Opioid-Associated Postoperative Nausea and Vomiting in Women Undergoing Laparoscopic Hysterectomy: A Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility
- (1)
- Population (P): women who underwent laparoscopic hysterectomy,
- (2)
- Intervention/Comparison (I/C): any pairwise comparisons of opioids or comparisons of opioids with non-opioid analgesics for postoperative pain management, and
- (3)
- Outcome (O): postoperative nausea or vomiting.
2.2. Search Strategy
2.3. Study Selection
2.4. Definition of Independent and Dependent Variables
2.5. Risk-of-Bias Assessment
2.6. Statistical Analysis
3. Results
3.1. Selected Studies
3.2. Study Characteristics
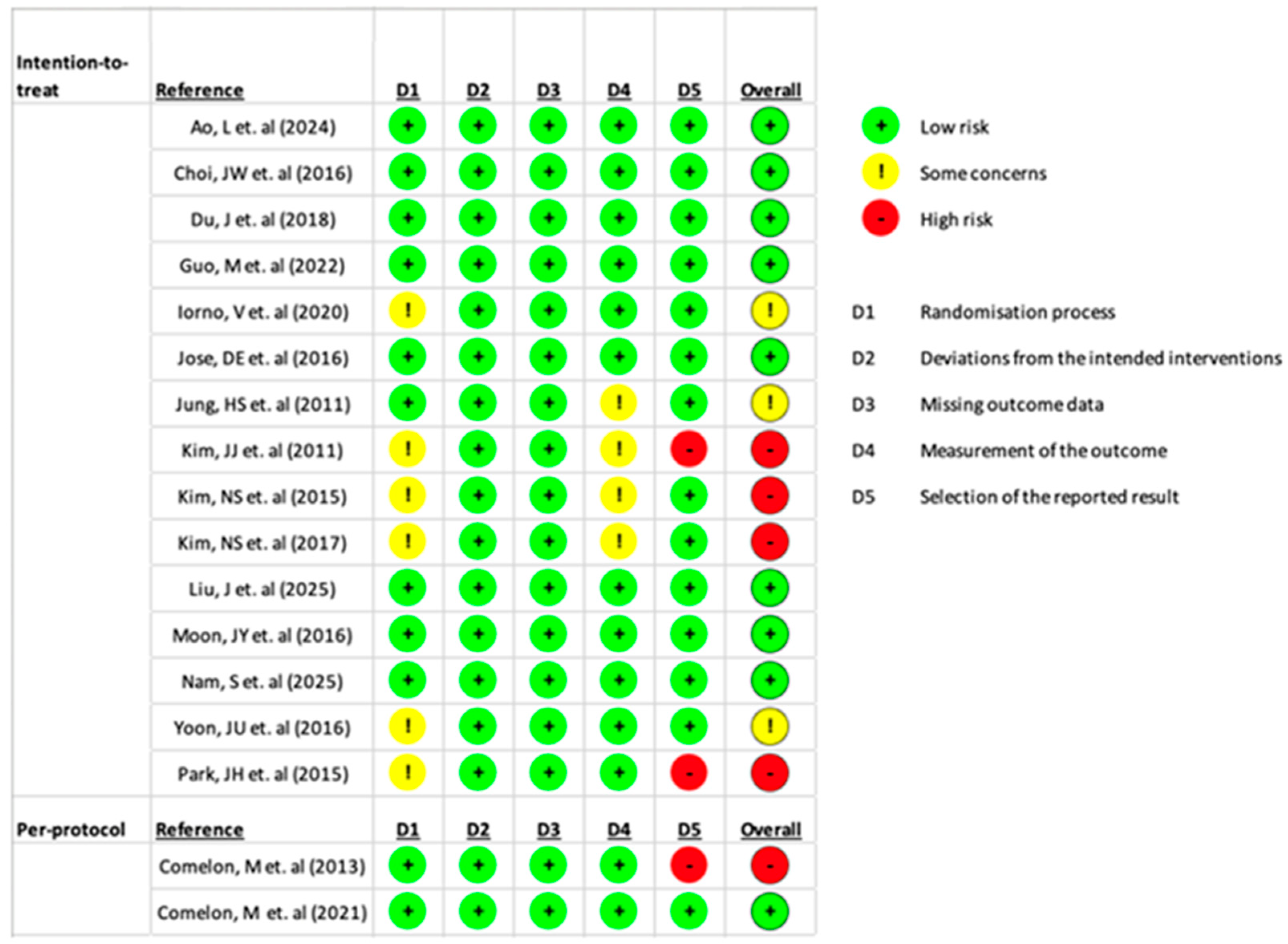
