Evolution in Laryngeal Cancer Mortality at the National and Subnational Level in Romania with 2030 Forecast
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Data Sources and Ethical Oversight
2.2. Case Definition, Variable Set and Data Cleaning
2.3. Rate Calculations and Age Standardisation
2.4. Meta-Analytic Pooling of County-Level Effects
2.5. Assessment of Spatial Autocorrelation
2.6. Temporal Trend Analysis
2.7. Forecasting Strategy Combining Classical Time-Series and Elliott–Fibonacci Heuristics
2.8. Validation, Software, Sensitivity Analyses and Scenario Modelling
3. Results
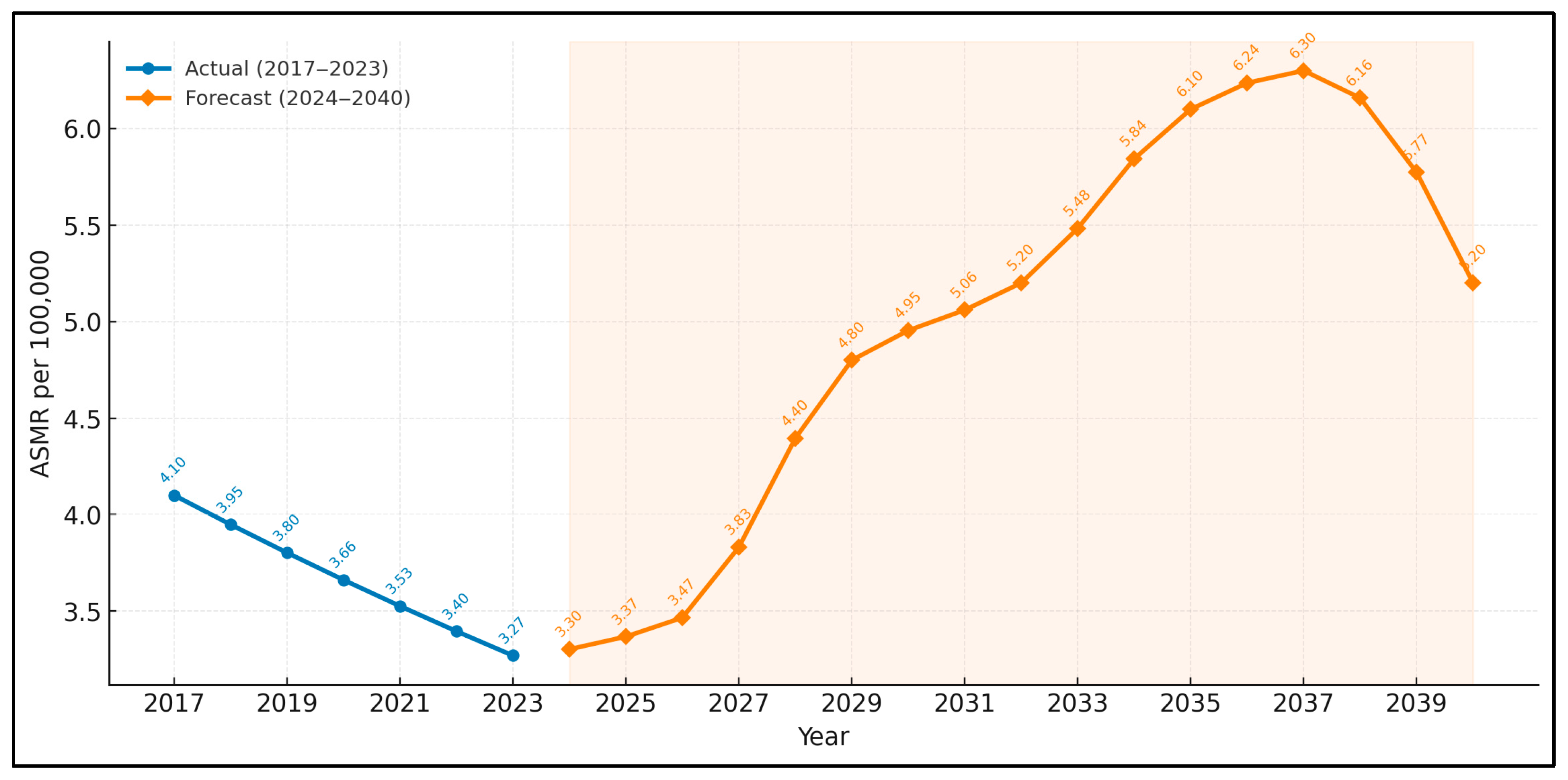
3.1. National Results
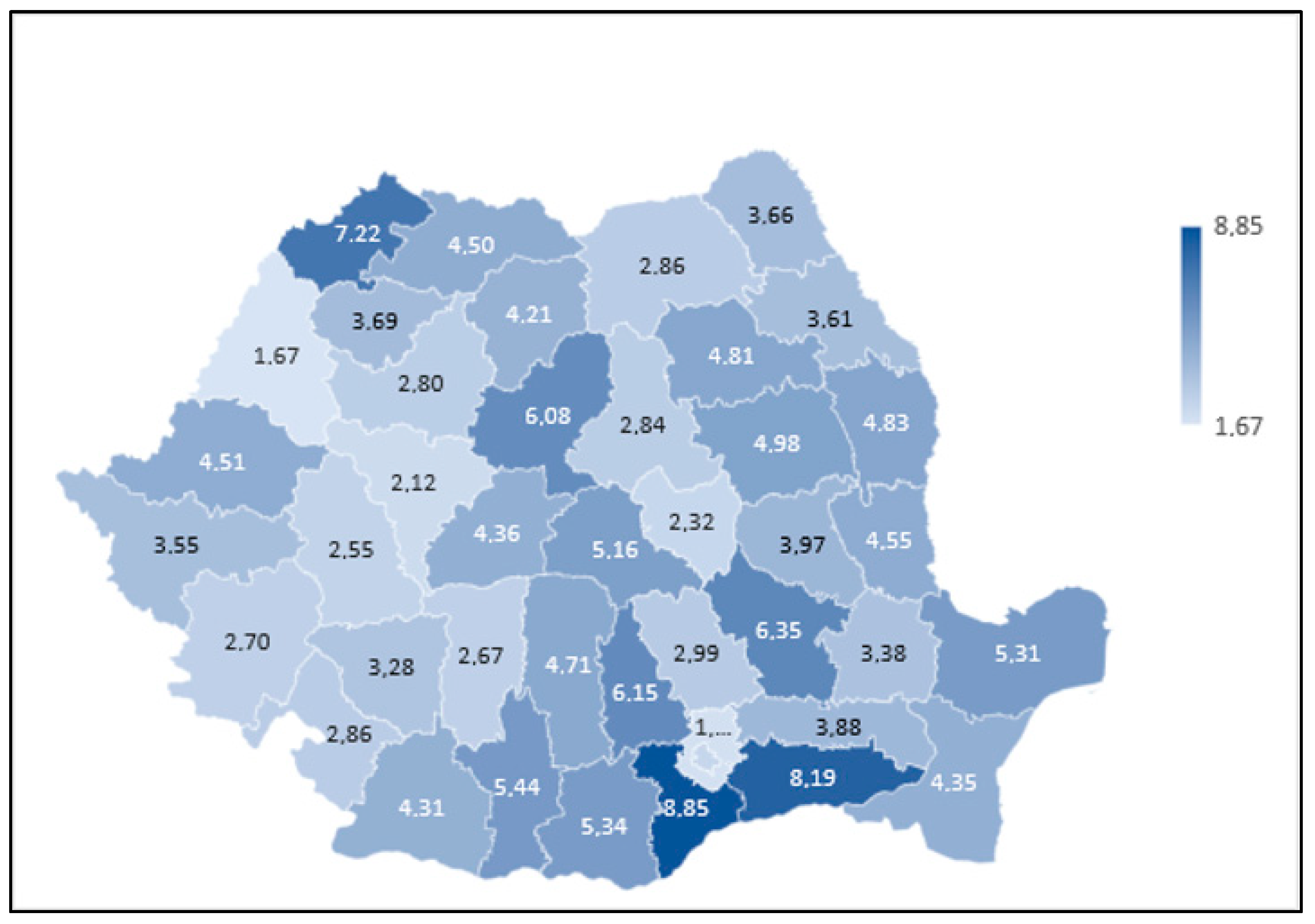
3.2. Regional Details
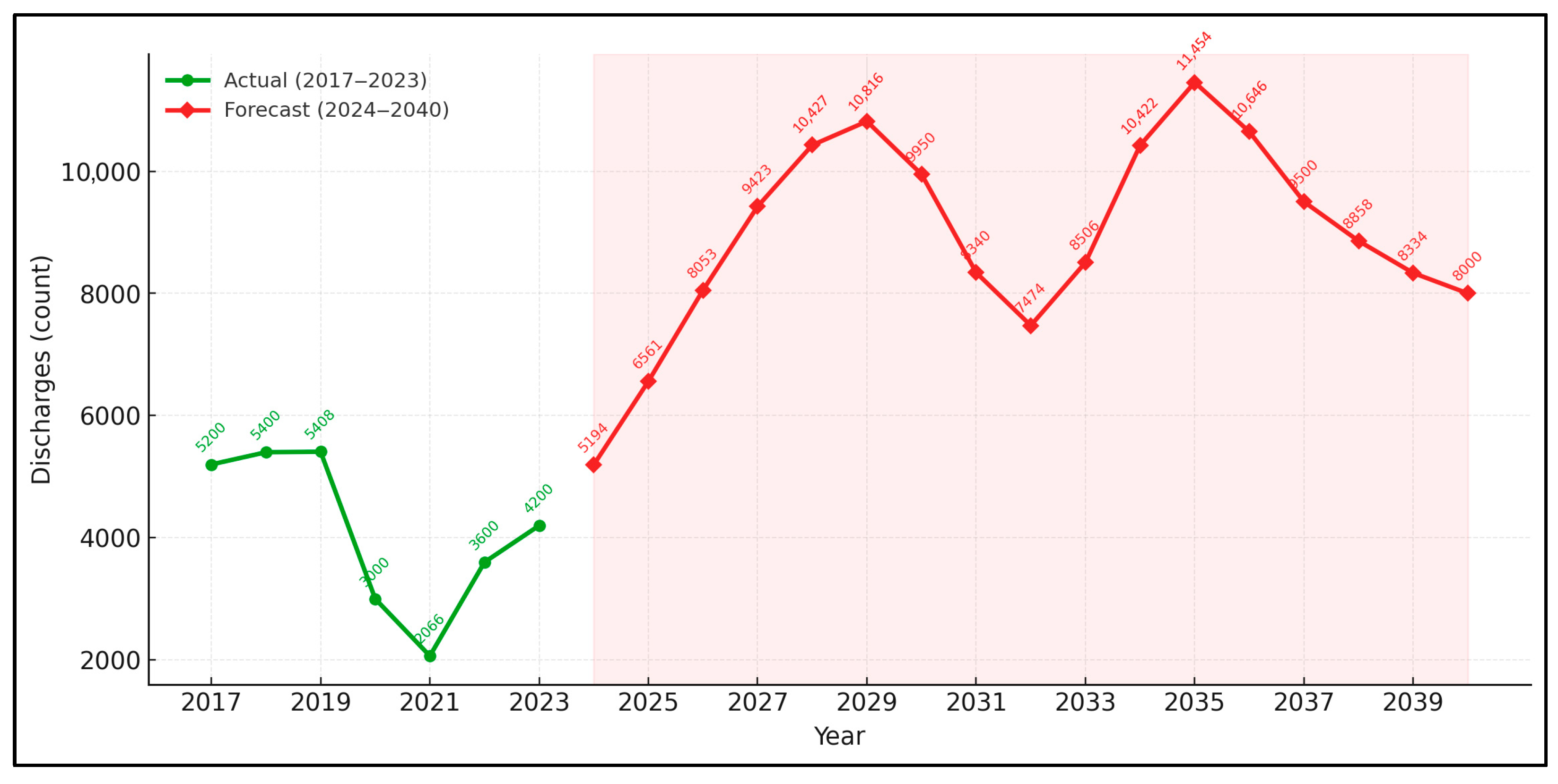
3.3. Service Delivery, Pandemic Backlog, Regional Model Sensitivity and Procedural Workload Trends
4. Discussion
4.1. Literature Findings
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Cheng, W.; Liu, X.; Li, H.; Song, Y. The global, regional, national burden of laryngeal cancer and its attributable risk factors (1990–2019) and predictions to 2035. Eur. J. Cancer Care 2022, 31, e13689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Wang, J.Y.; Qiao, X.F.; Li, T.L.; Li, X. Variations in disease burden of laryngeal cancer attributable to alcohol use and smoking in 204 countries or territories, 1990–2019. BMC Cancer 2021, 21, 1082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, A.; Wu, X.L.; Song, J.; Wang, Y.T.; Yao, Y.; Liu, Z.; Wang, H. Global trend and risk factors of the disease burden for pharynx and larynx cancers between 1990 and 2019: A systematic analysis of the global burden of disease study 2019. BMC Public Health 2022, 22, 2192. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, J.W.; Cao, J.L.; Wu, J.H.; Wang, L.L.; Gan, H.; Xu, J.H.; Ye, F. Tobacco and alcohol use are the risk factors responsible for the greatest burden of head and neck cancers: A study from the Global Burden of Disease Study 2019. Ann. Med. 2025, 57, 2500693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Wei, J.; Wang, B.; Meng, L.; Xin, Y.; Dong, L.; Jiang, X. Role of human papillomavirus in laryngeal squamous cell carcinoma: A meta-analysis of cohort study. Cancer Med. 2020, 9, 204–214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, E.; Xin, Z.; Jianrui, D.; Jinzhu, Y. Meta-Analysis of the Relationship Between Occupational/Environmental Exposure to Wood Dust and Laryngeal Cancer. Cancer Med. 2024, 13, e70330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tirmarche, M. Radon and cancer risk: Epidemiological studies after occupational or domestic exposure. Rev. Epidemiol. Sante Publique 1995, 43, 451–460. [Google Scholar] [PubMed]
- Ruano-Ravina, A.; Martin-Gisbert, L.; Kelsey, K.; Pérez-Ríos, M.; Candal-Pedreira, C.; Rey-Brandariz, J.; Varela-Lema, L. An overview on the relationship between residential radon and lung cancer: What we know and future research. Clin. Transl. Oncol. 