Modified Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction in 291 High-Level Athletes: Clinical Outcomes at Minimum 2.5-Year Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Eligibility Criteria
2.3. Surgical Technique
2.4. Rehabilitation Protocol
2.5. Outcome Measures
2.6. Data Collection
2.7. Statistical Analysis
3. Results
3.1. Rerupture Rate
3.2. Return to Sport
3.3. Tegner Activity Scale
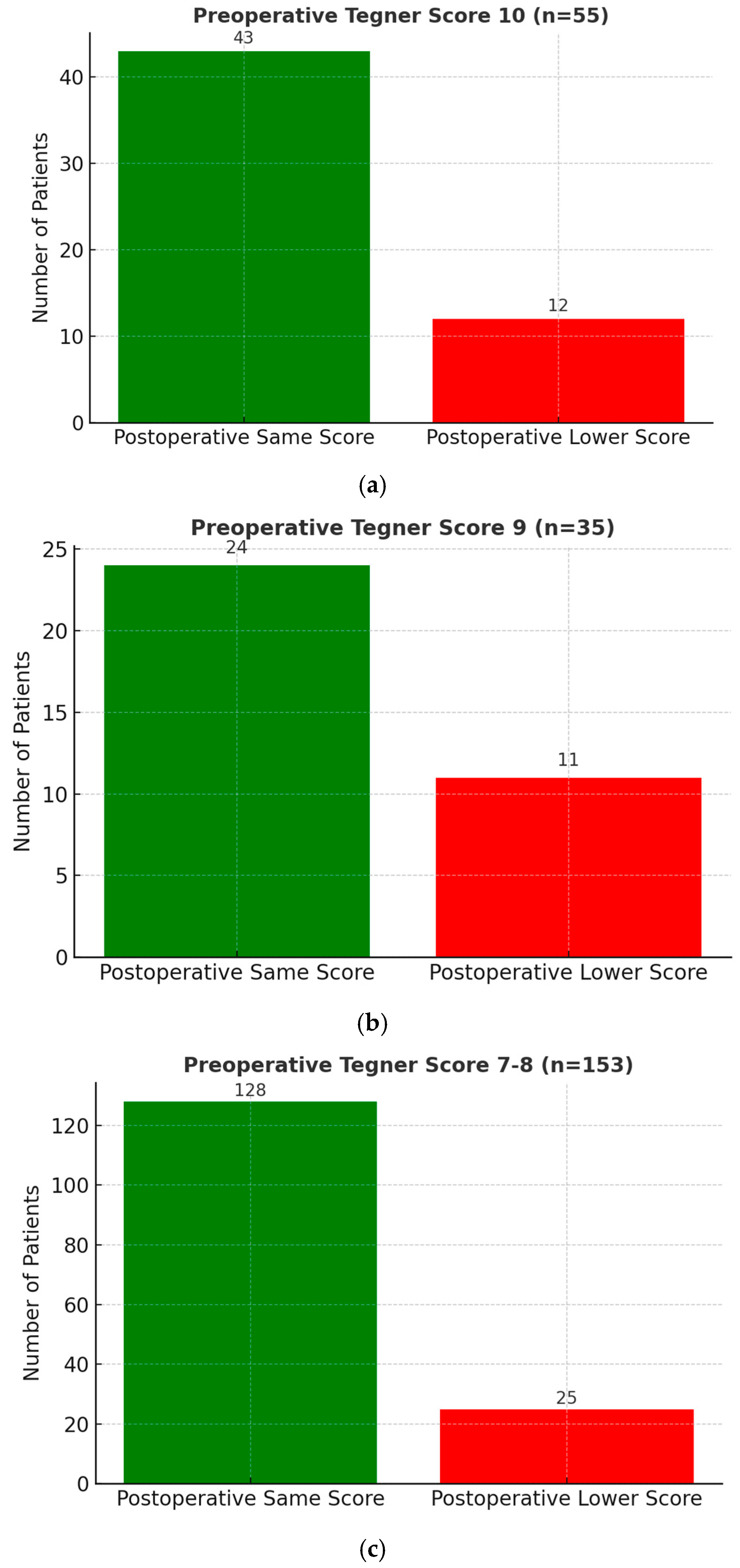
- Among 55 patients (14.0%) with a preoperative score of 10, 43 (78.2%) regained the same score postoperatively.
- Among 35 patients (8.9%) with a preoperative score of 9, 24 (68.6%) regained the same score.