3.3. Risk of Bias Assessment
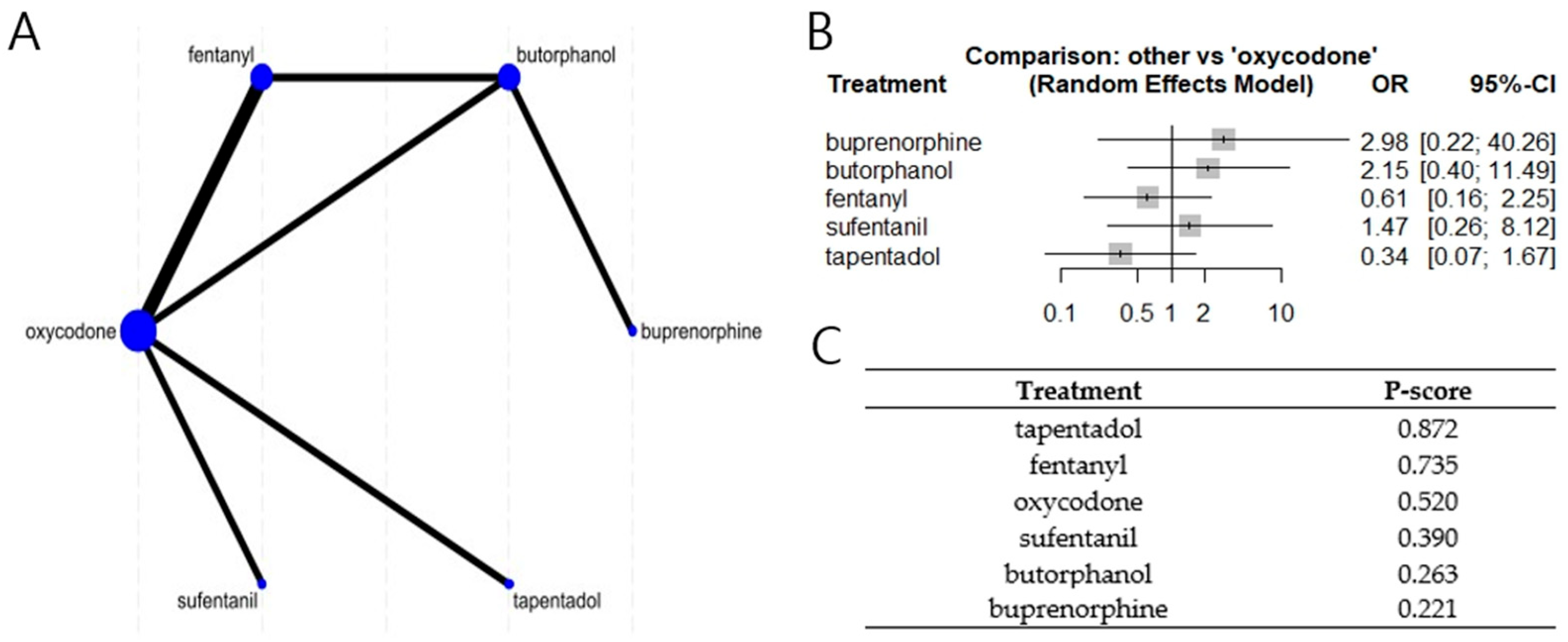
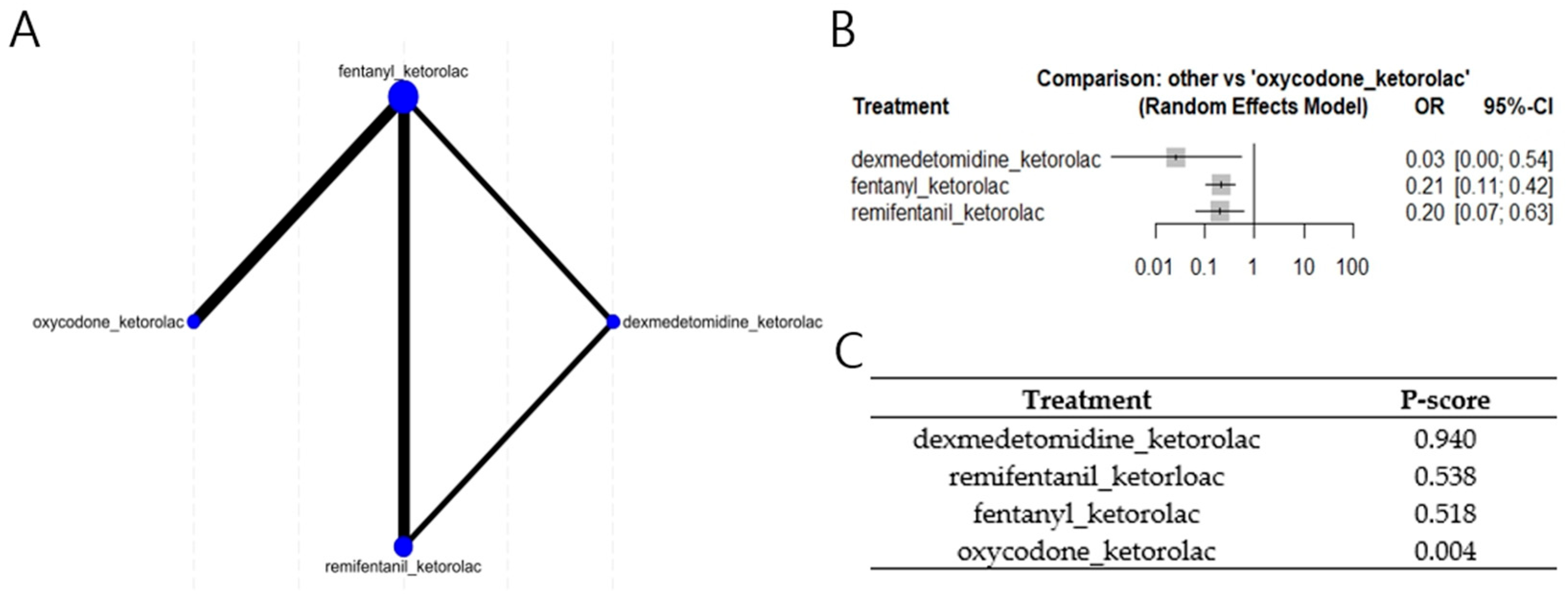
3.4. Network Meta Analyses (NMA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| O-PONV | Opioid-associated postoperative nausea and vomiting |
| OINV | Opioid-induced nausea and vomiting |
| PONV | Postoperative nausea and vomiting |
| RCT | Randomized controlled trial |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PICO | Population, Intervention, Comparison, and Outcome |
| RISS | Research Information Sharing Service |
| PACU | Post-Anesthesia Care Unit |
| NMA | Network meta-analysis |
| STATA/SE | Statistical software for data science/special edition |
| OR | Odds ratio |
| CI | Confidence interval |
References
- Capri, C.A.; Neto, A.G.G.; Gusmao, R.A.; Silva, T.A.M.; Gomez, M.V.; Castro-Junior, C.J. Intrathecal morphine versus ketamine in postoperative pain after hysterectomy: Double-blinded, randomized clinical trial. J. Perianesth. Nurs. 2020, 35, 580–585.e2. [Google Scholar]
- Gorina, Y.; Elgaddal, N.; Weeks, J.D. Hysterectomy Among Women Age 18 and Older: United States, 2021; NCHS Data Brief, No. 494; National Center for Health Statistics: Hyattsville, MD, USA, 2024. [Google Scholar]
- American College of Obstetricians and Gynecologists. Hysterectomy. Available online: https://www.acog.org/womens-health/faqs/hysterectomy (accessed on 11 August 2025).
