ICU Admission-Related Factors Affecting the Duration of Mechanical Ventilation After Elective Cardiac Surgery—Retrospective Cohort Study from a Tertiary Center in Croatia
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Outcome Measure
2.4. Weaning and Extubation Criteria
2.5. Data Collection
2.6. Clinical Scoring Tools
2.7. Statistical Analysis
3. Results
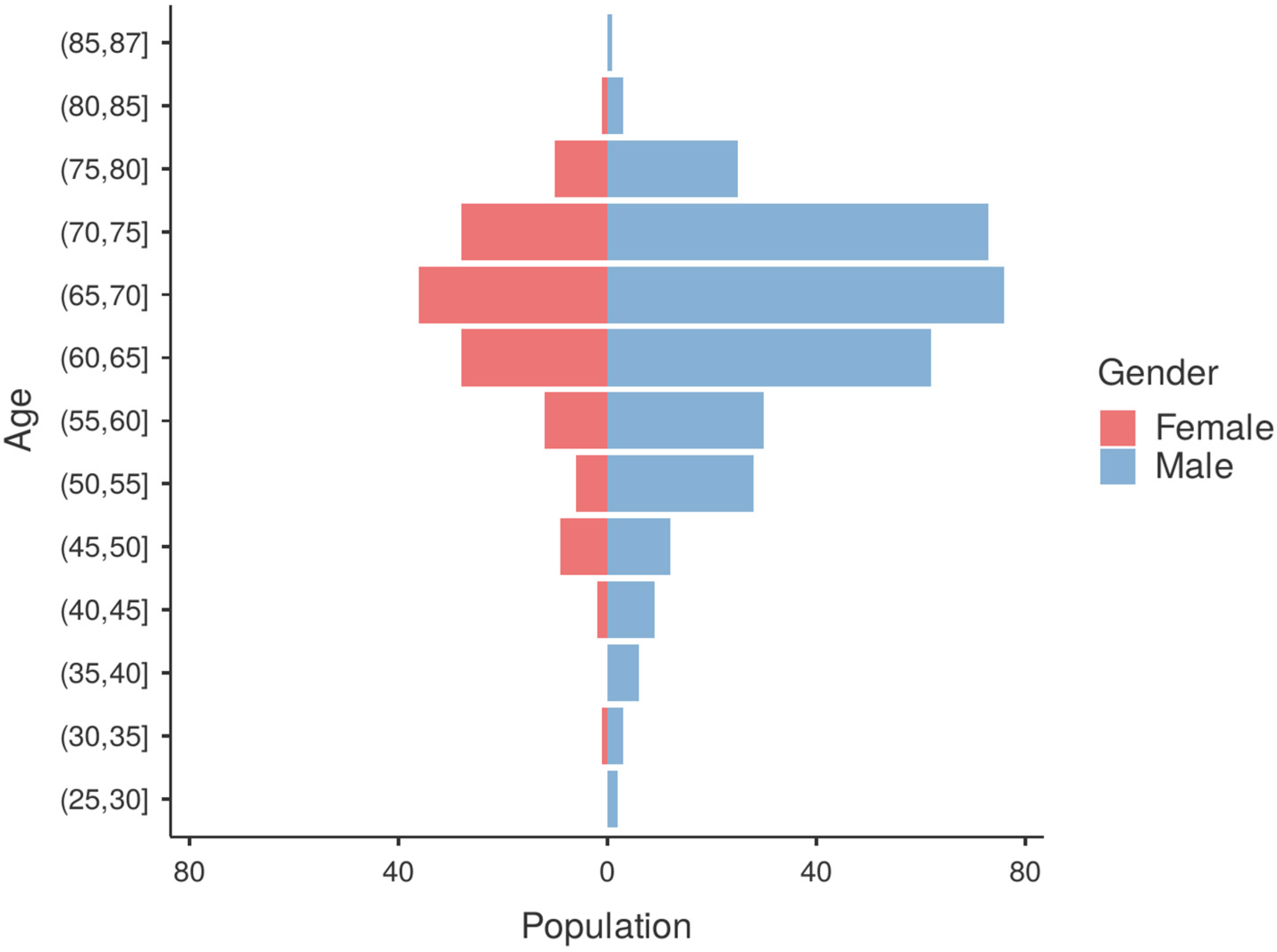
3.1. Patient Characteristics
3.2. Factors Affecting the Duration of Mechanical Ventilation
3.3. Prognostic Factors Determining the Duration of Mechanical Ventilation—Logistic Regression Model
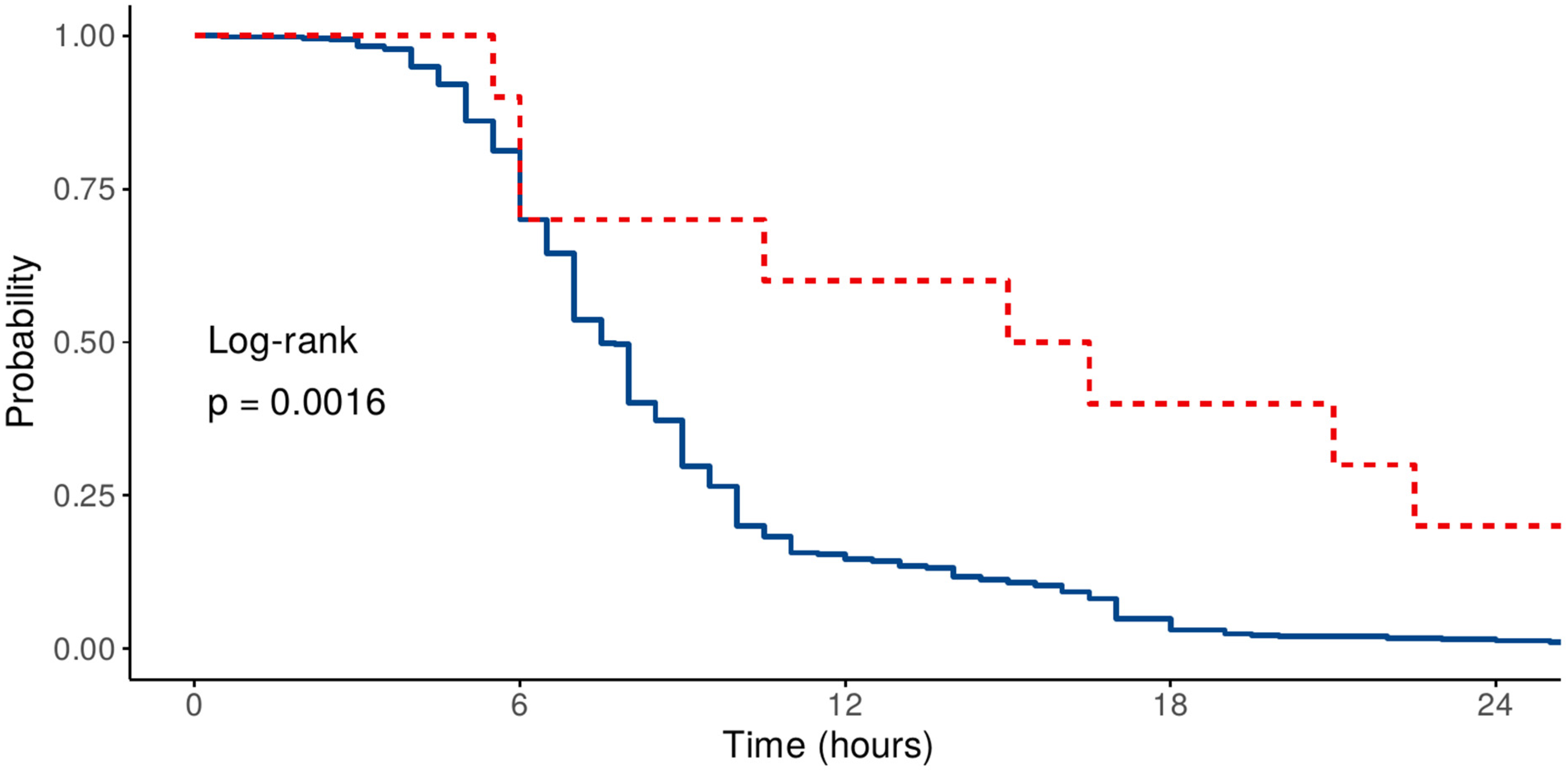
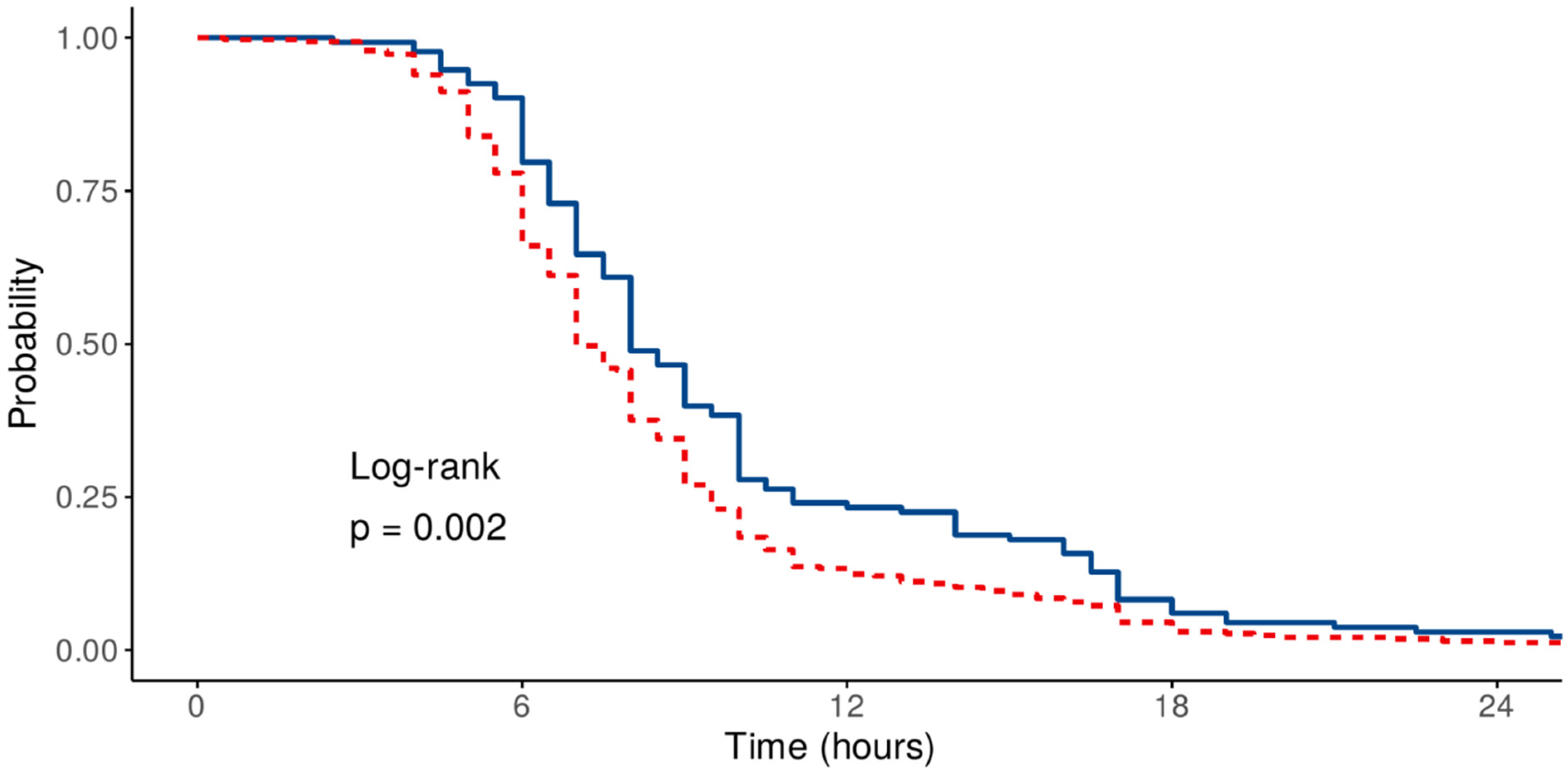
3.4. Prognostic Factors Determining the Duration of Mechanical Ventilation—Time-to-Event Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goecke, S.; Pitts, L.; Dini, M.; Montagner, M.; Wert, L.; Akansel, S.; Kofler, M.; Stoppe, C.; Ott, S.; Jacobs, S.; et al. Enhanced Recovery After Cardiac Surgery for Minimally Invasive Valve Surgery: A Systematic Review of Key Elements and Advancements. Medicina 2025, 61, 495. [Google Scholar] [CrossRef]