2023, 25, 3357–3368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lechien, J.R.; Hans, S. Epidemiological, clinical and oncological outcomes of laryngeal verrucous carcinomas: A systematic review. J. Otolaryngol. Head Neck Surg. 2023, 52, 81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cîrstea, A.I.; Berteșteanu, Ș.V.G.; Scăunașu, R.V.; Popescu, B.; Bejenaru, P.L.; Simion-Antonie, C.B.; Berteșteanu, G.S.; Diaconu, T.E.; Taher, P.B.; Rujan, S.A.; et al. Management of Locally Advanced Laryngeal Cancer-From Risk Factors to Treatment, the Experience of a Tertiary Hospital from Eastern Europe. Int. J. Environ. Res. Public Health. 2023, 20, 4737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crossley, J.R.; Nelson, L.L.; VanDolah, H.; Davidson, B.J.; Maxwell, J.H. The impact of COVID-19 on presentation and diagnosis of head and neck squamous cell carcinoma. Laryngoscope Investig. Otolaryngol. 2022, 7, 1436–1440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nadarajan, A.R.; George, N.A.; Thomas, S.; Varghese, B.T.; Iype, E.M.; Krishna K.M., J. Impact of COVID-19 on Disease Progression and Postoperative Complications in Patients with Head and Neck Cancer. Indian. J. Surg. Oncol. 2023, 16, 491–495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roman, R.C.; Faur, C.I.; Gordan, E.; Văleanu, M.; Moldovan, M.A. Impact of the COVID-19 Pandemic on the Surgical Management of Head and Neck Non-Melanoma Skin Cancers in a Maxillofacial Center of Cluj-Napoca. J. Clin. Med. 2024, 13, 3934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanna, G.J.; Chang, S.S.; Siddiqui, F.; Bain, P.A.; Takiar, V.; Ward, M.C.; Shukla, M.E.; Hu, K.S.; Robbins, J.; Witek, M.E.; et al. Imaging and Biomarker Surveillance for Head and Neck Squamous Cell Carcinoma: A Systematic Review and American Radium Society Appropriate Use Criteria Statement. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, E.; Dyba, T.; Martos, C.; Randi, G.; Rooney, R.; Bettio, M. The need for a rapid and comprehensive adoption of the revised European standard population in cancer incidence comparisons. Eur. J. Cancer Prev. 2017, 26, 447–452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatenoud, L.; Garavello, W.; Pagan, E.; Bertuccio, P.; Gallus, S.; La Vecchia, C.; Negri, E.; Bosetti, C. Laryngeal cancer mortality trends in European countries. Int. J. Cancer 2016, 138, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Nešić, V.; Krstić Nešić, D.; Šipetić Grujičić, S.; Bukurov, B.; Miljuš, D.; Živković Perišić, S.; Nikolić, A. Long-Term Trends in Laryngeal Cancer Incidence and Mortality in Central Serbia (1999–2023): A Joinpoint Regression Analysis. Healthcare 2025, 13, 1633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kokoska, M.S.; Piccirillo, J.F.; Haughey, B.H. Gender differences in cancer of the larynx. Ann. Otol. Rhinol. Laryngol. 1995, 104, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, S.A.; Lango, M.N. Effect of rural and urban geography on larynx cancer incidence and survival. Laryngoscope 2018, 128, 1874–1880. [Google Scholar] [CrossRef] [PubMed]
- Lein, A.; Liu, D.T.; Haas, M.; Salkic, A.; Ibrisevic, A.; Uscuplic, S.; Harcinovic, A.; Brkic, T.; Thurner, T.; Brkic, F.F. Impact of the COVID-19 pandemic on management of surgically treated laryngeal squamous cell carcinoma. Biomol. Biomed. 