Analysis of HER2 Expression in Different Histological Subtypes and IHC-Based Molecular Variants of Muscle-Invasive Bladder Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Clinical Cohort
2.2. Patient Characteristics
2.3. Histological and Molecular Subtyping of Tumors
2.4. Immunohistochemical Study of HER2
2.5. Interpretation of HER2 Status
2.6. Statistical Analysis
2.7. Ethical Aspects
3. Results
4. Discussion
4.1. HER2 Expression and Molecular Variant
4.2. HER2 Expression and Histological Subtype
4.3. Diagnostic Significance of HER2 in Bladder Carcinoma
4.4. Prognostic Significance of HER2 Expression
4.5. Therapeutic Significance and HER2-Targeted Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Bladder carcinoma |
| MIBC | Muscle-invasive bladder carcinoma |
| UC | Urothelial carcinoma |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| IHC | Immunohistochemistry |
| TURB | Transurethral resection |
| RC | Radical cystectomy |
| FGFR | Fibroblast growth factor receptor 3 |
| FISH/CISH | Fluorescence/chromogenic in situ hybridization |
| ADC | Antibody–drug conjugate |
| ASCO/CAP | American society of clinical oncology/College of American pathologists |
| EAU | European association of urology |
| ESMO | European society for medical oncology |
| NCCN | National comprehensive cancer network |
References
- International Agency for Research on Cancer. Cancer Factsheets: Bladder. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/30-bladder-fact-sheet.pdf (accessed on 8 February 2024).
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Netto, G.J.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs—Part B: Prostate and Urinary Tract Tumors. Eur. Urol. 2022, 82, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, A.; De Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.; Krishnamurti. Reporting Template for Reporting Results of Biomarker Testing of Specimens from Patients with Carcinoma of the Breast; Version 1.6.0.0; College of American Pathologists: Northfield, IL, USA, 2025; Available online: https://documents.cap.org/documents/New-Cancer-Protocols-March-2025/Breast.Bmk_1.6.0.0.REL.CAPCP.pdf (accessed on 8 March 2025).
