Key Biomarker Correlations in Cutaneous Melanoma: Implications for Diagnostic, Prognostic, and Therapeutic Strategies—A Retrospective Single-Centered Study
Abstract
1. Introduction
2. Materials and Methods
- -
- Only primary tumours that were completely surgically removed.
- -
- No prior therapy had been administered.
- -
- Available follow-up information, including full-body CT scans after surgery to detect metastatic lesions and survival data in 2025;
- -
- Availability of immunohistochemical analyses for standard markers, including Ki-67, along with sufficient tissue samples for additional studies on PRAME and p16.
3. Results
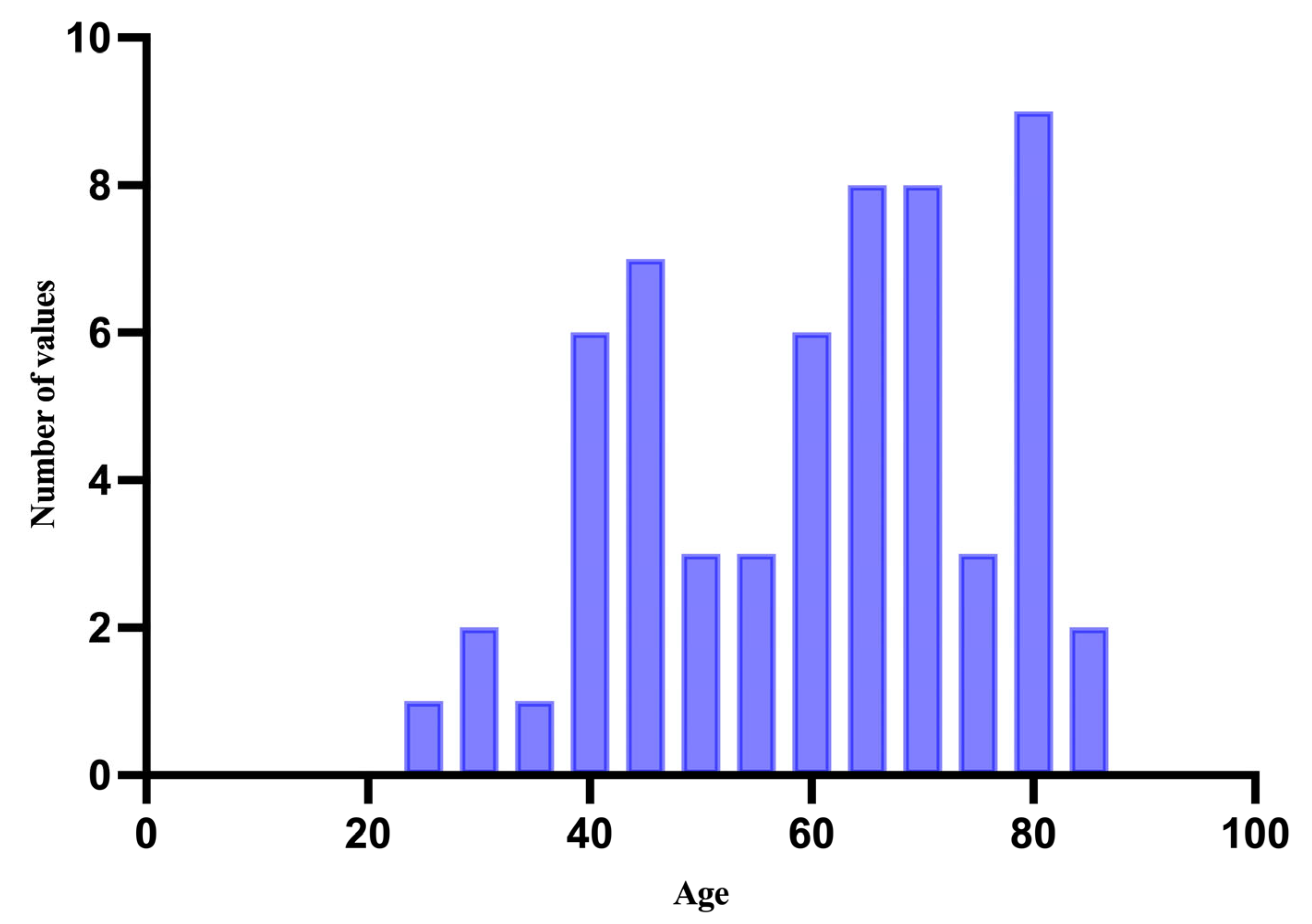
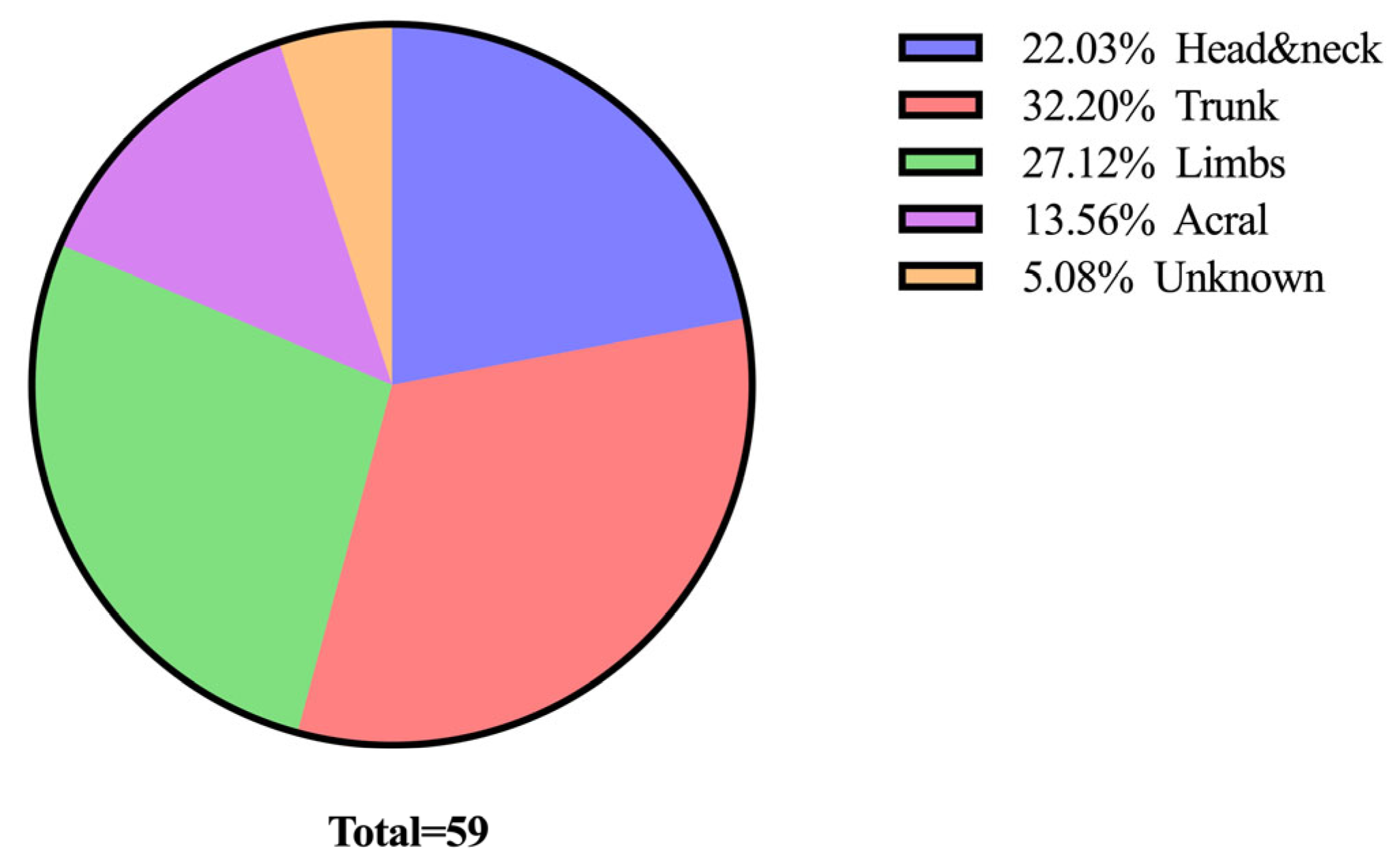
3.1. Demographic and Clinical Characteristics of the Study Population
3.2. Description and Analysis of the Correlation Matrix
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef]
- Perera, E.; Gnaneswaran, N.; Jennens, R.; Sinclair, R. Malignant Melanoma. Healthcare 2013, 2, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sboner, A.; Eccher, C.; Blanzieri, E.; Bauer, P.; Cristofolini, M.; Zumiani, G.; Forti, S. A multiple classifier system for early melanoma diagnosis. Artif. Intell. Med. 2003, 27, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Buja, A.; Bardin, A.; Damiani, G.; Zorzi, M.; De Toni, C.; Fusinato, R.; Spina, R.; Vecchiato, A.; Del Fiore, P.; Mocellin, S.; et al. Prognosis for Cutaneous Melanoma by Clinical and Pathological Profile: A Population-Based Study. Front. Oncol. 2021, 11, 737399. [Google Scholar] [CrossRef]
- Wong, V.K.; Lubner, M.G.; Menias, C.O.; Mellnick, V.M.; Kennedy, T.A.; Bhalla, S.; Pickhardt, P.J. Clinical and Imaging Features of Noncutaneous Melanoma. AJR Am. J. Roentgenol. 2017, 208, 942–959. [Google Scholar] [CrossRef]
- Viale, P.H. The American Cancer Society’s Facts & Figures: 2020 Edition. J. Adv. Pract. Oncol. 2020, 11, 135–136. [Google Scholar] [CrossRef]
- Mukkamalla, S.K.R.; Koya, S. Metastatic Melanoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Faries, M.B.; Thompson, J.F.; Cochran, A.J.; Andtbacka, R.H.; Mozzillo, N.; Zager, J.S.; Jahkola, T.; Bowles, T.L.; Testori, A.; Beitsch, P.D.; et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017, 376, 2211–2222. [Google Scholar] [CrossRef]
- Burns, D.; George, J.; Aucoin, D.; Bower, J.; Burrell, S.; Gilbert, R.; Bower, N. The Pathogenesis and Clinical Management of Cutaneous Melanoma: An Evidence-Based Review. J. Med. Imaging Radiat. Sci. 2019, 50, 460–469.e1. [Google Scholar] [CrossRef]
- Kauffmann, R.M.; Chen, S.L. Workup and staging of malignant melanoma. Surg. Clin. N. Am. 2014, 94, 963–972. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Dickson, P.V.; Gershenwald, J.E. Staging and prognosis of cutaneous melanoma. Surg. Oncol. Clin. N. Am. 2011, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Țăpoi, D.A.; Gheorghișan-Gălățeanu, A.-A.; Dumitru, A.V.; Ciongariu, A.M.; Furtunescu, A.R.; Marin, A.; Costache, M. Primary Undifferentiated/Dedifferentiated Cutaneous Melanomas—A Review on Histological, Immunohistochemical, and Molecular Features with Emphasis on Prognosis and Treatment. Int. J. Mol. Sci. 2023, 24, 9985. [Google Scholar] [CrossRef] [PubMed]