2024, 24, 188–195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galletti, C.; De Marco, L.; Ciodaro, F.; Freni, F.; Saraniti, C.; Galletti, F.; Galletti, B. Impact of the Sars-COVID-19 Pandemic on the “Early Diagnosis” of Laryngeal Tumors: Data from Monocentric Tertiary Care Hospital of South Italy. J. Voice 2024, 30. in press. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Bisogno, A.; Colacurcio, V.; Marra, P.; Cassandro, C.; Camaioni, A.; Cassandro, E.; Scarpa, A. Diagnosis and treatment delay of head and neck cancers during COVID-19 era in a tertiary care academic hospital: What should we expect? Eur. Arch. Oto Rhino Laryngol. 2022, 279, 961–965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roșioară, A.I.; Năsui, B.A.; Ciuciuc, N.; Sîrbu, D.M.; Curșeu, D.; Oprica, R.F.; Popescu, C.A.; Ungur, R.A.; Cheșcheș, T.; Popa, M. Beyond Vaccination: Exploring Young Adults’ Awareness, Knowledge, and Attitudes Related to Sexually Transmitted Infections in Romania. Vaccines 2025, 13, 322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
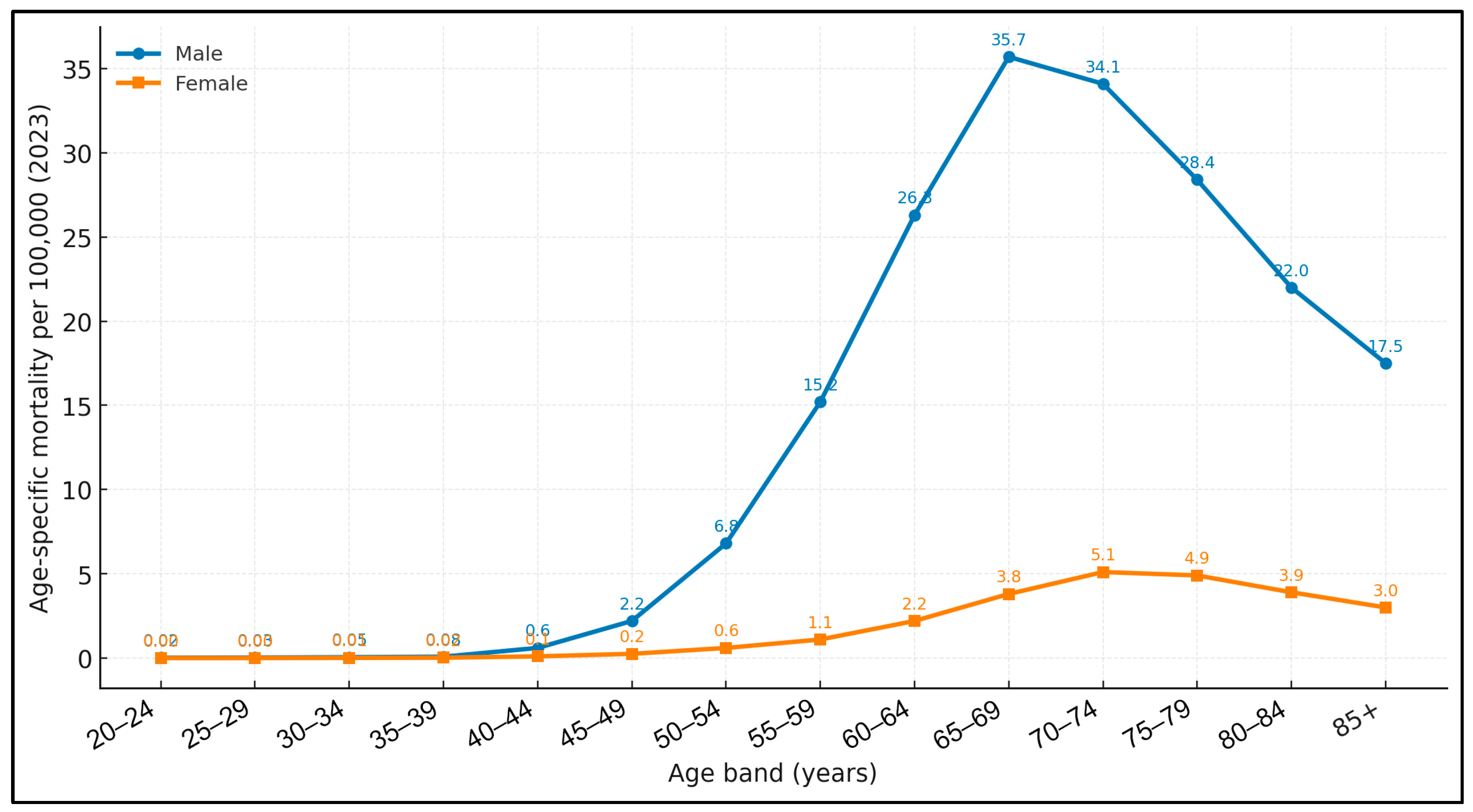
| 2023 | No. of Deaths by Laryngeal Cancer | % of Total | Laryngeal Cancer Death Rate (Deaths Per 100,000) | 95% CI for the Laryngeal Cancer Death Rate |
|---|---|---|---|---|
| Total | 798 | 100.0 | 3.65 | 3.41–3.91 |
| Male | 755 | 94.6 | 7.07 | 6.58–7.59 |
| Female | 43 | 5.4 | 0.39 | 0.28–0.53 |
| Rural | 411 | 51.5 | 4.26 | 3.86–4.69 |
| Urban | 344 | 43.1 | 3.04 | 2.98–3.44 |
| Group | County | SMR |
|---|---|---|
| Highest-risk corridor | Giurgiu | 1.82 |
| Călărași | 1.77 | |
| Satu Mare | 1.69 | |
| Buzău | 1.66 | |
| Brăila | 1.59 | |
| Lowest-risk quintile | Bucharest | 0.54 |