- Among 153 patients (38.7%) with a preoperative score of 7 or 8, 128 (83.7%) regained the same score.
3.4. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanders, T.L.; Maradit Kremers, H.; Bryan, A.J.; Larson, D.R.; Dahm, D.L.; Levy, B.A.; Stuart, M.J. Incidence of anterior cruciate ligament tears and reconstruction: A 21-year population-based study. Am. J. Sports Med. 2016, 44, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, S.; Urrizola, F.; Signorelli, C.; Raggi, F.; Di Sarsina, T.R.; Grassi, A. Residual rotatory laxity after anterior cruciate ligament reconstruction: How do we diagnose it and prevent it? Curr. Orthop. Pract. 2016, 27, 241–246. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Barbosa, N.C.; Vieira, T.D.; Saithna, A. Clinical outcomes of extra-articular tenodesis/anterolateral reconstruction in the ACL injured knee. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 596–604. [Google Scholar] [CrossRef]
- Cavaignac, E.; Ancelin, D.; Chiron, P.; Tricoire, J.L.; Wytrykowski, K.; Faruch, M.; Murgier, J. Historical perspective on the “discovery” of the anterolateral ligament of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 991–996. [Google Scholar] [CrossRef]
- Claes, S.; Vereecke, E.; Maes, M.; Victor, J.; Verdonk, P.; Bellemans, J. Anatomy of the anterolateral ligament of the knee. J. Anat. 2013, 223, 321–328. [Google Scholar] [CrossRef]
- Daggett, M.; Ockuly, A.C.; Cullen, M.; Busch, K.; Lutz, C.; Imbert, P.; Sonnery-Cottet, B. Femoral origin of the anterolateral ligament: An anatomic analysis. Arthroscopy 2016, 32, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Dodds, A.L.; Halewood, C.; Gupte, C.M.; Williams, A.; Amis, A.A. The anterolateral ligament: Anatomy, length changes and association with the Segond fracture. Bone Jt. J. 2014, 96-B, 325–331. [Google Scholar] [CrossRef]
- Parsons, E.M.; Gee, A.O.; Spiekerman, C.; Cavanagh, P.R. The biomechanical function of the anterolateral ligament of the knee. Am. J. Sports Med. 2015, 43, 669–674. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Lutz, C.; Daggett, M.; Dalmay, F.; Freychet, B.; Niglis, L.; Thaunat, M. The involvement of the anterolateral ligament in rotational control of the knee. Am. J. Sports Med. 2016, 44, 1209–1214. [Google Scholar] [CrossRef]
- Imbert, P.; Lutz, C.; Daggett, M.; Niglis, L.; Freychet, B.; Dalmay, F.; Sonnery-Cottet, B. Isometric characteristics of the anterolateral ligament of the knee: A cadaveric navigation study. Arthroscopy 2016, 32, 2017–2024. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Archbold, P.; Rezende, F.C.; Neto, A.M.; Fayard, J.M.; Thaunat, M. Arthroscopic identification of the anterolateral ligament of the knee. Arthrosc. Tech. 2014, 3, e389–e392. [Google Scholar] [CrossRef]
- Helito, C.P.; Helito, P.V.P.; Costa, H.P.; Bordalo-Rodrigues, M.; Pecora, J.R.; Camanho, G.L.; Demange, M.K. MRI evaluation of the anterolateral ligament of the knee: Assessment in routine 1.5-T scans. Skelet. Radiol. 2014, 43, 1421–1427. [Google Scholar] [CrossRef]
- Muramatsu, K.; Saithna, A.; Watanabe, H.; Sasaki, K.; Yokosawa, K.; Hachiya, Y.; Sonnery-Cottet, B. Three-dimensional magnetic resonance imaging of the anterolateral ligament of the knee: An evaluation of intact and ACL-deficient knees from the SANTI Study Group. Arthroscopy 2018, 34, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Cavaignac, E.; Wytrykowski, K.; Reina, N.; Pailhé, R.; Murgier, J.; Faruch, M.; Chiron, P. Ultrasonographic identification of the anterolateral ligament of the knee. Arthroscopy 2016, 32, 120–126. [Google Scholar] [CrossRef]
- Kittl, C.; El-Daou, H.; Athwal, K.K.; Gupte, C.M.; Weiler, A.; Williams, A.; Amis, A.A. The role of the anterolateral strutures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am. J. Sports Med. 2016, 44, 345–354. [Google Scholar] [CrossRef]
