4) | 0.120 | −0.20 |
| Heart failure | 0 | 10 (8.6) | 42 (12.5) | 0.313 | −0.13 |
| COPD | 0 | 6 (5.2) | 14 (4.2) | 0.610 | 0.05 |
| Peripheral artery disease | 0 | 3 (2.6) | 29 (8.7) | 0.034 | −0.27 |
| Cancer | 0 | 1 (0.9) | 17 (5.1) | 0.053 | −0.25 |
| Ischemic stroke | 0 | 4 (3.4) | 38 (11.3) | 0.009 | −0.31 |
| Hemorrhagic stroke | 0 | 6 (5.2) | 8 (2.4) | 0.209 | 0.15 |
| Lipid profile | |||||
| Troponin I (ng/mL) | 38 | 1.18 [0.17, 9.08] | 1.00 [0.09, 13.16] | 0.593 | NA |
| LDL-C (mg/dL) | 79 | 96.4 ± 29.7 | 92.9 ± 34.6 | 0.326 | 0.11 |
| Total cholesterol (mg/dL) | 60 | 163.5 ± 38.7 | 158.0 ± 42.0 | 0.216 | 0.14 |
| HDL-C (mg/dL) | 115 | 38.6 ± 10.5 | 39.1 ± 10.6 | 0.634 | −0.05 |
| Triglyceride (mg/dL) | 66 | 165.4 ± 123.7 | 154.7 ± 104.2 | 0.365 | 0.09 |
| NT-proBNP (mg/dL) | 381 | 9191 [3660, 16,460] | 14,620 [7552, 21,796] | <0.001 | NA |
| HbA1C (%) | 183 | 8.1 ± 1.5 | 7.8 ± 1.2 | 0.013 | 0.22 |
| Fasting glucose (mg/dL) | 136 | 214.8 ± 75.4 | 200.3 ± 83.6 | 0.099 | 0.18 |
| Hemoglobin (mg/dL) | 8 | 12.4 ± 2.1 | 11.8 ± 2.5 | 0.020 | 0.26 |
| Smoking, n (%) | 0 | 27 (23.3) | 79 (23.6) | 1.000 | −0.01 |
| Lowering glucose drug, n (%) | |||||
| Insulin | 0 | 52 (44.8) | 123 (36.7) | 0.124 | 0.17 |
| Sulfonylurea | 0 | 40 (34.5) | 106 (31.6) | 0.567 | 0.06 |
| Mitiglinide | 0 | 12 (10.3) | 47 (14.0) | 0.342 | −0.11 |
| Metformin | 0 | 34 (29.3) | 75 (22.4) | 0.166 | 0.16 |
| Thiazolidinedione | 0 | 1 (0.9) | 6 (1.8) | 0.683 | −0.08 |
| Acarbose | 0 | 11 (9.5) | 24 (7.2) | 0.424 | 0.08 |
| DPP4 inhibitor | 0 | 49 (42.2) | 130 (38.8) | 0.512 | 0.07 |
| Non-DM medication, n (%) | |||||
| Aspirin | 0 | 109 (94.0) | 294 (87.8) | 0.079 | 0.22 |
| Anticoagulant | 0 | 2 (1.7) | 10 (3.0) | 0.739 | −0.09 |
| GP IIb/IIIa Inhibitor | 0 | 7 (6.0) | 9 (2.7) | 0.140 | 0.16 |
| Heparin or LMWH | 0 | 76 (65.5) | 223 (66.6) | 0.909 | −0.02 |
| ACEI or ARB | 0 | 76 (65.5) | 192 (57.3) | 0.126 | 0.17 |
| Beta-blocker | 0 | 86 (74.1) | 222 (66.3) | 0.133 | 0.17 |
| Statin | 0 | 86 (74.1) | 257 (76.7) | 0.614 | −0.06 |
| Calcium channel blocker | 0 | 25 (21.6) | 139 (41.5) | <0.001 | −0.44 |
| Digoxin | 0 | 2 (1.7) | 11 (3.3) | 0.530 | −0.10 |
| Diuretic | 0 | 37 (31.9) | 155 (46.3) | 0.009 | −0.30 |
| IV Inotropic agent | 0 | 5 (4.3) | 35 (10.4) | 0.057 | −0.24 |
| Nitrate | 0 | 49 (42.2) | 156 (46.6) | 0.450 | −0.09 |
| Antiarrhythmic drug | 0 | 11 (9.5) | 38 (11.3) | 0.729 | −0.06 |
| Proton pump inhibitor | 0 | 42 (36.2) | 125 (37.3) | 0.911 | −0.02 |
| Follow up duration (year) | 0 | 1.3 ± 0.7 | 1.5 ± 0.6 | 0.027 | −0.31 |
| Number of Event (%) | Unadjusted Analysis | Adjusted Analysis * | ||||
|---|---|---|---|---|---|---|
| Outcome | Ticagrelor (n = 116) | Clopidogrel (n = 335) | HR (95% CI) of Ticagrelor | p | HR (95% CI) of Ticagrelor | p |
| 6 month follow-up | ||||||
| Death | 7 (6.0) | 14 (4.2) | 1.56 (0.63–3.87) | 0.334 | 2.35 (0.80–6.87) | 0.118 |
| CV death | 1 (0.9) | 4 (1.2) | 0.79 (0.09–7.03) | 0.829 | 0.79 (0.06–9.60) | 0.850 |
| Readmission | 39 (33.6) | 87 (26.0) | 1.46 (1.00–2.13) | 0.049 | 1.76 (1.12–2.77) | 0.014 |
| CV-related readmission | 28 (24.1) | 48 (14.3) | 1.95 (1.22–3.10) | 0.005 | 2.16 (1.24–3.77) | 0.007 |