| Hunedoara | 0.56 | |
| Covasna | 0.58 | |
| Alba | 0.6 | |
| Bihor | 0.63 |
| Year | Direct ASMR (Romania 2017) | Direct ASMR (ESP-2013) | Direct ASMR (WHO World 2000–2025) |
|---|---|---|---|
| 2017 | 4.10 | 3.20 | 2.90 |
| 2023 | 3.23 | 2.62 | 2.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banța, A.-M.; Balica, N.-C.; Pîrvu, S.; Marin, K.-C.; Guran, K.; Barcan, I.-D.; Moț, C.-I.; Hîrtie, B.; Banța, V.; Horhat, D.I. Evolution in Laryngeal Cancer Mortality at the National and Subnational Level in Romania with 2030 Forecast. Medicina 2025, 61, 1743. https://doi.org/10.3390/medicina61101743
Banța A-M, Balica N-C, Pîrvu S, Marin K-C, Guran K, Barcan I-D, Moț C-I, Hîrtie B, Banța V, Horhat DI. Evolution in Laryngeal Cancer Mortality at the National and Subnational Level in Romania with 2030 Forecast. Medicina. 2025; 61(10):1743. https://doi.org/10.3390/medicina61101743
Chicago/Turabian StyleBanța, Andreea-Mihaela, Nicolae-Constantin Balica, Simona Pîrvu, Karina-Cristina Marin, Kristine Guran, Ingrid-Denisa Barcan, Cristian-Ion Moț, Bogdan Hîrtie, Victor Banța, and Delia Ioana Horhat. 2025. "Evolution in Laryngeal Cancer Mortality at the National and Subnational Level in Romania with 2030 Forecast" Medicina 61, no. 10: 1743. https://doi.org/10.3390/medicina61101743
APA StyleBanța, A.-M., Balica, N.-C., Pîrvu, S., Marin, K.-C., Guran, K., Barcan, I.-D., Moț, C.-I., Hîrtie, B., Banța, V., & Horhat, D. I. (2025). Evolution in Laryngeal Cancer Mortality at the National and Subnational Level in Romania with 2030 Forecast. Medicina, 61(10), 1743. https://doi.org/10.3390/medicina61101743