- De Bie, A.J.R.; Neto, A.S.; van Meenen, D.M.; Bouwman, A.R.; Roos, A.N.; Lameijer, J.R.; Korsten, E.H.M.; Schultz, M.J.; Bindels, A.J.G.H. Fully Automated Postoperative Ventilation in Cardiac Surgery Patients: A Randomised Clinical Trial. Br. J. Anaesth. 2020, 125, 739–749. [Google Scholar] [CrossRef]
- Bernardi, M.H.; Bettex, D.; Buiteman-Kruizinga, L.A.; de Bie, A.; Hoffmann, M.; de Kleijn, J.; Serafini, S.C.; Molenaar, M.A.; Paulus, F.; Peršec, J.; et al. POStoperative INTELLiVENT-Adaptive Support VEntilation in Cardiac Surgery Patients (POSITiVE) II-Study Protocol of a Randomized Clinical Trial. Trials 2024, 25, 449. [Google Scholar] [CrossRef]
- Cordeiro, A.L.L.; Oliveira, L.F.D.L.; Queiroz, T.C.; Santana, V.L.L.D.; Melo, T.A.D.; Guimarães, A.R.; Martinez, B.P. Association of Respiratory Mechanics with Oxygenation and Duration of Mechanical Ventilation After Cardiac Surgery. Int. J. Cardiovasc. Sci. 2018, 31, 244–249. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Cui, R.; An, B. A Study of Mechanical Ventilation in the ICU after Cardiac Surgery: A Bibliometric Analysis. J. Thorac. Dis. 2022, 14, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Ceriani, R.; Mazzoni, M.; Bortone, F.; Gandini, S.; Solinas, C.; Susini, G.; Parodi, O. Application of the Sequential Organ Failure Assessment Score to Cardiac Surgical Patients. Chest 2003, 123, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Chen, S.-W.; Fan, P.-C.; Lee, C.-C.; Yang, H.-Y.; Chang, S.-W.; Pan, H.-C.; Tsai, F.-C.; Yang, C.-W.; Chen, Y.-C. Sequential Organ Failure Assessment Score Predicts Mortality after Coronary Artery Bypass Grafting. BMC Surg. 2017, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Schoe, A.; Bakhshi-Raiez, F.; de Keizer, N.; van Dissel, J.T.; de Jonge, E. Mortality Prediction by SOFA Score in ICU-Patients after Cardiac Surgery; Comparison with Traditional Prognostic–Models. BMC Anesthesiol. 2020, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Baris, O.; Oksuzler Kizilbay, G.; Holat, C.M.; Uzturk, M.E.; Canikoglu, M.; Durmaz, A.; Omay, O.; Yavuz, S. The Efficacy of the Charlson Comorbidity Index and Its Age-Adjusted Version in Forecasting Mortality and Postoperative Outcomes Following Isolated Coronary Artery Bypass Grafting. J. Clin. Med. 2025, 14, 395. [Google Scholar] [CrossRef] [PubMed]
- Koponen, T.; Karttunen, J.; Musialowicz, T.; Pietiläinen, L.; Uusaro, A.; Lahtinen, P. Vasoactive-Inotropic Score and the Prediction of Morbidity and Mortality after Cardiac Surgery. BJA Br. J. Anaesth. 2019, 122, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, W.; Yao, Y. The Association of Vasoactive-Inotropic Score and Surgical Patients’ Outcomes: A Systematic Review and Meta-Analysis. Syst. Rev. 2024, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- The Jamovi Project. Jamovi. 2019. Available online: www.jamovi.org (accessed on 15 June 2025).
- Balci, S. Comprehensive Analysis for Clinicopathological Research 2025. Available online: https://www.serdarbalci.com/ClinicoPathJamoviModule/ (accessed on 15 June 2025).
- Therneau, T.M.; Lumley, T.; Atkinson, E.; Crowson, C. Survival: Survival Analysis, version 3.8-3; R Foundation for Statistical Computing: Vienna, Austria, 2004.
