Effectiveness of Classic Triple Therapy Compared with Alternative Regimens for Eradicating H. pylori: A Systematic Review
Abstract
1. Introduction
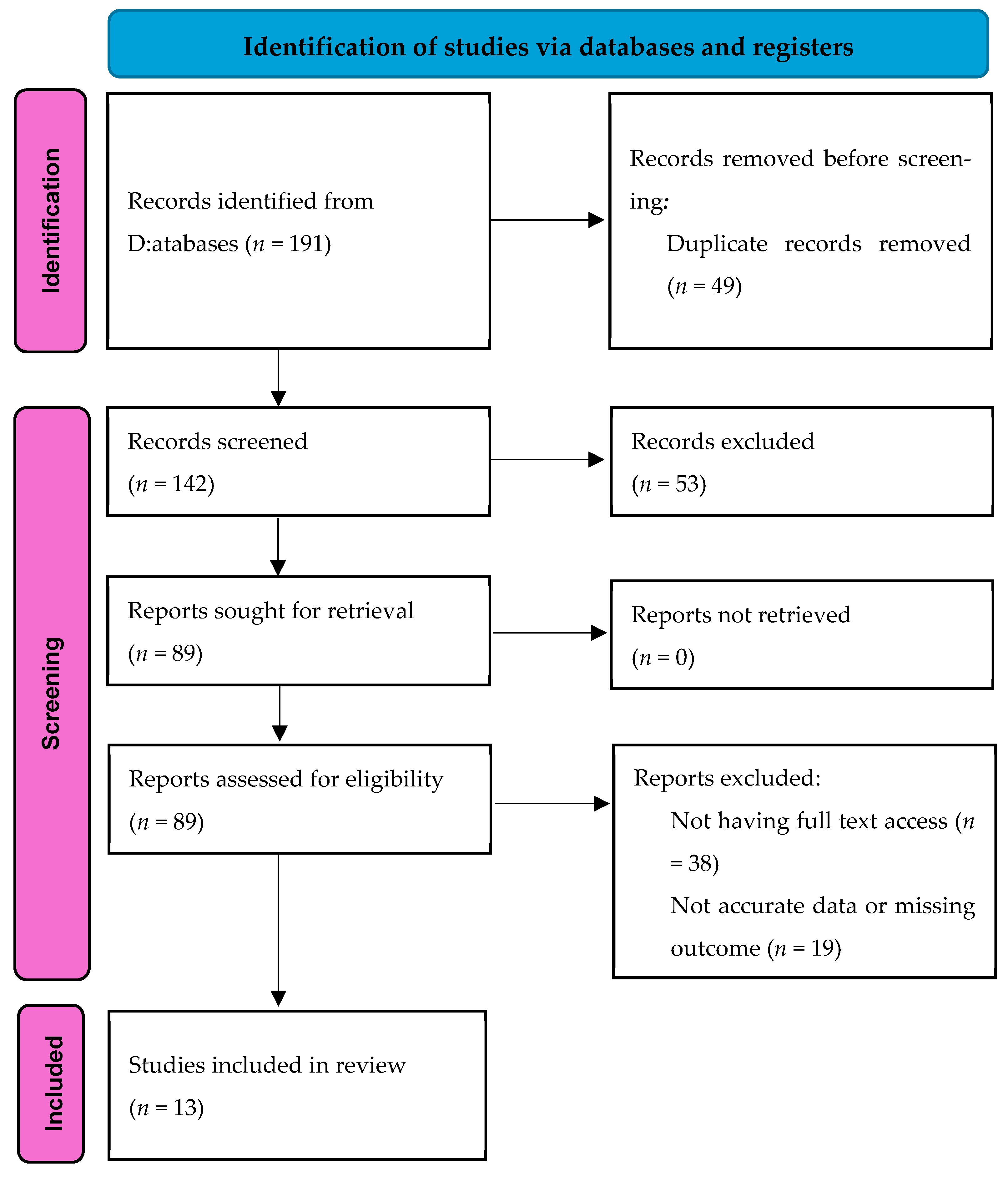
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| H. pylori | Helicobacter pylori |
| GI | Gastrointestinal |
| CTT | Classic triple therapy |
| PPI | Proton pump inhibitor |
| RCTs | Randomized controlled trials |
References
- Reyes, V.E. Helicobacter pylori and Its Role in Gastric Cancer. Microorganisms 2023, 11, 1312. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M.; Wilson, K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef]
- Ali, A.; AlHussaini, K.I. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms 2024, 12, 222. [Google Scholar] [CrossRef]
- Chang, S.S.; Hu, H.-Y. Helicobacter pylori Eradication within 120 Days Is Associated with Decreased Complicated Recurrent Peptic Ulcers in Peptic Ulcer Bleeding Patients. Gut Liver 2015, 9, 346–352. [Google Scholar] [CrossRef]
- Marcus, E.A.; Sachs, G.; Scott, D.R. Eradication of Helicobacter pylori Infection. Curr. Gastroenterol. Rep. 2016, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Morcillo-Muñoz, J.A.; Regino-Otero, W.A.; Gómez Zuleta, M.A. Helicobacter pylori: ¿cómo mejorar las terapias de erradicación? Rev. Colomb. Gastroenterol. 2018, 33, 437. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Lu, N.-H. Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 168. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef] [PubMed]
- Abdoh, Q.; Alnees, M.; Kharraz, L.; Ayoub, K.; Darwish, A.; Awwad, M.; Najajra, D.; Khraim, J.; Awad, W.; Sbaih, A.; et al. Prevalence of Helicobacter pylori resistance to certain antibiotics at An-Najah University Hospital: A cross-sectional study. Sci. Rep. 2024, 14, 14542. [Google Scholar] [CrossRef]
- Bang, C.S. Attempts to enhance the eradication rate of Helicobacter pylori infection. World J. Gastroenterol. 2014, 20, 5252. [Google Scholar] [CrossRef]
- Kim, S.Y.; Chung, J.-W. Best Helicobacter pylori Eradication Strategy in the Era of Antibiotic Resistance. Antibiotics 2020, 9, 436. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Graham, D.Y. Bismuth-containing quadruple therapy for Helicobacter pylori. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1134–1140. [Google Scholar] [CrossRef]
- Guo, B.; Cao, N.-W.; Zhou, H.-Y.; Chu, X.-J.; Li, B.-Z. Efficacy and safety of bismuth-containing quadruple treatment and concomitant treatment for first-line Helicobacter pylori eradication: A systematic review and meta-analysis. Microb Pathog. 2021, 152, 104661. [Google Scholar] [CrossRef]