- Maddodi, N.; Setaluri, V. Prognostic significance of melanoma differentiation and trans-differentiation. Cancers 2010, 2, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, Y.; Uchikawa, M.; Kondoh, H. Pairing SOX off: With partners in the regulation of embryonic development. Trends Genet. 2000, 16, 182–187. [Google Scholar] [CrossRef]
- Schepers, G.E.; Teasdale, R.D.; Koopman, P. Twenty pairs of sox: Extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev. Cell 2002, 3, 167–170. [Google Scholar] [CrossRef]
- Wegner, M. From head to toes: The multiple facets of Sox proteins. Nucleic Acids Res. 1999, 27, 1409–1420. [Google Scholar] [CrossRef]
- Schock, E.N.; LaBonne, C. Sorting Sox: Diverse Roles for Sox Transcription Factors During Neural Crest and Craniofacial Development. Front. Physiol. 2020, 11, 606889. [Google Scholar] [CrossRef]
- Cronin, J.C.; Watkins-Chow, D.E.; Incao, A.; Hasskamp, J.H.; Schönewolf, N.; Aoude, L.G.; Hayward, N.K.; Bastian, B.C.; Dummer, R.; Loftus, S.K.; et al. SOX10 ablation arrests cell cycle, induces senescence, and suppresses melanomagenesis. Cancer Res. 2013, 73, 5709–5718. [Google Scholar] [CrossRef]
- Cronin, J.C.; Wunderlich, J.; Loftus, S.K.; Prickett, T.D.; Wei, X.; Ridd, K.; Vemula, S.; Burrell, A.S.; Agrawal, N.S.; Lin, J.C.; et al. Frequent mutations in the MITF pathway in melanoma. Pigment Cell Melanoma Res. 2009, 22, 435–444. [Google Scholar] [CrossRef]
- Gambichler, T.; Petig, A.L.; Stockfleth, E.; Stücker, M. Expression of SOX10, ABCB5 and CD271 in melanocytic lesions and correlation with survival data of patients with melanoma. Clin. Exp. Dermatol. 2016, 41, 709–716. [Google Scholar] [CrossRef]
- Hemminger, J.A.; Toland, A.E.; Scharschmidt, T.J.; Mayerson, J.L.; Guttridge, D.C.; Iwenofu, O.H. Expression of cancer-testis antigens MAGEA1, MAGEA3, ACRBP, PRAME, SSX2, and CTAG2 in myxoid and round cell liposarcoma. Mod. Pathol. 2014, 27, 1238–1245. [Google Scholar] [CrossRef]
- Zhang, W.; Barger, C.J.; Eng, K.H.; Klinkebiel, D.; Link, P.A.; Omilian, A.; Bshara, W.; Odunsi, K.; Karpf, A.R. PRAME expression and promoter hypomethylation in epithelial ovarian cancer. Oncotarget 2016, 7, 45352–45369. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.L.; De Pas, T.; Rittmeyer, A.; Vallières, E.; Kubisa, B.; Levchenko, E.; Wiesemann, S.; Masters, G.A.; Shen, R.; Tjulandin, S.A.; et al. Safety and Immunogenicity of the PRAME Cancer Immunotherapeutic in Patients with Resected Non-Small Cell Lung Cancer: A Phase I Dose Escalation Study. J. Thorac. Oncol. 2016, 11, 2208–2217. [Google Scholar] [CrossRef] [PubMed]
- Goodison, S.; Urquidi, V. The cancer testis antigen PRAME as a biomarker for solid tumor cancer management. Biomark. Med. 2012, 6, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Rivoltini, L.; Levchenko, E.; Testori, A.; Utikal, J.; Ascierto, P.A.; Demidov, L.; Grob, J.J.; Ridolfi, R.; Schadendorf, D.; et al. Safety and immunogenicity of the PRAME cancer immunotherapeutic in metastatic melanoma: Results of a phase I dose escalation study. ESMO Open 2016, 1, e000068. [Google Scholar] [CrossRef]