- Papadopoulos, M.S.; Tolikas, A.C.; Miliaras, D.E. Hysterectomy–current methods and alternatives for benign indications. Obstet. Gynecol. Int. 2010, 2010, 356740. [Google Scholar] [CrossRef]
- Lenfant, L.; Canlorbe, G.; Belghiti, J.; Kreaden, U.S.; Hebert, A.E.; Nikpayam, M. Robotic-assisted benign hysterectomy compared with laparoscopic, vaginal, and open surgery: A systematic review and meta-analysis. J. Robot. Surg. 2023, 17, 2647–2662. [Google Scholar] [CrossRef]
- Wright, J.D.; Ananth, C.V.; Lewin, S.N.; Burke, W.M.; Lu, Y.S.; Neugut, A.I. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA 2013, 309, 689–698. [Google Scholar] [CrossRef]
- Brandsborg, B.; Nikolajsen, L. Chronic pain after hysterectomy. Curr. Opin. Anaesthesiol. 2018, 31, 268–273. [Google Scholar] [CrossRef]
- Lirk, P.; Thiry, J.; Bonnet, M.P.; Joshi, G.P.; Bonnet, F.; Group, P.W. Pain management after laparoscopic hysterectomy: Systematic review of literature and PROSPECT recommendations. Reg. Anesth. Pain Med. 2019, 44, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.A.; Walsh, S.R.; Tang, T.Y.; Slack, M. Total abdominal hysterectomy versus total laparoscopic hysterectomy for benign disease: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Bijen, C.B.; Vermeulen, K.M.; Mourits, M.J.; de Bock, G.H. Costs and effects of abdominal versus laparoscopic hysterectomy: Systematic review of controlled trials. PLoS ONE 2009, 4, e7340. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations–2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef]
- Cozowicz, C.; Gerner, H.D.; Zhong, H.; Illescas, A.; Reisinger, L.; Poeran, J. Multimodal analgesia and outcomes in hysterectomy surgery–a population-based analysis. J. Clin. Med. 2024, 13, 5431. [Google Scholar] [CrossRef]
- Dinges, H.C.; Otto, S.; Stay, D.K.; Baumlein, S.; Waldmann, S.; Kranke, P. Side effect rates of opioids in equianalgesic doses via intravenous patient-controlled analgesia: A systematic review and network meta-analysis. Anesth. Analg. 2019, 129, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.S.; Smith, J.M.; Seidner, P. Opioid-induced nausea and vomiting. Ann. Palliat. Med. 2012, 1, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.N. Risk factors for postoperative nausea and vomiting. Anaesthesia 1994, 49, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Schwarzer, G. Netmeta: Network Meta-Analysis Using Frequentist Methods (Updated 10 April 2025). Available online: https://cran.r-project.org/web/packages/netmeta/index.html (accessed on 11 August 2025).
- Shim, S.; Yoon, B.H.; Shin, I.S.; Bae, J.M. Network meta-analysis: Application and practice using Stata. Epidemiol. Health 2017, 39, e2017047. [Google Scholar] [CrossRef]
- Jung, H.S.; Joo, J.D.; Jeon, Y.S.; Lee, J.A.; Kim, D.W.; In, J.H. Comparison of an intraoperative infusion of dexmedetomidine or remifentanil on perioperative haemodynamics, hypnosis and sedation, and postoperative pain control. J. Int. Med. Res. 2011, 39, 1890–1899. [Google Scholar] [CrossRef]
- Kim, J.J.; Ha, M.H.; Jung, S.H.; Song, N.W. The efficiency of IV PCA with remifentanil and ketorolac after laparoscopic-assisted vaginal hysterectomy. Korean J. Anesthesiol. 2011, 61, 42–49. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, C.; Shin, Y.; An, J.H.; Ban, J.S.; Lee, J.H. Comparison of oxycodone and fentanyl for postoperative patient-controlled analgesia after laparoscopic gynecological surgery. Korean J. Anesthesiol. 2015, 68, 153–158. [Google Scholar] [CrossRef]
- Kim, N.S.; Kang, K.S.; Yoo, S.H.; Chung, J.H.; Chung, J.W.; Seo, Y. A comparison of oxycodone and fentanyl in intravenous patient-controlled analgesia after laparoscopic hysterectomy. Korean J. Anesthesiol. 2015, 68, 261–266. [Google Scholar] [CrossRef]
- Choi, J.W.; Joo, J.D.; Kim, D.W.; In, J.H.; Kwon, S.Y.; Seo, K. Comparison of an intraoperative infusion of dexmedetomidine, fentanyl, and remifentanil on perioperative hemodynamics, sedation quality, and postoperative pain control. J. Korean Med. Sci. 2016, 31, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Choi, S.S.; Lee, S.Y.; Lee, M.K.; Kim, J.E.; Lee, J.E. The effect of nefopam on postoperative fentanyl consumption: A randomized, double-blind study. Korean J. Pain 2016, 29, 110–118. [Google Scholar] [CrossRef]
- Yoon, J.U.; Byeon, G.J.; Cheon, J.H.; Choi, Y.M.; Ri, H.S.; Baik, S.W. Post-operative intravenous patient-controlled analgesic efficacy of morphine with ketorolac versus nefopam after laparoscopic gynecologic surgery: A randomized non-inferiority trial. Korean J. Anesthesiol. 2016, 69, 161–166. [Google Scholar] [CrossRef]