- Scherrer, E.; Kang, A.; Bloudek, L.M.; Koshkin, V.S. HER2 expression in urothelial carcinoma, a systematic literature review. Front. Oncol. 2022, 12, 1011885. [Google Scholar] [CrossRef] [PubMed]
- Kiss, B.; Wyatt, A.W.; Douglas, J.; Skuginna, V.; Mo, F.; Anderson, S.; Rotzer, D.; Fleischmann, A.; Genitsch, V.; Hayashi, T.; et al. Her2 alterations in muscle-invasive bladder cancer: Patient selection beyond protein expression for targeted therapy. Sci. Rep. 2017, 7, 42713. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, G.; Song, B.; Lee, C.; Park, J.H.; Moon, K.C. HER2 Protein Overexpression and Gene Amplification in Plasmacytoid Urothelial Carcinoma of the Urinary Bladder. Dis. Markers 2016, 2016, 8463731. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.A.; Sukov, W.R.; Frank, I.; Boorjian, S.A.; Costello, B.A.; Tarrell, R.F.; Thapa, P.; Houston Thompson, R.; Tollefson, M.K.; Jeffrey Karnes, R.; et al. Outcome of patients with micropapillary urothelial carcinoma following radical cystectomy: ERBB2 (HER2) amplification identifies patients with poor outcome. Mod. Pathol. 2014, 27, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Bolenz, C.; Shariat, S.F.; Karakiewicz, P.I.; Ashfaq, R.; Ho, R.; Sagalowsky, A.I.; Lotan, Y. Human epidermal growth factor receptor 2 expression status provides independent prognostic information in patients with urothelial carcinoma of the urinary bladder. BJU Int. 2010, 106, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Oudard, S.; Culine, S.; Vano, Y.; Goldwasser, F.; Théodore, C.; Nguyen, T.; Voog, E.; Banu, E.; Vieillefond, A.; Priou, F.; et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur. J. Cancer 2015, 51, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, L.; He, Z.; Shi, Y.; Luo, H.; Han, W.; Yao, X.; Shi, B.; Liu, J.; Hu, C.; et al. Efficacy and Safety of Disitamab Vedotin in Patients with Human Epidermal Growth Factor Receptor 2–Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J. Clin. Oncol. 