- Ferris, L.K.; Jansen, B.; Ho, J.; Busam, K.J.; Gross, K.; Hansen, D.D.; Alsobrook, J.P., II; Yao, Z.; Peck, G.L.; Gerami, P. Utility of a Noninvasive 2-Gene Molecular Assay for Cutaneous Melanoma and Effect on the Decision to Biopsy. JAMA Dermatol. 2017, 153, 675–680. [Google Scholar] [CrossRef]
- Clarke, L.E.; Flake, D.D., II; Busam, K.; Cockerell, C.; Helm, K.; McNiff, J.; Reed, J.; Tschen, J.; Kim, J.; Barnhill, R.; et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer 2017, 123, 617–628. [Google Scholar] [CrossRef]
- Gerdes, J.; Schwab, U.; Lemke, H.; Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer. 1983, 31, 13–20. [Google Scholar] [CrossRef]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Luo, Y.; Ren, F.; Liu, Y.; Shi, Z.; Tan, Z.; Xiong, H.; Dang, Y.; Chen, G. Clinicopathological and prognostic significance of high Ki-67 labeling index in hepatocellular carcinoma patients: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10235–10247. [Google Scholar]
- Richards-Taylor, S.; Ewings, S.M.; Jaynes, E.; Tilley, C.; Ellis, S.G.; Armstrong, T.; Pearce, N.; Cave, J. The assessment of Ki-67 as a prognostic marker in neuroendocrine tumours: A systematic review and meta-analysis. J. Clin. Pathol. 2016, 69, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Flørenes, V.A.; Maelandsmo, G.M.; Faye, R.; Nesland, J.M.; Holm, R. Cyclin A expression in superficial spreading malignant melanomas correlates with clinical outcome. J. Pathol. 2001, 195, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Peng, Z.; Shen, L.; Shen, L. Prognostic and Clinicopathological Value of Ki-67 in Melanoma: A Meta-Analysis. Front. Oncol. 2021, 11, 737760. [Google Scholar] [CrossRef] [PubMed]
- Asato, M.A.; Moraes Neto, F.A.; Moraes, M.P.T.; Ocanha-Xavier, J.P.; Takita, L.C.; Fung, M.A.; Marques, M.E.A.; Xavier-Júnior, J.C.C. The Utility of PRAME and Ki-67 as Prognostic Markers for Cutaneous Melanoma. Am. J. Dermatopathol. 2025, 47, 9–16. [Google Scholar] [CrossRef]
- Ming, Z.; Lim, S.Y.; Rizos, H. Genetic Alterations in the INK4a/ARF Locus: Effects on Melanoma Development and Progression. Biomolecules 2020, 10, 1447. [Google Scholar] [CrossRef]
- Ricci, C.; Dika, E.; Ambrosi, F.; Lambertini, M.; Veronesi, G.; Barbara, C. Cutaneous Melanomas: A Single Center Experience on the Usage of Immunohistochemistry Applied for the Diagnosis. Int. J. Mol. Sci. 2022, 23, 5911. [Google Scholar] [CrossRef]
- Gkionis, I.G.; Tzardi, M.; Alegakis, A.; Datseri, G.; Moustou, E.; Saridakis, G.; Michelakis, D.; Kruger-Krasagakis, S.; Krasagakis, K.; DEBree, E. Cyclin E Expression and p16 Loss Are Strong Prognostic Biomarkers in Primary Invasive Cutaneous Melanoma. Anticancer Res. 2025, 45, 625–637. [Google Scholar] [CrossRef]
- Koh, S.S.; Cassarino, D.S. Immunohistochemical Expression of p16 in Melanocytic Lesions: An Updated Review and Meta-analysis. Arch. Pathol. Lab. Med. 2018, 142, 815–828. [Google Scholar] [CrossRef]
- Ricci, C.; Dika, E.; Corti, B.; Lambertini, M.; Ambrosi, F.; Cappilli, S.; Grillini, M.; Filippo, G.D.; Franchini, E.; Maloberti, T.; et al. “Paradoxical” p16 overexpression in cutaneous melanoma: Molecular and immunohistochemical analysis of a rare phenomenon with a focus on cell cycle regulatory molecules. Pathol. Res. Pract. 2023, 247, 154564. [Google Scholar] [CrossRef]