- Sherkatolabbasieh, H.; Shafizadeh, S.; Azadbakht, S.; Moradniani, M.; Maleki, H.; Jaferian, S.; Roozbahany, M.M.; Mirbeik-Sabzevari, Z.; Baharvand, P. Levofloxacin-based sequential therapy versus classic triple therapy in Helicobacter pylori eradication: A randomized clinical trial. Biomed. Res. Ther. 2017, 4, 1785. [Google Scholar] [CrossRef]
- Hassan, M.; Noureddine, M.; Assi, F.; Houmani, Z. Eradication rate of Helicobacter pylori by classic triple therapy in Lebanon: Is it still effective? Integr. Clin. Med. 2018, 2, 129. [Google Scholar] [CrossRef]
- Yang, Q.; He, C.; Hu, Y.; Hong, J.; Zhu, Z.; Xie, Y.; Shu, X.; Lu, N.; Zhu, Y. 14-day pantoprazole- and amoxicillin-containing high-dose dual therapy for Helicobacter pylori eradication in elderly patients: A prospective, randomized controlled trial. Front. Pharmacol. 2023, 14, 1096103. [Google Scholar] [CrossRef] [PubMed]
- Felga, G.; Silva, F.M.; Barbuti, R.C.; Tomás, N.-R.; Zaterka, S.; Eisig, J.N. Clarithromycin-based triple therapy for Helicobacter pylori treatment in peptic ulcer patients. J. Infect. Dev. Ctries. 2010, 4, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Ozbalci, G.S.; Yuruker, S.S.; Tarim, I.A.; Cinar, H.; Polat, A.K.; Ozbalci, A.B.; Karabulut, K.; Erzurumlu, K. First-line therapy in Helicobacter pylori eradication therapy: Experience of a surgical clinic. Turk. J. Surg. 2014, 30, 133–137. [Google Scholar] [CrossRef]
- Prasertpetmanee, S.; Mahachai, V.; Vilaichone, R. Improved Efficacy of Proton Pump Inhibitor–Amoxicillin–Clarithromycin Triple Therapy for Helicobacter pylori Eradication in Low Clarithromycin Resistance Areas or for Tailored Therapy. Helicobacter 2013, 18, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Shiotani, A.; Katsumata, R.; Fujita, M.; Nakato, R.; Murao, T.; Ishii, M.; Kamada, T.; Haruma, K.; Graham, D.Y. Helicobacter pylori Eradication with Proton Pump Inhibitors or Potassium-Competitive Acid Blockers: The Effect of Clarithromycin Resistance. Dig. Dis. Sci. 2016, 61, 3215–3220. [Google Scholar] [CrossRef]
- Salamah, A.M.; Gad, M.; Deghady, A.; Elgayar, N.H. Effectiveness of 14-days course of clarithromycin-based triple therapy as first line therapy for h.pylori infection in egyptian elderly patients. EJGG 2015, 2, 19–26. Available online: https://ejgg.journals.ekb.eg/article_5342_cb1615ad79f7bf72890bdf89a907d2ca.pdf (accessed on 16 June 2025).
- Kim, S.Y.; Jung, S.W.; Kim, J.H.; Koo, J.S.; Yim, H.J.; Park, J.J.; Chun, H.J.; Lee, S.W.; Choi, J.H. Effectiveness of three times daily lansoprazole/amoxicillin dual therapy for Helicobacter pylori infection in Korea. Br. J. Clin. Pharmacol. 2012, 73, 140–143. [Google Scholar] [CrossRef] [PubMed]