2024, 42, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Chang, Y.; Zhao, D.; Guo, A.; Cao, J.; Wu, C.; Guan, Y.; Ding, S. Expression of HER2 in high-grade urothelial carcinoma based on Chinese expert consensus and the clinical effects of disitamab vedotin-tislelizumab combination therapy in the treatment of advanced patients. Front. Pharmacol. 2024, 15, 1355081. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Bisagni, A.; Zizzo, M.; Ascani, S.; Pedicillo, M.C.; Cormio, A.; Falagario, U.G.; Carrieri, G.; et al. HER2 Expression in Bladder Cancer: A Focused View on Its Diagnostic, Prognostic, and Predictive Role. Int. J. Mol. Sci. 2023, 24, 3720. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Indini, A.; Rijavec, E.; Grossi, F. Trastuzumab Deruxtecan: Changing the Destiny of HER2 Expressing Solid Tumors. Int. J. Mol. Sci. 2021, 22, 4774. [Google Scholar] [CrossRef] [PubMed]
- Uzunparmak, B.; Haymaker, C.; Raso, G.; Masciari, S.; Wang, L.; Lin, H.; Gorur, A.; Kirby, B.; Cimo, A.-M.; Kennon, A.; et al. HER2-low expression in patients with advanced or metastatic solid tumors. Ann. Oncol. 2023, 34, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Zinnall, U.; Weyerer, V.; Compérat, E.; Camparo, P.; Gaisa, N.T.; Knuechel-Clarke, R.; Perren, A.; Lugli, A.; Toma, M.; Baretton, G.; et al. Micropapillary urothelial carcinoma: Evaluation of HER2 status and immunohistochemical characterization of the molecular subtype. Hum. Pathol. 2018, 80, 55–64. [Google Scholar] [CrossRef] [PubMed]
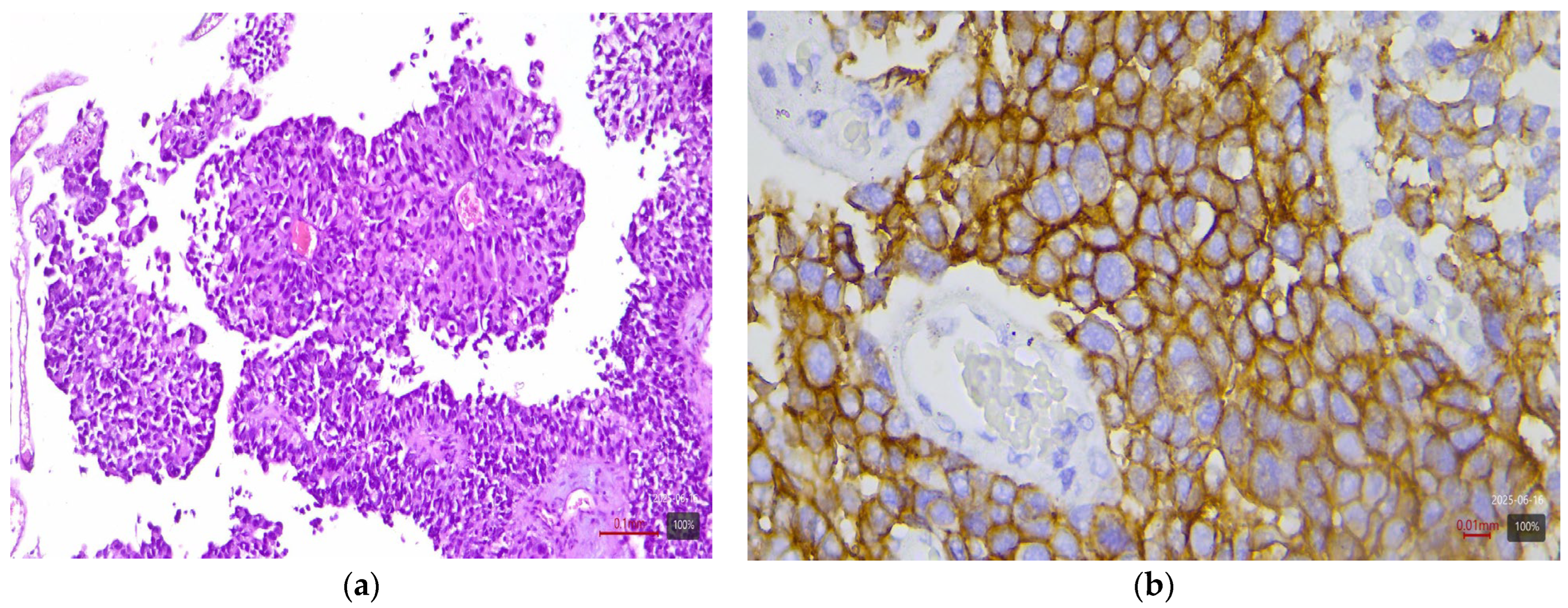

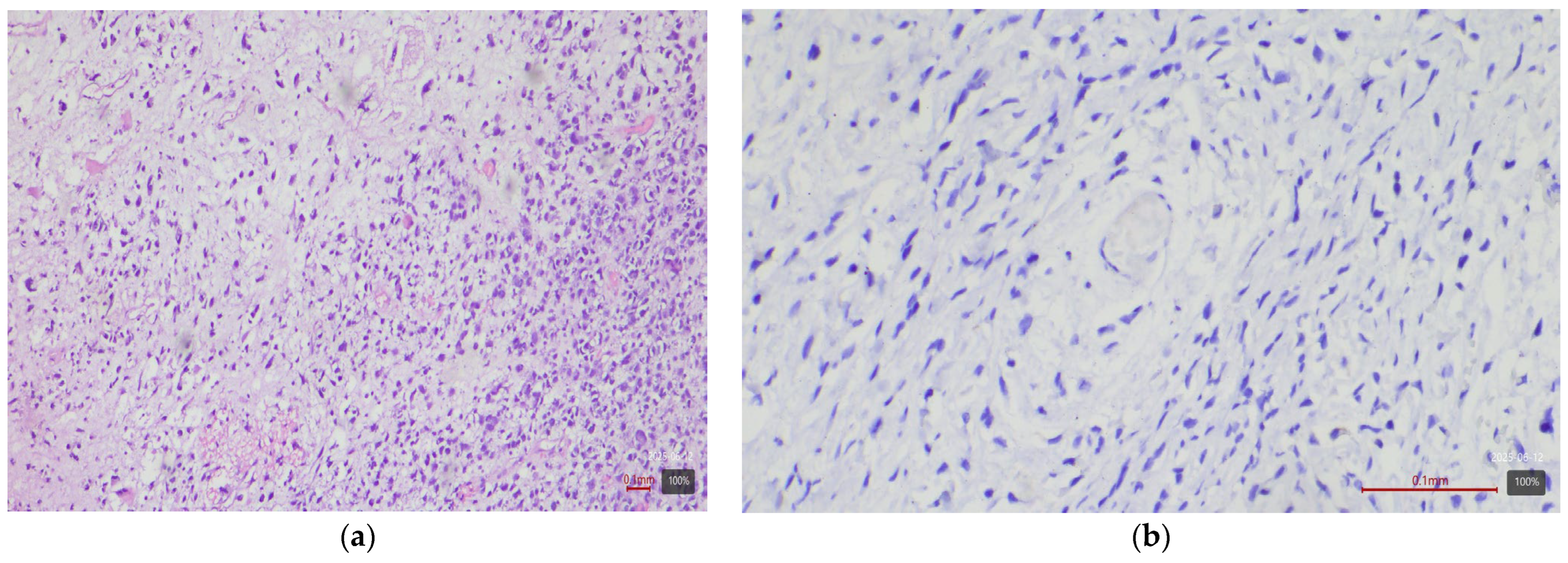



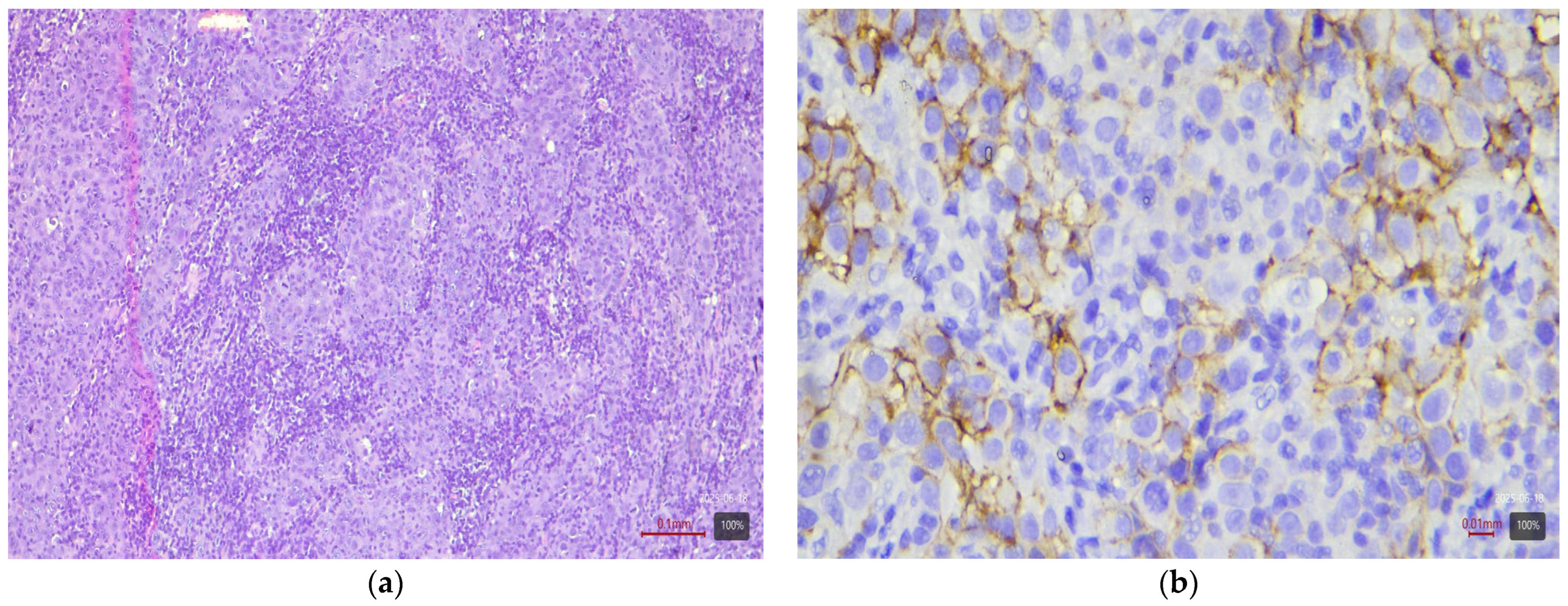
| Histological Subtype | Number of Cases (n) | HER2 3+ | HER2 2+ |
|---|---|---|---|
| Urothelial carcinoma (NOS) | 4 | 0% | 25% |
| Papillary urothelial carcinoma | 27 | 44% | 26% |