- Elder, D.E.; Barnhill, R.Y. (Eds.) Chapter III: Melanocytic neoplasms. In WHO Classification of Tumours Editorial Board. Skin Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2023; Volume 12, Available online: https://tumourclassification.iarc.who.int/chapters/64 (accessed on 20 July 2025).
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Thompson, J.F.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Atkins, M.B.; Balch, C.M.; Barnhill, R.L.; et al. Melanoma of the Skin. In AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 563–585. [Google Scholar]
- Semiz, Y.; Aktas, E.; Topal, I.O. Demographic and clinical features of cutaneous malignant melanoma patients: A single center cohort study. North. Clin. Istanb. 2023, 10, 687–696. [Google Scholar] [CrossRef]
- Ghazawi, F.M.; Cyr, J.; Darwich, R.; Le, M.; Rahme, E.; Moreau, L.; Netchiporouk, E.; Zubarev, A.; Roshdy, O.; Glassman, S.J.; et al. Cutaneous malignant melanoma incidence and mortality trends in Canada: A comprehensive population-based study. J. Am. Acad. Dermatol. 2019, 80, 448–459. [Google Scholar] [CrossRef]
- Bucchi, L.; Mancini, S.; Zamagni, F.; Crocetti, E.; Dal Maso, L.; Ferretti, S.; Baldacchini, F.; Giuliani, O.; Ravaioli, A.; Vattiato, R.; et al. Patient presentation, skin biopsy utilization and cutaneous malignant melanoma incidence and mortality in northern Italy: Trends and correlations. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, S.; Maddukuri, S.; Cadwell, J.B.; Lambert, W.C.; Schwartz, R.A. Gender differences in cutaneous melanoma: Demographics, prognostic factors, and survival outcomes. Dermatol. Ther. 2020, 33, e14131. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Önefäldt, D.; Zommorodi, S.; Delgado, A.F. Location of cutaneous malignant melanoma in Sweden 2004–2018—Mortality and sex differences. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 3398–3405. [Google Scholar] [CrossRef]
- Voinea, S.; Blidaru, A.; Panaitescu, E.; Sandru, A. Impact of gender and primary tumor location on outcome of patients with cutaneous melanoma. J. Med. Life 2016, 9, 444–448. [Google Scholar]
- Nasser, N.; Silva, J.L.D.; Corrêa, G. Epidemiology of cutaneous melanoma in Blumenau, Santa Catarina state, Brazil from 1980 to 2019. Bras. Dermatol. 2023, 98, 611–619. [Google Scholar] [CrossRef]
- van Niekerk, C.C.; Otten, J.H.D.M.; van Rossum, M.M.; van den Reek, J.M.P.A.; Brummelkamp, E.; Mol, M.; Groenewoud, J.H.M.M.; Verbeek, A.L.M. Trends in three major histological subtypes of cutaneous melanoma in the Netherlands between 1989 and 2016. Int. J. Dermatol. 2023, 62, 508–513. [Google Scholar] [CrossRef]
- Di Carlo, V.; Stiller, C.A.; Eisemann, N.; Bordoni, A.; Matz, M.; Curado, M.P.; Daubisse-Marliac, L.; Valkov, M.; Bulliard, J.L.; Morrison, D.; et al. Does the morphology of cutaneous melanoma help to explain the international differences in survival? Results from 1 578 482 adults diagnosed during 2000–2014 in 59 countries (CONCORD-3). Br. J. Dermatol. 2022, 187, 364–380. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Cutaneous Melanoma (Version 2.2025). In NCCN Clinical Practice Guidelines in Oncology; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2025; Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1492 (accessed on 10 September 2025).