- McNicholl, A.G.; Bordin, D.S.; Lucendo, A.; Fadeenko, G.; Fernandez, M.C.; Voynovan, I.; Zakharova, N.V.; Sarsenbaeva, A.S.; Bujanda, L.; Perez-Aisa, Á.; et al. Combination of Bismuth and Standard Triple Therapy Eradicates Helicobacter pylori Infection in More than 90% of Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 89–98. [Google Scholar] [CrossRef]
- Onyekwere, C.A. Rabeprazole, clarithromycin, and amoxicillin Helicobacter pylori eradication therapy: Report of an efficacy study. World J. Gastroenterol. 2014, 20, 3615. [Google Scholar] [CrossRef]
- Lee, Y.D.; Kim, S.E.; Park, S.J.; Park, M.I.; Moon, W.; Kim, J.H.; Jung, K.; Song, J. Efficacy of Seven-day High-dose Esomeprazole-based Triple Therapy versus Seven-day Standard Dose Non-esomeprazole-based Triple Therapy as the First-line Treatment of Patients with Helicobacter pylori Infection. Korean J. Gastroenterol. 2020, 76, 142–149. [Google Scholar] [CrossRef]
- Kamal, A.; Ghazy, R.M.; Sherief, D.; Ismail, A.; Ellakany, W.I. Helicobacter pylori eradication rates using clarithromycin and levofloxacin-based regimens in patients with previous COVID-19 treatment: A randomized clinical trial. BMC Infect. Dis. 2023, 23, 36. [Google Scholar] [CrossRef]
- Kato, M.; Yamaoka, Y.; Kim, J.J.; Reddy, R.; Asaka, M.; Kashima, K.; Osato, M.S.; El-Zaatari, F.A.K.; Graham, D.Y.; Kwon, D.H. Regional Differences in Metronidazole Resistance and Increasing Clarithromycin Resistance among Helicobacter pylori Isolates from Japan. Antimicrob. Agents Chemother. 2000, 44, 2214–2216. [Google Scholar] [CrossRef]
- Kalach, N.; Bergeret, M.; Benhamou, P.H.; Dupont, C.; Raymond, J. High Levels of Resistance to Metronidazole and Clarithromycin in Helicobacter pylori Strains in Children. J. Clin. Microbiol. 2001, 39, 394–397. [Google Scholar] [CrossRef]
- Huang, Q.; Shi, Z.; Cheng, H.; Ye, H.; Zhang, X. Efficacy and Safety of Modified Dual Therapy as the First-line Regimen for the Treatment of Helicobacter pylori Infection. J. Clin. Gastroenterol. 2021, 55, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Losurdo, G.; La Fortezza, R.F.; Principi, M.; Barone, M.; Di Leo, A. Optimizing proton pump inhibitors in Helicobacter pylori treatment: Old and new tricks to improve effectiveness. World J. Gastroenterol. 2019, 25, 5097–5104. [Google Scholar] [CrossRef]
- Fallone, C.A.; Barkun, A.N.; Szilagyi, A.; Herba, K.M.; Sewitch, M.; Martel, M.; Fallone, S.S. Prolonged Treatment Duration is Required for Successful Helicobacter pylori Eradication with Proton Pump Inhibitor Triple Therapy in Canada. Can. J. Gastroenterol. 2013, 27, 397–402. [Google Scholar] [CrossRef]