- Sharma, V.; Rao, V.; Manlhiot, C.; Boruvka, A.; Fremes, S.; Wąsowicz, M. A Derived and Validated Score to Predict Prolonged Mechanical Ventilation in Patients Undergoing Cardiac Surgery. J. Thorac. Cardiovasc. Surg. 2017, 153, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Warren, O.J.; Smith, A.J.; Alexiou, C.; Rogers, P.L.; Jawad, N.; Vincent, C.; Darzi, A.W.; Athanasiou, T. The inflammatory response to cardiopulmonary bypass: Part 1—Mechanisms of pathogenesis. J. Cardiothorac. Vasc. Anesth. 2009, 23, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Kermani, M.S.; Dehesh, T.; Pouradeli, S.; Esmaili, B.S. Factors Affecting the Prolongation of Mechanical Ventilation in Patients after Cardiac Surgery. J. Cardiothorac. Surg. 2025, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Hessels, L.; Coulson, T.G.; Seevanayagam, S.; Young, P.; Pilcher, D.; Marhoon, N.; Bellomo, R. Development and Validation of a Score to Identify Cardiac Surgery Patients at High Risk of Prolonged Mechanical Ventilation. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Zamora, M.D.; Gordillo-Brenes, A.; Banderas-Bravo, E.; Arboleda-Sánchez, J.A.; Hinojosa-Pérez, R.; Aguilar-Alonso, E.; Herruzo-Aviles, Á.; Curiel-Balsera, E.; Sánchez-Rodríguez, Á.; Rivera-Fernández, R.; et al. Prolonged Mechanical Ventilation as a Predictor of Mortality After Cardiac Surgery. Respir. Care 2018, 63, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Lellouche, F.; Bouchard, P.-A.; Simard, S.; L’Her, E.; Wysocki, M. Evaluation of Fully Automated Ventilation: A Randomized Controlled Study in Post-Cardiac Surgery Patients. Intensive Care Med. 2013, 39, 463–471. [Google Scholar] [CrossRef] [PubMed]

| Clinical Scoring Tool | |||
|---|---|---|---|
| VIS | CCI | SOFA | |
| Measured Parameters (Score): | Vasoactive or Inotropic Drugs | Comorbidity Burden | Acute Organ System Status/Degree of Failure |
| 10,000 × vasopressin dose (IU/kg/min) | Age: <50, 50–59, 60–69, 70–79, >80 years (0–4 pts) | Respiratory (paO2/FiO2): 0–4 pts | |
| 100 × epinephrine or norepinephrine dose (μg/kg/min) | Myocardial infarction: no/yes (0/1 pts) | Cardiovascular (MAP or vasoactive drug dose): 0–4 pts | |
| 50 × levosimendan dose (μg/kg/min) | Congestive heart failure: no/yes (0/1 pts) | Neurologic (Glasgow Coma Score): 0–4 pts | |
| 25 × olprinone dose (μg/kg/min) | Peripheral vascular disease: no/yes (0/1 pts) | Hepatic (bilirubin): 0–4 pts | |