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2019, 80, 208–250. [Google Scholar] [CrossRef]
- Kashani-Sabet, M.; Leachman, S.A.; Stein, J.A.; Arbiser, J.L.; Berry, E.G.; Celebi, J.T.; Curiel-Lewandrowski, C.; Ferris, L.K.; Grank-Kels, J.M.; Grossman, D.; et al. Early detection and prognostic assessment of cutaneous melanoma: Consensus on optimal practice and the role of gene expression profile testing. JAMA Dermatol. 2023, 159, 545–553. [Google Scholar] [CrossRef]
- Bartlett, E.K.; O’Donoghue, C.; Boland, G.; Bowles, T.; Delman, K.A.; Hieken, T.J.; Moncrieff, M.; Wong, S.; White, R.L.; Karakousis, G.; et al. Society of Surgical Oncology consensus statement: Assessing the evidence for and utility of gene expression profiling of primary cutaneous melanoma. Ann. Surg. Oncol. 2025, 32, 1429–1442. [Google Scholar] [CrossRef]
- Mokos, M.; Prkačin, I.; Gaćina, K.; Brkić, A.; Pondeljak, N.; Šitum, M. Therapeutic Opportunities in Melanoma Through PRAME Expression. Biomedicines 2025, 13, 1988. [Google Scholar] [CrossRef] [PubMed]
- Wermke, M.; Tsimberidou, A.M.; Mohamed, A.; Mayer-Mokler, A.; Satelli, A.; Reinhardt, C.; Araujo, D.; Maurer, D.; Blumenschein, G.J.; Singh, H.; et al. Safety and anti-tumour activity of TCR-engineered autologous, PRAME-directed T cells across multiple advanced solid cancers at low doses—Clinical update on the ACTengine® IMA203 trial. J. Immunother. Cancer 2021, 9 (Suppl. S2), A1009. [Google Scholar]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Parra, O.; Ma, W.; Li, Z.; Coffing, B.N.; Linos, K.; LeBlanc, R.E.; Momtahen, S.; Sriharan, A.; Cloutier, J.M.; Wells, W.A.; et al. PRAME expression in cutaneous melanoma does not correlate with disease-specific survival. J. Cutan. Pathol. 2023, 50, 903–912. [Google Scholar] [CrossRef]
- Ronchi, A.; Cazzato, G.; Ingravallo, G.; D’Abbronzo, G.; Argenziano, G.; Moscarella, E.; Brancaccio, G.; Franco, R. PRAME Is an Effective Tool for the Diagnosis of Nevus-Associated Cutaneous Melanoma. Cancers 2024, 16, 278. [Google Scholar] [CrossRef]
- Blount, S.L.; Liu, X.; McBride, J.D. The Utilization of PRAME in the Diagnosis, Prognosis, and Treatment of Melanoma. Cells 2024, 13, 1740. [Google Scholar] [CrossRef]
- Tapoi, D.A.; Gheorghișan-Gălățeanu, A.-A.; Gosman, L.M.; Derewicz, D.; Costache, M. The Prognostic Value of Proliferative Activity in Cutaneous Melanoma: A Pilot Study Evaluating the Mitotic Rate and Ki67 Index to Predict Patient Outcomes. Biomedicines 2024, 12, 1318. [Google Scholar] [CrossRef]
- Blakely, A.M.; Cohen, J.T.; Comissiong, D.S.; Vezeridis, M.P.; Miner, T.J. Prognosis and Management of Thick and Ultrathick Melanoma. Am. J. Clin. Oncol. 2019, 42, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Vidri, R.J.; MacGillivray, D.C.; Fitzgerald, T.L. Tumor mitotic rate is an independent predictor of survival for nonmetastatic melanoma. Surgery 2018, 164, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Buja, A.; Rugge, M.; Cozzolino, C.; Dossi, F.; Zorzi, M.; Vecchiato, A.; de Luca, G.; Del Fiore, P.; Tropea, S.; dall’Olmo, L.; et al. Could the mitotic count improve personalized prognosis in melanoma patients? PLoS ONE 2024, 19, e0302309. [Google Scholar] [CrossRef] [PubMed]