- Daggett, M.; Claes, S.; Helito, C.P.; Imbert, P.; Monaco, E.; Lutz, C.; Sonnery-Cottet, B. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee: Letter to the editor. Am. J. Sports Med. 2016, 44, NP14–NP15. [Google Scholar] [CrossRef]
- Musahl, V.; Rahnemai-Azar, A.A.; van Eck, C.F.; Guenther, D.; Fu, F.H. Anterolateral ligament of the knee, fact or fiction? Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2–3. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Saithna, A.; Helito, C.; Daggett, M.; Thaunat, M. Regarding “Anterolateral ligament of the knee, fact or fiction?”. Arthroscopy 2016, 32, 1740–1741. [Google Scholar] [CrossRef]
- Musahl, V.; Getgood, A.; Neyret, P.; Claes, S.; Burnham, J.M.; Batailler, C.; Sonnery-Cottet, B. Contributions of the anterolateral complex and the anterolateral ligament to rotatory knee stability in the setting of ACL injury: A roundtable discussion. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Claes, S.; Blakeney, W.G.; Cavaignac, E.; Saithna, A.; Daggett, M.; Thaunat, M. Anterolateral ligament: Let’s stick to the facts! Arthroscopy 2018, 34, 2259–2262. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Daggett, M.; Helito, C.P.; Fayard, J.M.; Thaunat, M. Combined anterior cruciate ligament and anterolateral ligament reconstruction. Arthrosc. Tech. 2016, 5, e1253–e1259. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Thaunat, M.; Freychet, B.; Pupim, B.H.B.; Murphy, C.G.; Claes, S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am. J. Sports Med. 2015, 43, 1598–1605. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Saithna, A.; Cavalier, M.; Kajetanek, C.; Temponi, E.F.; Daggett, M.; Thaunat, M. Anterolateral ligament reconstruction is associated with significantly reduced ACL graft rupture rates at a minimum follow-up of 2 years: A prospective comparative study of 502 patients from the SANTI Study Group. Am. J. Sports Med. 2017, 45, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Saithna, A.; Blakeney, W.G.; Ouanezar, H.; Borade, A.; Daggett, M.; Thaunat, M. Anterolateral ligament reconstruction protects the repaired medial meniscus: A comparative study of 383 anterior cruciate ligament reconstructions from the SANTI Study Group with a minimum follow-up of 2 years. Am. J. Sports Med. 2018, 46, 1819–1826. [Google Scholar] [CrossRef]
- Saithna, A.; Daggett, M.; Helito, C.P.; Monaco, E.; Franck, F.; Vieira, T.D.; Sonnery-Cottet, B. Clinical results of combined ACL and anterolateral ligament reconstruction: A narrative review from the SANTI Study Group. J. Knee Surg. 2021, 34, 962–970. [Google Scholar] [CrossRef]
- Delaloye, J.R.; Murar, J.; Gonzalez, M.; Amaral, T.; Kakatkar, V.; Sonnery-Cottet, B. Clinical outcomes after combined anterior cruciate ligament and anterolateral ligament reconstruction. Tech. Orthop. 2018, 33, 225–231. [Google Scholar] [CrossRef]
- Thaunat, M.; Clowez, G.; Saithna, A.; Cavalier, M.; Choudja, E.; Vieira, T.D.; Sonnery-Cottet, B. Reoperation rates after combined anterior cruciate ligament and anterolateral ligament reconstruction: A series of 548 patients from the SANTI Study Group with a minimum follow-up of 2 years. Am. J. Sports Med. 2017, 45, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Sonnery-Cottet, B.; Haidar, I.; Rayes, J.; Fradin, T.; Ngbilo, C.; Vieira, T.D.; Thaunat, M. Long-term graft rupture rates after combined ACL and anterolateral ligament reconstruction versus isolated ACL reconstruction: A matched-pair analysis from the SANTI Study Group. Am. J. Sports Med. 2021, 49, 2889–2897. [Google Scholar] [CrossRef]
- Coquard, M.; Carrozzo, A.; Saithna, A.; Vigne, G.; Le Guen, M.; Fournier, Y.; Sonnery-Cottet, B. Anterolateral ligament reconstruction does not delay functional recovery, rehabilitation, and return to sport after anterior cruciate ligament reconstruction: A matched-pair analysis from the SANTI Study Group. Arthrosc. Sports Med. Rehabil. 2022, 4, e9–e16. [Google Scholar] [CrossRef]