- Gisbert, J.P. Optimization Strategies Aimed to Increase the Efficacy of Helicobacter pylori Eradication Therapies with Quinolones. Molecules 2020, 25, 5084. [Google Scholar] [CrossRef] [PubMed]
- Kouroumalis, E.; Tsomidis, I.; Voumvouraki, A. Helicobacter pylori and gastric cancer: A critical approach to who really needs eradication. Explor. Dig. Dis. 2024, 3, 107–142. [Google Scholar] [CrossRef]
- Saleem, N.; Howden, C.W. Update on the Management of Helicobacter pylori Infection. Curr. Treat. Options Gastroenterol. 2020, 18, 476–487. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | Sample Size | Treatment Groups | Duration | Follow-Up Method |
|---|---|---|---|---|---|
| Sherkatolabbasieh H et al., 2017 [14] | Randomized Clinical Trial | 192 | CTT * (Omeprazole, Amoxicillin, Clarithromycin) vs. Levofloxacin-based sequential therapy (Omeprazole, Amoxicillin for 7 days, then Omeprazole, Levofloxacin, Metronidazole for 7 days) | 14 days | Urea Breath Test |
| Hassan M et al., 2018 [15] | Retrospective Study | 168 | CTT (varied durations: 7, 10, or 14 days) | 7–14 days | Urea Breath Test (UBT) |
| Prasertpetmanee S et al., 2013 [19] | Prospective pilot single-center study | 110 | 7-day vs. 14-day high-dose PPI triple therapy | 7 or 14 days | 13C-UBT test 4+ weeks post-treatment |
| Matsumoto H et al., 2016 [20] | Prospective sequential cohort study | 420 | PPI **-based vs. vanoprazan-based triple therapy | 7 days | 13C-UBT test |
| Salamah A et al., 2015 [21] | Experimental study | 34 | 14-day Clarithromycin-based triple therapy | 14 days | H. pylori stool antigen test 4 weeks post-treatment |
| Kim S et al., 2011 [22] | Randomized controlled trial (RCT) | 204 | Triple therapy (amoxicillin, clarithromycin, lansoprazole) vs. Dual therapy (amoxicillin, lansoprazole) | 2 weeks | 4–5 weeks post-treatment |
| McNicholl A et al., 2020 [23] | Prospective multi-center registry study | 1141 | Bismuth-based quadruple therapy (bismuth, amoxicillin, clarithromycin, proton pump inhibitor) | 10 or 14 days | Data from the European Registry on H. pylori Management |
| Onyekware C et al., 2014 [24] | Open-label randomized trial | 50 | 7-day triple therapy (amoxicillin, clarithromycin, rabeprazole) 10-day triple therapy (amoxicillin, clarithromycin, rabeprazole) | 7 or 10 days | Urea breath test after 1 month |
| Lee Y et al., 2020 [25] | Retrospective study | 223 | 7-NEAC (standard dose triple therapy) 7-HEAC (high-dose esomeprazole-based triple therapy) | 7 days | Urea breath test or rapid urease test after 4 weeks |
| Kamal A et al., 2023 [26] | Randomized controlled trial | 270 | Clarithromycin-based triple therapy (clarithromycin, esomeprazole, amoxicillin) Levofloxacin-based triple therapy (levofloxacin, esomeprazole, amoxicillin) | - | Not specified |