- Marsch, A.F.; McKee, R.M.; Werbel, T.; Ruo, B.; Hinds, B.R. The Relationship Between Epidermal Mitotic Density, Atypical Mitotic Figure Density, Breslow Depth, Ulceration, and Dermal Mitotic Rate in Cutaneous Melanoma: A Retrospective Cohort Study. Int. J. Surg. Pathol. 2021, 29, 592–599. [Google Scholar] [CrossRef]
- Jurmeister, P.; Bockmayr, M.; Treese, C.; Stein, U.; Lenze, D.; Jöhrens, K.; Friedling, F.; Dietel, M.; Klauschen, F.; Marsch, W.; et al. Immunohistochemical analysis of Bcl-2, nuclear S100A4, MITF, and Ki67 for risk stratification of early-stage melanoma—A combined IHC score for melanoma risk stratification. J. Dtsch. Dermatol. Ges. 2019, 17, 800–808. [Google Scholar] [CrossRef]
- Robinson, E.M.; Rosenbaum, B.E.; Zhang, Y.; Rogers, R.; Tchack, J.; Berman, R.S.; Darvishian, F.; Osman, I.; Shapiro, R.L.; Shao, Y.; et al. Association between Ki-67 expression and clinical outcomes among patients with clinically node-negative, thick primary melanoma who underwent nodal staging. J. Surg. Oncol. 2018, 118, 150–156. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Ding, M.; Xu, K.; Li, L.; Mao, L.; Zheng, J. Ki67 targeted strategies for cancer therapy. Clin. Transl. Oncol. 2018, 20, 570–575. [Google Scholar] [CrossRef]
- Rees, M.J.; Liao, H.; Spillane, J.; Speakman, D.; McCormack, C.; Donahoe, S.; Pohl, M.; Webb, A.; Gyorki, D.; Henderson, M.A. Melanoma in the very elderly: Management in patients 85 years of age and over. J. Geriatr. Oncol. 2018, 9, 488–493. [Google Scholar] [CrossRef]
- Ferhatoglu, F.; Erturk, K.; Faruk, T. Cutaneous melanoma survival rates of the elderly are not worse than those of the young, yet they have some specific differences. J. Cancer Res. Ther. 2023, 19 (Suppl. S1), S349–S354. [Google Scholar] [CrossRef]
- Țăpoi, D.A.; Derewicz, D.; Gheorghișan-Gălățeanu, A.-A.; Dumitru, A.V.; Ciongariu, A.M.; Costache, M. The Impact of Clinical and Histopathological Factors on Disease Progression and Survival in Thick Cutaneous Melanomas. Biomedicines 2023, 11, 2616. [Google Scholar] [CrossRef]
- Boada, A.; Tejera-Vaquerizo, A.; Ribero, S.; Puig, S.; Moreno-Ramírez, D.; Osella-Abate, S.; Cassoni, P.; Malvehy, J.; Podlipnik, S.; Requena, C.; et al. Age as a prognostic factor in thick and ultrathick melanomas without lymph node metastasis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e513–e517. [Google Scholar] [CrossRef] [PubMed]
- Lade-Keller, J.; Riber-Hansen, R.; Guldberg, P.; Schmidt, H.; Hamilton-Dutoit, S.J.; Steiniche, T. Immunohistochemical analysis of molecular drivers in melanoma identifies p16 as an independent prognostic biomarker. J. Clin. Pathol. 2014, 67, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Obadofin, O.; Badmos, K.; Orsi, N.; Bipin, M.; Rotimi, O.; Banjo, A. Immunohistochemical Analysis of BRAF (V600E) Mutation and P16 Expression in Malignant Melanoma in Lagos, Nigeria: A 10-Year Retrospective Study. J. Ski. Cancer 2019, 2019, 1628247. [Google Scholar] [CrossRef]