- Jankovic, S.; Vrgoc, G.; Vuletic, F.; Ivkovic, A. Modified technique for combined reconstruction of anterior cruciate ligament and anterolateral ligament. Arthrosc. Tech. 2021, 10, e599–e604. [Google Scholar] [CrossRef] [PubMed]
- Helito, C.P.; Camargo, D.B.; Sobrado, M.F.; Bonadio, M.B.; Giglio, P.N.; Pécora, J.R.; Camanho, G.L. Combined reconstruction of the anterolateral ligament in chronic ACL injuries leads to better clinical outcomes than isolated ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3652–3659. [Google Scholar] [CrossRef]
- Helito, C.P.; Sobrado, M.F.; Giglio, P.N.; Bonadio, M.B.; Pécora, J.R.; Camanho, G.L.; Demange, M.K. Combined reconstruction of the anterolateral ligament in patients with anterior cruciate ligament injury and ligamentous hyperlaxity leads to better clinical stability and a lower failure rate than isolated anterior cruciate ligament reconstruction. Arthroscopy 2019, 35, 2648–2654. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Pioger, C.; Vieira, T.D.; Franck, F.; Kajetanek, C.; Fayard, J.M.; Thaunat, M. Combined ACL and anterolateral reconstruction is not associated with a higher risk of adverse outcomes: Preliminary results from the SANTI randomized controlled trial. Orthop. J. Sports Med. 2020, 8, 2325967120924183. [Google Scholar] [CrossRef]
- Laboudie, P.; Douiri, A.; Bouguennec, N.; Biset, A.; Graveleau, N. Combined ACL and ALL reconstruction reduces the rate of reoperation for graft failure or secondary meniscal lesions in young athletes. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3488–3498. [Google Scholar] [CrossRef]
- Roessler, P.P.; Schüttler, K.F.; Heyse, T.J.; Wirtz, D.C.; Efe, T. The anterolateral ligament (ALL) and its role in rotational extra-articular stability of the knee joint: A review of anatomy and surgical concepts. Arch. Orthop. Trauma Surg. 2016, 136, 305–313. [Google Scholar] [CrossRef]
- Cavaignac, E.; Mesnier, T.; Marot, V.; Fernandez, A.; Faruch, M.; Bérard, E.; Chiron, P. Effect of lateral extra-articular tenodesis on anterior cruciate ligament graft incorporation. Orthop. J. Sports Med. 2020, 8, 2325967120964499. [Google Scholar] [CrossRef] [PubMed]
- Devitt, B.M.; Bouguennec, N.; Barfod, K.W.; Porter, T.; Webster, K.E.; Feller, J.A. Combined anterior cruciate ligament reconstruction and lateral extra-articular tenodesis does not result in an increased rate of osteoarthritis: A systematic review and best evidence synthesis. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.E.; Feller, J.A. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am. J. Sports Med. 2016, 44, 2827–2832. [Google Scholar] [CrossRef]
- Kamath, G.V.; Murphy, T.; Creighton, R.A.; Viradia, N.; Taft, T.N.; Spang, J.T. Anterior cruciate ligament injury, return to play, and reinjury in the elite collegiate athlete: Analysis of an NCAA Division I cohort. Am. J. Sports Med. 2014, 42, 1638–1643. [Google Scholar] [CrossRef]
- Lai, C.C.H.; Ardern, C.L.; Feller, J.A.; Webster, K.E. Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: A systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br. J. Sports Med. 2018, 52, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Ardern, C.L.; Taylor, N.F.; Feller, J.A.; Webster, K.E. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: An updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br. J. Sports Med. 2014, 48, 1543–1552. [Google Scholar] [CrossRef]
- Rosenstiel, N.; Praz, C.; Ouanezar, H.; Saithna, A.; Fournier, Y.; Hager, J.P.; Sonnery-Cottet, B. Combined anterior cruciate and anterolateral ligament reconstruction in the professional athlete: Clinical outcomes from the Scientific Anterior Cruciate Ligament Network International Study Group in a series of 70 patients with a minimum follow-up of 2 years. Arthroscopy 2019, 35, 885–892. [Google Scholar] [CrossRef]