| Repeat revascularization | 21 (18.1) | 24 (7.2) | 2.87 (1.60–5.16) | <0.001 | 3.34 (1.66–6.68) | 0.001 |
| PCI | 20 (17.2) | 22 (6.6) | 3.03 (1.65–5.54) | <0.001 | 3.80 (1.86–7.79) | <0.001 |
| CABG | 2 (1.7) | 2 (0.6) | 3.07 (0.43–21.82) | 0.261 | 2.16 (0.21–22.07) | 0.516 |
| Composite outcome # | 29 (25.0) | 52 (15.5) | 1.86 (1.18–2.93) | 0.008 | 1.97 (1.15–3.40) | 0.014 |
| 1 year follow-up | ||||||
| Death | 9 (7.8) | 30 (9.0) | 0.94 (0.45–1.98) | 0.870 | 1.26 (0.53–2.98) | 0.603 |
| CV death | 2 (1.7) | 8 (2.4) | 0.78 (0.17–3.68) | 0.755 | 0.46 (0.08–2.61) | 0.383 |
| Readmission | 57 (49.1) | 140 (41.8) | 1.34 (0.99–1.83) | 0.059 | 1.72 (1.19–2.49) | 0.004 |
| CV-related readmission | 43 (37.1) | 78 (23.3) | 1.59 (1.09–2.32) | 0.015 | 1.62 (1.04–2.51) | 0.032 |
| Repeat revascularization | 35 (30.2) | 47 (14.0) | 2.53 (1.63–3.91) | <0.001 | 2.63 (1.56–4.43) | <0.001 |
| PCI | 35 (30.2) | 45 (13.4) | 2.67 (1.71–4.15) | <0.001 | 2.84 (1.68–4.80) | <0.001 |
| CABG | 2 (1.7) | 2 (0.6) | 3.07 (0.43–21.82) | 0.261 | 2.16 (0.21–22.07) | 0.516 |
| Composite outcome # | 44 (37.9) | 85 (25.4) | 1.49 (1.03–2.15) | 0.034 | 1.49 (0.97–2.29) | 0.069 |
| 2 year follow-up | ||||||
| Death | 11 (9.5) | 41 (12.2) | 0.85 (0.44–1.66) | 0.638 | 1.29 (0.60–2.80) | 0.514 |
| CV death | 2 (1.7) | 9 (2.7) | 0.71 (0.15–3.28) | 0.659 | 0.48 (0.09–2.66) | 0.403 |
| Readmission | 60 (51.7) | 161 (48.1) | 1.25 (0.93–1.68) | 0.139 | 1.59 (1.12–2.28) | 0.010 |
| CV-related readmission | 44 (37.9) | 92 (27.5) | 1.67 (1.16–2.39) | 0.005 | 1.72 (1.12–2.65) | 0.014 |
| Repeat revascularization | 35 (30.2) | 58 (17.3) | 2.09 (1.37–3.18) | 0.001 | 2.24 (1.36–3.68) | 0.002 |
| PCI | 35 (30.2) | 56 (16.7) | 2.19 (1.43–3.33) | <0.001 | 2.39 (1.45–3.95) | 0.001 |
| CABG | 2 (1.7) | 2 (0.6) | 3.07 (0.43–21.82) | 0.261 | 2.16 (0.21–22.07) | 0.516 |
| Composite outcome # | 45 (38.8) | 99 (29.6) | 1.59 (1.12–2.27) | 0.010 | 1.63 (1.06–2.48) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-L.; Wu, V.C.-C.; Shyu, K.-G.; Hsieh, I.-C.; Chen, T.-H.; Tsai, M.-L. Ticagrelor Versus Clopidogrel in Patients with Acute Coronary Syndrome and Chronic Kidney Disease: A Real-World Analysis from a National Registry. Medicina 2025, 61, 1804. https://doi.org/10.3390/medicina61101804
Wang T-L, Wu VC-C, Shyu K-G, Hsieh I-C, Chen T-H, Tsai M-L. Ticagrelor Versus Clopidogrel in Patients with Acute Coronary Syndrome and Chronic Kidney Disease: A Real-World Analysis from a National Registry. Medicina. 2025; 61(10):1804. https://doi.org/10.3390/medicina61101804
Chicago/Turabian StyleWang, Tzu-Lin, Victor Chien-Chia Wu, Kou-Gi Shyu, I-Chang Hsieh, Tien-Hsing Chen, and Ming-Lung Tsai. 2025. "Ticagrelor Versus Clopidogrel in Patients with Acute Coronary Syndrome and Chronic Kidney Disease: A Real-World Analysis from a National Registry" Medicina 61, no. 10: 1804. https://doi.org/10.3390/medicina61101804
APA StyleWang, T.-L., Wu, V. C.-C., Shyu, K.-G., Hsieh, I.-C., Chen, T.-H., & Tsai, M.-L. (2025). Ticagrelor Versus Clopidogrel in Patients with Acute Coronary Syndrome and Chronic Kidney Disease: A Real-World Analysis from a National Registry. Medicina, 61(10), 1804. https://doi.org/10.3390/medicina61101804