- Fauri, J.; Ricardi, F.; Diehl, E.; Cartell, A.; Furian, R.; Bakos, L.; Edelweiss, M. P16 protein expression in primary cutaneous melanoma with positive and negative lymph node biopsies: Particular aspects of a study performed at the Hospital de Clinicas de Porto Alegre, Brazil. Can. J. Plast. Surg. 2011, 19, 77–81. [Google Scholar] [CrossRef]
- Ghiorzo, P.; Villaggio, B.; Sementa, A.R.; Hansson, J.; Platz, A.; Nicoló, G.; Spina, B.; Canepa, M.; Palmer, J.M.; Hayward, N.K.; et al. Expression and localization of mutant p16 proteins in melanocytic lesions from familial melanoma patients. Hum. Pathol. 2004, 35, 25–33. [Google Scholar] [CrossRef]
- Straume, O.; Sviland, L.; Akslen, L.A. Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and poor prognosis in patients with vertical growth phase melanoma. Clin. Cancer Res. 2000, 6, 1845–1853. [Google Scholar]
- Hilliard, N.J.; Krahl, D.; Sellheyer, K. p16 expression differentiates between desmoplastic Spitz nevus and desmoplastic melanoma. J. Cutan. Pathol. 2009, 36, 753–759. [Google Scholar] [CrossRef]
- Pavey, S.J.; Cummings, M.C.; Whiteman, D.C.; Castellano, M.; Walsh, M.D.; Gabrielli, B.G.; Green, A.; Hayward, N.K. Loss of p16 expression is associated with histological features of melanoma invasion. Melanoma Res. 2002, 12, 539–547. [Google Scholar] [CrossRef]
- Sirigu, P.; Piras, F.; Minerba, L.; Murtas, D.; Maxia, C.; Colombari, R.; Corbu, A.; Perra, M.T.; Ugalde, J. Prognostic prediction of the immunohistochemical expression of p16 and p53 in cutaneous melanoma: A comparison of two populations from different geographical regions. Eur. J. Histochem. 2006, 50, 191–198. [Google Scholar]
- Dhanyamraju, P.K.; Patel, T.N. Melanoma therapeutics: A literature review. J. Biomed. Res. 2022, 36, 77–97. [Google Scholar] [CrossRef]
- Soo, J.K.; Castle, J.T.; Bennett, D.C. Preferential killing of melanoma cells by a p16-related peptide. Biol. Open. 2023, 12, bio059965. [Google Scholar] [CrossRef] [PubMed]
- Pavlick, A.C.; Ariyan, C.E.; Buchbinder, E.I.; Davar, D.; Gibney, G.T.; Hamid, O.; Hieken, T.J.; Izar, B.; Johnson, D.B.; Kulkarni, R.P.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of melanoma, version 3.0. J. Immunother. Cancer 2023, 11, e006947. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, V.; Pizzimenti, C.; Franchina, M.; Pepe, L.; Russotto, F.; Tralongo, P.; Micali, M.G.; Militi, G.B.; Lentini, M. Programmed Cell Death Ligand 1 Immunohistochemical Expression and Cutaneous Melanoma: A Controversial Relationship. Int. J. Mol. Sci. 2024, 25, 676. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Haruki, K.; Tsukihara, S.; Ito, D.; Kanno, H.; Son, K.; Hanyu, N.; Eto, K. The impact of low serum cholinesterase levels on survival in patients with colorectal cancer. Int. J. Color. Dis. 2022, 37, 869–877. [Google Scholar] [CrossRef]
- Nicolae, C.; Nicolae, I.; Tampa, M.; Matei, C.; Georgescu, S.R. Butyrylcholinesterase in UV reactions. Rev. Chim. 2013, 64, 654–658. [Google Scholar]
- Zhang, Q.; Yang, H.; Gao, J.; Lv, R.; Zheng, K.; Zhang, P.; Ding, C. NIR fluorescence strategy for the early diagnosis of melanoma liver metastasis based on the cascade reaction of two specific bioenzymes. Anal. Chem. 2025, 97, 3125–3135. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef]