- Hopper, G.P.; Pioger, C.; Philippe, C.; El Helou, A.; Campos, J.P.; Gousopoulos, L.; Sonnery-Cottet, B. Risk factors for anterior cruciate ligament graft failure in professional athletes: An analysis of 342 patients with a mean follow-up of 100 months from the SANTI Study Group. Am. J. Sports Med. 2022, 50, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Getgood, A.M.J.; Bryant, D.M.; Litchfield, R.; Heard, M.; McCormack, R.G.; Rezansoff, A.; Peterson, D.; Willits, K.; Firth, A.; MacDonald, P. Lateral extra-articular tenodesis reduces failure of hamstring tendon autograft anterior cruciate ligament reconstruction: 2-year outcomes from the STABILITY Study randomized clinical trial. Am. J. Sports Med. 2020, 48, 285–297. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Carrozzo, A.; Saithna, A.; Monaco, E.; Vieira, T.D.; Musahl, V.; Getgood, A.; Helito, C.P.; International Experts Panel. Surgical treatment and complications of lateral extra-articular procedures in the anterior cruciate ligament–reconstructed knee: Part II of an international consensus statement. Arthroscopy 2025, in press. [Google Scholar] [CrossRef]
- Nedder, V.J.; Raju, A.G.; Moyal, A.J.; Calcei, J.G.; Voos, J.E. Impact of psychological factors on rehabilitation after anterior cruciate ligament reconstruction: A systematic review. Sports Health 2025, 17, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Colombet, P.; Graveleau, N.; Jambou, S. Incorporation of hamstring grafts within the tibial tunnel after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2016, 44, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Papachristou, G.; Nikolaou, V.; Efstathopoulos, N.; Sourlas, J.; Lazarettos, J.; Fragia, K.; Papalois, A. ACL reconstruction with semitendinosus tendon autograft without detachment of its tibial insertion: A histologic study in a rabbit model. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 1175–1180. [Google Scholar] [CrossRef]
- Bahlau, D.; Clavert, P.; Favreau, H.; Ollivier, M.; Lustig, S.; Bonnomet, F.; Ehlinger, M. Mechanical advantage of preserving the hamstring tibial insertion for anterior cruciate ligament reconstruction: A cadaver study. Orthop. Traumatol. Surg. Res. 2019, 105, 89–93. [Google Scholar] [CrossRef]
- Vari, N.; Cavaignac, E.; Cavaignac, M.; Bérard, É.; Marot, V. Outcomes of hamstring graft with preserved tibial insertion for ACL reconstruction: Systematic review and meta-analysis. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 67–73. [Google Scholar] [CrossRef]


| Outcome | n (%) of Patients |
|---|---|
| Graft rerupture | 11 (3.78%) |
| Return to sport at pre-injury level | 220 (75.6%) |
| Tegner Activity Scale | |
| Preoperative score 10 (n = 55) | 43 regained same score (78.2%) |
| Preoperative score 9 (n = 35) | 24 regained same score (68.6%) |
| Preoperative score 7–8 (n = 153) | 128 regained same score (83.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kottek, T.; Bulat, S.; Vrgoč, G.; Ivković, A.; Bukvić, F.; Jeličić, J.; Janković, S. Modified Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction in 291 High-Level Athletes: Clinical Outcomes at Minimum 2.5-Year Follow-Up. Medicina 2025, 61, 1762. https://doi.org/10.3390/medicina61101762
Kottek T, Bulat S, Vrgoč G, Ivković A, Bukvić F, Jeličić J, Janković S. Modified Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction in 291 High-Level Athletes: Clinical Outcomes at Minimum 2.5-Year Follow-Up. Medicina. 2025; 61(10):1762. https://doi.org/10.3390/medicina61101762
Chicago/Turabian StyleKottek, Tomislav, Stjepan Bulat, Goran Vrgoč, Alan Ivković, Frane Bukvić, Joško Jeličić, and Saša Janković. 2025. "Modified Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction in 291 High-Level Athletes: Clinical Outcomes at Minimum 2.5-Year Follow-Up" Medicina 61, no. 10: 1762. https://doi.org/10.3390/medicina61101762
APA StyleKottek, T., Bulat, S., Vrgoč, G., Ivković, A., Bukvić, F., Jeličić, J., & Janković, S. (2025). Modified Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction in 291 High-Level Athletes: Clinical Outcomes at Minimum 2.5-Year Follow-Up. Medicina, 61(10), 1762. https://doi.org/10.3390/medicina61101762