| 20 × methylene blue dose (mg/kg/h) | Cerebrovascular disease: no/yes (0/1 pts) | Renal (creatinine or diuresis): 0–4 mL | |
| 10 × milrinone or phenylephrine dose (μg/kg/min) | Dementia: no/yes (0/1 pts) | ||
| 10 × terlipressin dose (μg/min) | Chronic pulmonary disease: no/yes (0/1 pts) | ||
| 1 × Dobutamine, dopamine, or enoximone dose (μg/kg/min) | Connective tissue disease: no/yes (0/1 pts) | ||
| 0.25 × angiotensin II dose (ng/kg/min) | Peptic ulcer disease: no/yes (0/1 pts) | ||
| Liver disease: no/mild/moderate to severe (0, 1, 3 pts) | |||
| Diabetes mellitus: no, uncomplicated, end-organ damage (0–2 pts) | |||
| Hemiplegia: no/yes (0/2 pts) | |||
| Moderate-to-severe CKD: no/yes (0/2 pts) | |||
| Solid tumor: none/localized/metastatic (0/2/6 pts) | |||
| Leukemia: no/yes (0/2 pts) | |||
| Lymphoma: no/yes (0/2 pts) | |||
| AIDS: no/yes (0/2 pts) | |||
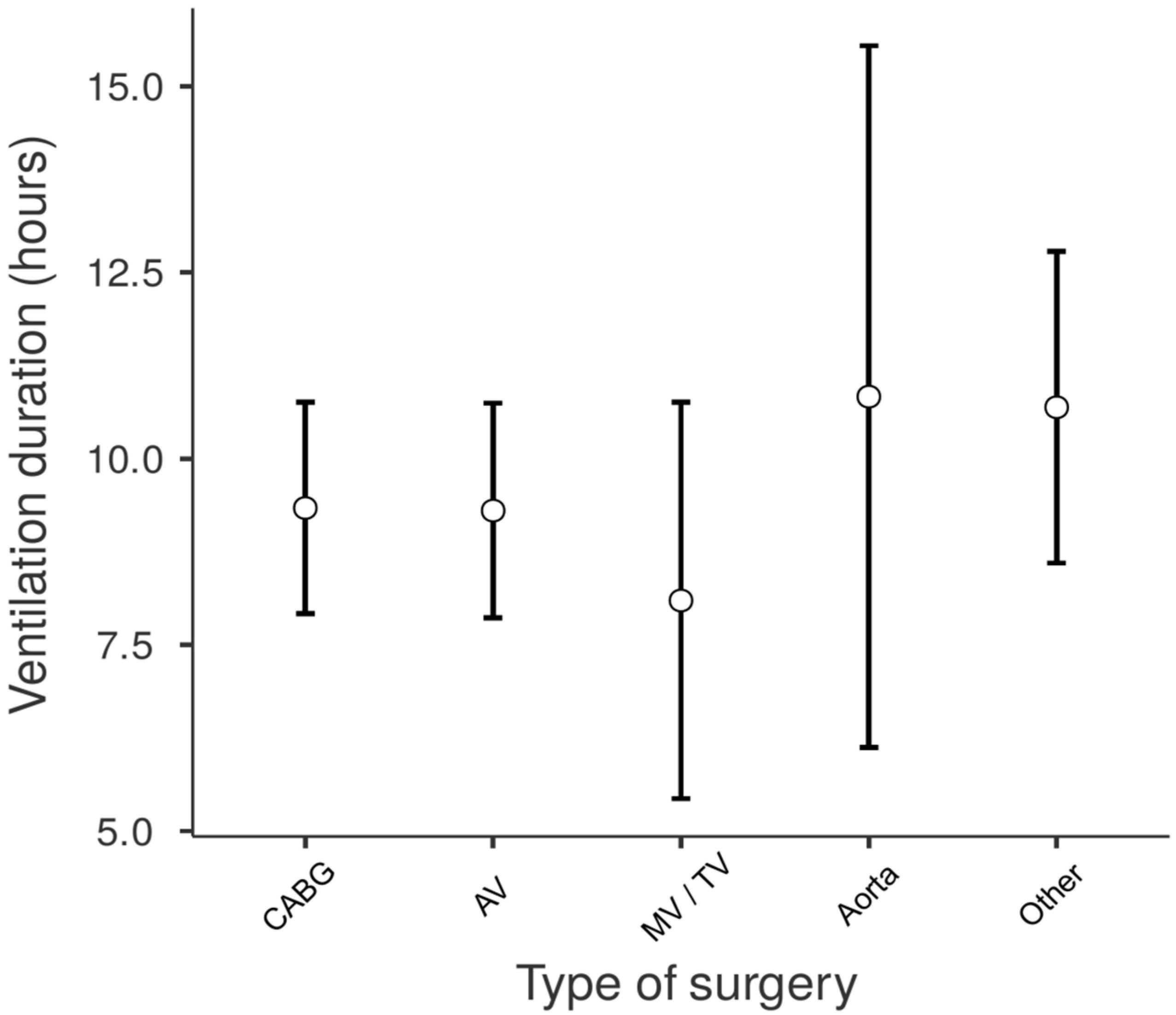
| Type of Surgery | Counts (%) | Duration of Mechanical Ventilation (Hours, Median, IQR) |
|---|---|---|
| CABG | 165 (35.6%) | 8 (6–10) |
| Aortic valve | 160 (34.6%) | 7.5 (6–10) |
| Mitral (+/− tricuspid) valve | 47 (10.2%) | 7 (5–9.25) |
| Ascendent aorta or aortic arch | 15 (3.2%) | 7 (5.5–13) |
| Other | 76 (16.4%) | 8 (6–10.6) |
| Age | CCI | VIS | Corrected SOFA | SOFA | ||
|---|---|---|---|---|---|---|
| Duration of ventilation | Spearman’s rho | 0.277 | 0.216 | 0.187 | 0.148 | 0.232 |
| p-value | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
| Prolonged Ventilation | |||||
|---|---|---|---|---|---|
| Yes | No | OR (95% CI)—Univariable | OR (95% CI)—Multivariable | ||
| Age | Mean (SD) | 68.1 (7.2) | 63.3 (10.3) | 0.94 (0.92–0.97, p < 0.001) | 0.95 (0.92–0.98, p = 0.003) |
| Sex | F | 51 (38.3) | 82 (61.7) | - | - |
| M | 76 (23%) | 254 (77%) | 2.08 (1.35–3.21, p = 0.001) | 1.98 (1.24–3.15, p = 0.004) | |
| Previous cardiac surgery | No | 120 (26.5%) | 333 (73.5%) | - | - |
| Yes | 7 (70%) | 3 (30%) | 0.15 (0.03–0.57, p = 0.007) | 0.15 (0.03–0.65, p = 0.015) | |
| Corrected SOFA | Mean (SD) | 3.4 (1.8) | 3.0 (1.7) | 0.85 (0.76–0.96, p = 0.009) | 0.91 (0.80–1.03, p = 0.141) |
| CCI | Mean (SD) | 4.6 (1.7) | 3.6 (1.9) | 0.75 (0.67–0.84, p < 0.001) | 0.86 (0.75–1.00, p = 0.041) |
| VIS | Mean (SD) | 5.4 (8.5) | 2.7 (5.3) | 0.94 (0.91–0.97, p < 0.001) | 0.95 (0.91–0.98, p = 0.001) |