| Yang Q et al., 2023 [16] | Randomized Study | 150 | High-dose dual therapy (HT), Bismuth quadruple therapy (BQT) | 14 days | 13C-urea breath test (4 weeks post-treatment) |
| Felga G et al., 2010 [17] | Observational Study | 493 | PPI/Amoxicillin/Clarithromycin (PPI/AC) | 7 days | Urease test & gastric biopsy (12 weeks post-treatment) |
| Ozbalci G et al., 2014 [18] | Comparative Study | 85 | PPI-based triple therapy (LAC), Bismuth quadruple therapy (BPMT) | 14 days | Not specified |
| Study | Treatment Group | Per Protocol Eradication Rate | Intention-to-Treat Eradication Rate | Statistical Significance |
|---|---|---|---|---|
| Sherkatolabbasieh H et al., 2017 [14] | Classic Triple Therapy | 68.4% | 65% | p = 0.001 |
| Levofloxacin-based Sequential Therapy | 87.6% | 85% | ||
| Hassan M et al., 2018 [15] | Classic Triple Therapy | 61.9% | Not Reported | p > 0.05 (No significance) |
| Prasertpetmanee S et al., 2013 [19] | 7-day high-dose PPI triple therapy | 92.7% | 92.7% | Not specified |
| 14-day high-dose PPI triple therapy | 100% | 100% | Not specified | |
| Matsumoto H et al., 2016 [20] | PPI-based triple therapy | 73.1% | 71.9% | p < 0.001 |
| Vonoprazan-based triple therapy | 89.6% | 89.6% | p < 0.001 | |
| Salamah A et al., 2015 [21] | 14-day Clarithromycin-based triple therapy | 88.2% | 88.2% | Not specified |
| Kim S et al., 2011 [22] | Triple Therapy | 82.8% | 74.0% | p = 0.573 |
| Dual Therapy | 78.4% | 67.3% | p = 0.573 | |
| McNicholl A et al., 2020 [23] | Bismuth-based Quadruple Therapy | 94.0% | 88.0% | p < 0.05 |
| Onyekware C et al., 2014 [24] | 7-day triple therapy 10-day triple therapy | 87.2% | Not reported | p = 0.78 |
| Lee Y et al., 2020 [25] | 7-NEAC | 67.7% | Not reported | p = 0.045 |
| 7-HEAC | 80.9% | |||
| Kamal A et al., 2023 [26] | Clarithromycin-based | 64.66% | 55.56% | p = 0.11 |
| Levofloxacin-based | 74.36% | 64.44% | ||
| Yang Q et al., 2023 [16] | HT | 93.0% | 89.3% | p = 0.484 |
| BQT | 90.3% | 86.6% | ||
| Felga G et al., 2010 [17] | PPI/AC | 88.8% | 82.7% | Not reported |
| Ozbalci G et al., 2014 [18] | LAC | 53.4% | Not reported | p < 0.05 |
| BPMT | 78.5% | Not reported | p < 0.05 |
| Study | Treatment Group | Adverse Event Rate | Common Adverse Events | Adherence Rate |
|---|---|---|---|---|
| Sherkatolabbasieh H et al., 2017 [14] | Classic Triple Therapy | 17.8% | Abdominal pain, loss of appetite, bad oral taste | 95% |
| Levofloxacin-based Sequential Therapy | 19.5% | Nausea, anorexia, abdominal pain | 97% | |
| Hassan M et al., 2018 [15] | Classic Triple Therapy | No significant adverse events reported | Not reported | Good adherence |