| Micropapillary subtype | 4 | 75% | 25% |
| Plasmacytoid subtype | 3 | 33% | 33% |
| UC with squamous differentiation | 17 | 0% | 0% |
| UC with glandular differentiation | 3 | 0% | 33% |
| Nested subtype | 2 | 50% | 0% |
| Sarcomatoid subtype | 5 | 0% | 0% |
| Small cell carcinoma | 5 | 0% | 0% |
| Molecular Variant | Number of Cases (n) | HER2 3+ (%) | HER2 2+ (%) |
|---|---|---|---|
| Luminal Papillary | 24 (24%) | 33% | 38% |
| Luminal Unstable | 16 (16%) | 56% | 31% |
| Luminal Non-Specifiend (LumNS) | 10 (10%) | 50% | 30% |
| Basal/Squamous | 33 (33%) | 0% | 3% |
| BasoLuminal (Double-positive) | 9 (9%) | 33% | 22% |
| NE-like | 6 (6%) | 0% | 0% |
| Stroma-rich | 2 (2%) | 0% | 0% |
| Total | 100 (100%) | - | - |
| HER2 IHC Category | Number of Cases (n) | Percentage (%) |
|---|---|---|
| 0 (Negative) | 31 | 31% |
| 0+ (Ultra-low) | 9 | 9% |
| 1+ (Weak) | 13 | 13% |
| 2+ (Moderate) | 22 | 22% |
| 3+ (Strong) | 25 | 25% |
| Total | 100 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraevska, E.; Popovska, S. Analysis of HER2 Expression in Different Histological Subtypes and IHC-Based Molecular Variants of Muscle-Invasive Bladder Carcinoma. Medicina 2025, 61, 1759. https://doi.org/10.3390/medicina61101759
Kraevska E, Popovska S. Analysis of HER2 Expression in Different Histological Subtypes and IHC-Based Molecular Variants of Muscle-Invasive Bladder Carcinoma. Medicina. 2025; 61(10):1759. https://doi.org/10.3390/medicina61101759
Chicago/Turabian StyleKraevska, Elitsa, and Savelina Popovska. 2025. "Analysis of HER2 Expression in Different Histological Subtypes and IHC-Based Molecular Variants of Muscle-Invasive Bladder Carcinoma" Medicina 61, no. 10: 1759. https://doi.org/10.3390/medicina61101759
APA StyleKraevska, E., & Popovska, S. (2025). Analysis of HER2 Expression in Different Histological Subtypes and IHC-Based Molecular Variants of Muscle-Invasive Bladder Carcinoma. Medicina, 61(10), 1759. https://doi.org/10.3390/medicina61101759