| SOX10 | Melan-A | HMB45 | S100 | PRAME | p16 | |
|---|---|---|---|---|---|---|
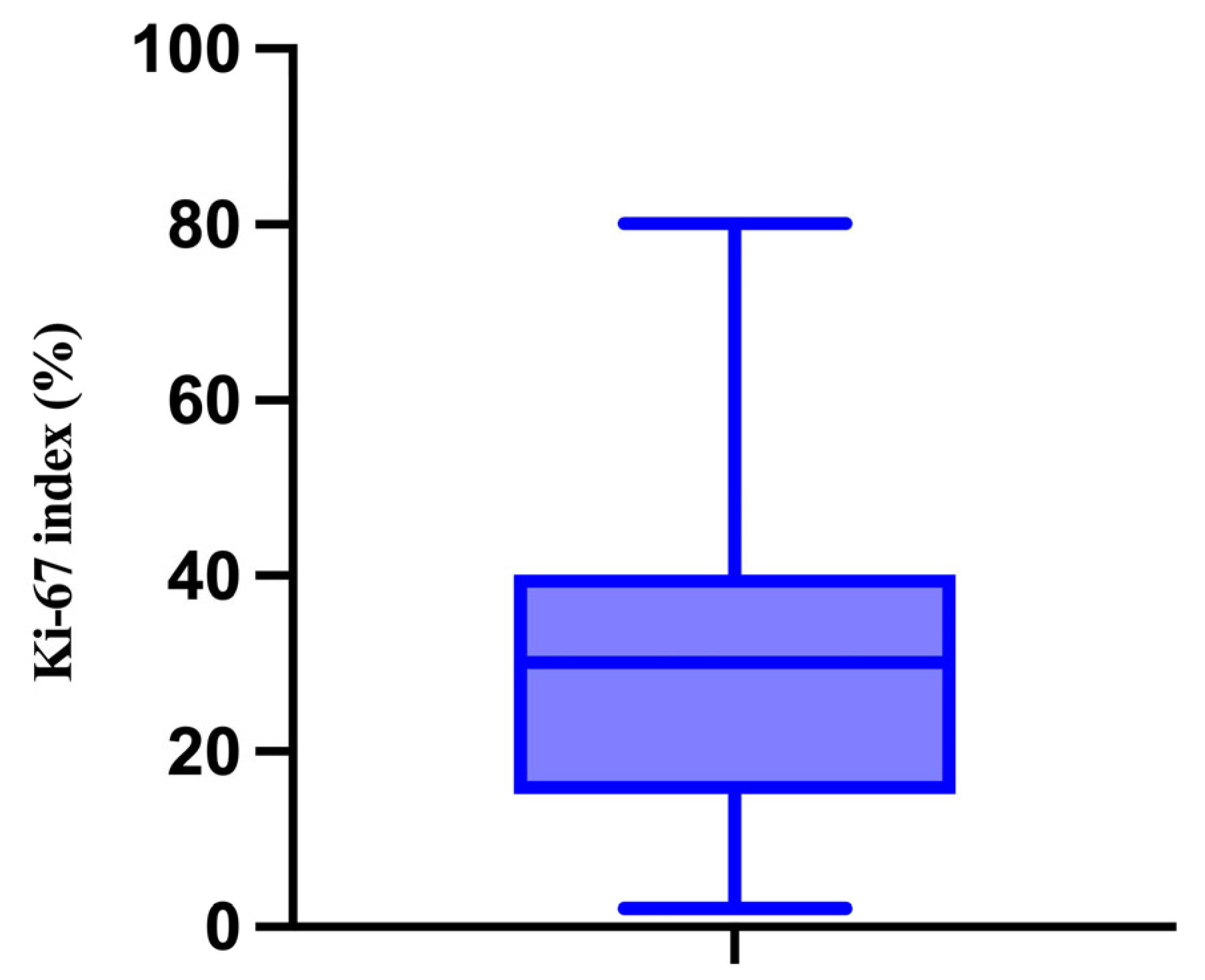
| Minimum | 70% | 0% | 0% | 30% | 0% | 0% |
| Maximum | 100% | 100% | 100% | 100% | 100% | 90% |
| Median | 85% | 90% | 80% | 90% | 90% | 50% |
| Age | Mitoses | Breslow Depth | Ki-67 | SOX10 | Melan-A | HMB45 | S100 | PRAME | p16 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.01 | 0.091 | 0.012 | 0.859 | 0.043 | 0.002 | 0.380 | 0.803 | 0.022 | |
| Mitoses | 0.010 | <0.0001 | <0.0001 | 0.524 | <0.0001 | <0.0001 | 0.020 | 0.007 | 0.090 | |
| Breslow Depth | 0.091 | <0.0001 | <0.0001 | 0.486 | <0.0001 | 0.002 | 0.031 | 0.002 | 0.032 | |
| Ki-67 | 0.012 | <0.0001 | <0.0001 | 0.437 | 0.001 | 0.004 | 0.125 | 0.001 | 0.044 | |
| SOX10 | 0.859 | 0.524 | 0.486 | 0.437 | 0.437 | 0.268 | 0.100 | 0.004 | 0.348 | |
| Melan-A | 0.043 | <0.0001 | <0.0001 | 0.001 | 0.437 | <0.0001 | <0.0001 | 0.846 | 0.233 | |
| HMB45 | 0.002 | <0.0001 | 0.002 | 0.004 | 0.268 | <0.0001 | 0.001 | 0.695 | 0.182 | |
| S100 | 0.380 | 0.002 | 0.031 | 0.125 | 0.100 | <0.0001 | 0.001 | 0.200 | 0.850 | |
| PRAME | 0.803 | 0.007 | 0.002 | 0.001 | 0.004 | 0.846 | 0.695 | 0.200 | 0.883 | |
| p16 | 0.022 | 0.09 | 0.032 | 0.044 | 0.3348 | 0.233 | 0.182 | 0.850 | 0.883 |
| Parameter | OR | 95%CI | p Value |
|---|---|---|---|
| Age | 1.037 | 0.99–1.08 | 0.0595 |
| Breslow depth | 1.175 | 1.06–1.33 | 0.0003 |
| Mitoses | 1.302 | 1.13–1.57 | <0.0001 |
| Ki-67 | 1.047 | 1.02–1.08 | 0.0006 |
| SOX10 | 1.009 | 0.89–1.15 | 0.882 |
| Melan-A | 0.9646 | 0.93–0.99 | 0.0116 |
| HMB45 | 0.9756 | 0.95–0.99 | 0.0153 |
| S100 | 0.9602 | 0.91–1.01 | 0.0806 |
| PRAME | 1.059 | 1.001–1.21 | 0.0449 |
| p16 | 0.9476 | 0.86–0.99 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costache, M.; Gheorghişan-Gălățeanu, A.-A.; Derewicz, D.; Cîrstoiu, C.; Ilieșiu, A. Key Biomarker Correlations in Cutaneous Melanoma: Implications for Diagnostic, Prognostic, and Therapeutic Strategies—A Retrospective Single-Centered Study. Medicina 2025, 61, 1733. https://doi.org/10.3390/medicina61101733
Costache M, Gheorghişan-Gălățeanu A-A, Derewicz D, Cîrstoiu C, Ilieșiu A. Key Biomarker Correlations in Cutaneous Melanoma: Implications for Diagnostic, Prognostic, and Therapeutic Strategies—A Retrospective Single-Centered Study. Medicina. 2025; 61(10):1733. https://doi.org/10.3390/medicina61101733
Chicago/Turabian StyleCostache, Mariana, Ancuța-Augustina Gheorghişan-Gălățeanu, Diana Derewicz, Cătălin Cîrstoiu, and Andreea Ilieșiu. 2025. "Key Biomarker Correlations in Cutaneous Melanoma: Implications for Diagnostic, Prognostic, and Therapeutic Strategies—A Retrospective Single-Centered Study" Medicina 61, no. 10: 1733. https://doi.org/10.3390/medicina61101733
APA StyleCostache, M., Gheorghişan-Gălățeanu, A.-A., Derewicz, D., Cîrstoiu, C., & Ilieșiu, A. (2025). Key Biomarker Correlations in Cutaneous Melanoma: Implications for Diagnostic, Prognostic, and Therapeutic Strategies—A Retrospective Single-Centered Study. Medicina, 61(10), 1733. https://doi.org/10.3390/medicina61101733