| Prasertpetmanee S et al., 2013 [19] | 7-day high-dose PPI triple therapy | Not specified | Nausea, metallic taste (minor, not significant) | 100% |
| 14-day high-dose PPI triple therapy | Not specified | Nausea, metallic taste (higher incidence, not significant) | 100% | |
| Matsumoto H et al., 2016 [20] | PPI-based triple therapy | Not specified | Not significantly different from the vonoprazan group | Dropouts: 5 |
| Vonoprazan-based triple therapy | Not specified | Not significantly different from the PPI group | No dropouts | |
| Salamah A et al., 2015 [21] | 14-day Clarithromycin-based triple therapy | None reported | None reported | 100% (no dropouts) |
| Kim S et al., 2011 [22] | Triple Therapy | 35.6% | Mild side effects | 100% |
| Dual Therapy | 18.3% | Mild side effects | 96% (4 patients < 80% compliance) | |
| McNicholl A et al., 2020 [23] | Bismuth-based Quadruple Therapy | 36.0% | Mild GI side effects (76% mild, avg. duration 6 days) | High adherence, associated with improved eradication rates |
| Onyekware C et al., 2014 [24] | 7-day triple therapy 10-day triple therapy | 0% | None reported | High |
| Lee Y et al., 2020 [25] | 7-NEAC | 5.8% | Diarrhea, nausea, vomiting, skin rash | Not specified |
| 7-HEAC | 7.4% | |||
| Kamal A et al., 2023 [26] | Clarithromycin-based Levofloxacin-based | Not specified | Side effects leading to dropout in 19 (Clarithromycin) and 18 (Levofloxacin) patients | Not specified |
| Yang Q et al., 2023 [16] | HT | 10.6% | Nausea, vomiting, bloating, abdominal pain, diarrhea, skin rash | 98.7% |
| BQT | 26.6% | Same as HT | 97.3% | |
| Felga G et al., 2010 [17] | PPI/AC | 35.5% | Not specified | Not reported |
| Ozbalci G et al., 2014 [18] | LAC | Lower than BPMT | Not specified | Not reported |
| BPMT | Higher than LAC | Not specified | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darraj, M. Effectiveness of Classic Triple Therapy Compared with Alternative Regimens for Eradicating H. pylori: A Systematic Review. Medicina 2025, 61, 1745. https://doi.org/10.3390/medicina61101745
Darraj M. Effectiveness of Classic Triple Therapy Compared with Alternative Regimens for Eradicating H. pylori: A Systematic Review. Medicina. 2025; 61(10):1745. https://doi.org/10.3390/medicina61101745
Chicago/Turabian StyleDarraj, Majid. 2025. "Effectiveness of Classic Triple Therapy Compared with Alternative Regimens for Eradicating H. pylori: A Systematic Review" Medicina 61, no. 10: 1745. https://doi.org/10.3390/medicina61101745
APA StyleDarraj, M. (2025). Effectiveness of Classic Triple Therapy Compared with Alternative Regimens for Eradicating H. pylori: A Systematic Review. Medicina, 61(10), 1745. https://doi.org/10.3390/medicina61101745