| HR (95% CI)—Univariable | HR (95% CI)—Multivariable | |||
|---|---|---|---|---|
| Age | Mean (SD) | 64.7 (9.8) | 1.00 (0.98–1.02, p = 0.970) | 0.99 (0.96–1.01, p = 0.326) |
| Sex | Female | 133 (28.7) | - | - |
| Male | 330 (71.3) | 1.05 (0.74–1.50, p = 0.787) | 0.81 (0.56–1.19, p = 0.291) | |
| Previous cardiac surgery | No | 453 (97.8) | - | - |
| Yes | 10 (2.2) | 0.42 (0.19–0.91, p = 0.028) | 0.27 (0.11–0.64, p = 0.003) | |
| Corrected SOFA | Mean (SD) | 3.1 (1.7) | 0.89 (0.81–0.98, p = 0.020) | 0.88 (0.79–0.98, p = 0.017) |
| CCI | Mean (SD) | 3.8 (1.9) | 0.96 (0.86–1.06, p = 0.395) | 1.02 (0.91–1.15, p = 0.708) |
| VIS | Mean (SD) | 3.4 (6.4) | 0.96 (0.94–0.99, p = 0.002) | 0.95 (0.92–0.98, p < 0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristović, D.; Mikecin, V.; Presečki, I.; Šafarić Oremuš, Z.; Sojčić, N.; Gospić, I.; Lasić, H.; Sakan, S.; Kralj Husajna, D.; Bradić, N.; et al. ICU Admission-Related Factors Affecting the Duration of Mechanical Ventilation After Elective Cardiac Surgery—Retrospective Cohort Study from a Tertiary Center in Croatia. Medicina 2025, 61, 1778. https://doi.org/10.3390/medicina61101778
Kristović D, Mikecin V, Presečki I, Šafarić Oremuš Z, Sojčić N, Gospić I, Lasić H, Sakan S, Kralj Husajna D, Bradić N, et al. ICU Admission-Related Factors Affecting the Duration of Mechanical Ventilation After Elective Cardiac Surgery—Retrospective Cohort Study from a Tertiary Center in Croatia. Medicina. 2025; 61(10):1778. https://doi.org/10.3390/medicina61101778
Chicago/Turabian StyleKristović, Darko, Verica Mikecin, Ivana Presečki, Zrinka Šafarić Oremuš, Nataša Sojčić, Ivan Gospić, Hrvoje Lasić, Sanja Sakan, Danijela Kralj Husajna, Nikola Bradić, and et al. 2025. "ICU Admission-Related Factors Affecting the Duration of Mechanical Ventilation After Elective Cardiac Surgery—Retrospective Cohort Study from a Tertiary Center in Croatia" Medicina 61, no. 10: 1778. https://doi.org/10.3390/medicina61101778
APA StyleKristović, D., Mikecin, V., Presečki, I., Šafarić Oremuš, Z., Sojčić, N., Gospić, I., Lasić, H., Sakan, S., Kralj Husajna, D., Bradić, N., Peršec, J., & Šribar, A. (2025). ICU Admission-Related Factors Affecting the Duration of Mechanical Ventilation After Elective Cardiac Surgery—Retrospective Cohort Study from a Tertiary Center in Croatia. Medicina, 61(10), 1778. https://doi.org/10.3390/medicina61101778