- Kim, N.S.; Lee, J.S.; Park, S.Y.; Ryu, A.; Chun, H.R.; Chung, H.S. Oxycodone versus fentanyl for intravenous patient-controlled analgesia after laparoscopic supracervical hysterectomy: A prospective, randomized, double-blind study. Medicine 2017, 96, e6286. [Google Scholar] [CrossRef]
- Nam, S.; Yoo, S.; Park, S.K.; Kim, J.T. Additive effect of a single intravenous dose of acetaminophen administered at the end of laparoscopic hysterectomy on postoperative pain control with nefopam and fentanyl-based patient-controlled analgesia: A double-blind, randomized controlled trial. BMC Anesthesiol. 2025, 25, 88. [Google Scholar] [CrossRef]
- Ao, L.; Shi, J.; Gan, J.; Yu, W.; Du, H. Effects of dexmedetomidine and ketorolac applied for patient-controlled analgesia on the balance of Th1/Th2 and level of VEGF in patients undergoing laparoscopic surgery for cervical cancer: A randomized controlled trial. Oncol Lett. 2024, 28, 379. [Google Scholar] [CrossRef]
- Du, J.; Li, J.W.; Jin, J.; Shi, C.X.; Ma, J.H. Intraoperative and postoperative infusion of dexmedetomidine combined with intravenous butorphanol patient-controlled analgesia following total hysterectomy under laparoscopy. Exp. Ther. Med. 2018, 16, 4063–4069. [Google Scholar] [CrossRef]
- Guo, M.; Liu, S.; Gao, J.; Han, C.; Yang, C.; Liu, C. The effects of fentanyl, oxycodone, and butorphanol on gastrointestinal function in patients undergoing laparoscopic hysterectomy: A prospective, double-blind, randomized controlled trial. BMC Anesthesiol. 2022, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, S.; Chen, J.; Liu, H.; Li, W.; Chi, H. Effects of perioperative oxycodone as the sole opioid on immunity within a multimodal analgesia framework in patients undergoing cervical cancer surgery: A randomised controlled trial. Indian J. Anaesth. 2025, 69, 191–199. [Google Scholar] [CrossRef]
- Comelon, M.; Raeder, J.; Drægni, T.; Lieng, M.; Lenz, H. Tapentadol versus oxycodone analgesia and side effects after laparoscopic hysterectomy: A randomised controlled trial. Eur. J. Anaesthesiol. 2021, 38, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Comelon, M.; Wisloeff-Aase, K.; Raeder, J.; Drægni, T.; Undersrud, H.; Qvigstad, E. A comparison of oxycodone prolonged-release vs. oxycodone plus naloxone prolonged-release after laparoscopic hysterectomy. Acta Anaesthesiol. Scand. 2013, 57, 509–517. [Google Scholar] [CrossRef]
- Iorno, V.; Landi, L.; Marchesi, T.; Porro, G.A.; Egan, C.G.; Calderini, E. Long-term effect of oxycodone/naloxone on the management of postoperative pain after hysterectomy: A randomized prospective study. Minerva Anestesiol. 2020, 86, 488–497. [Google Scholar] [CrossRef]
- Jose, D.E.; Ganapathi, P.; Anish Sharma, N.G.; Shankaranarayana, P.; Aiyappa, D.S.; Nazim, M. Postoperative pain relief with epidural buprenorphine versus epidural butorphanol in laparoscopic hysterectomies: A comparative study. Anesth. Essays Res. 2016, 10, 82–87. [Google Scholar] [CrossRef]
- Park, J.; Park, E.Y.; Han, S.S.; Park, H.M.; Lee, M.; Lee, S.A. Randomized controlled study comparing the analgesic effects of intravenous patient-controlled analgesia and patient-controlled epidural analgesia after open major surgery for pancreatobiliary cancer. HPB 2022, 24, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Laara, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A simplified risk score for predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology 1999, 91, 693–700. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Yuan, C. Comparative efficacy of opioid and non-opioid analgesics in labor pain management: A network meta-analysis. PLoS ONE 2024, 19, e0303174. [Google Scholar] [CrossRef] [PubMed]
- Ladha, K.S.; Neuman, M.D.; Broms, G.; Bethell, J.; Bateman, B.T.; Wijeysundera, D.N. Opioid prescribing after surgery in the United States, Canada, and Sweden. JAMA Netw. Open 2019, 2, e1910734. [Google Scholar] [CrossRef]
- Horn, C.C.; Wallisch, W.J.; Homanics, G.E.; Williams, J.P. Pathophysiological and neurochemical mechanisms of postoperative nausea and vomiting. Eur. J. Pharmacol. 2014, 722, 55–66. [Google Scholar] [CrossRef]
- Jayawardana, S.; Forman, R.; Johnston-Webber, C.; Campbell, A.; Berterame, S.; de Joncheere, C. Global consumption of prescription opioid analgesics between 2009–2019: A country-level observational study. EClinicalMedicine 2021, 42, 101198. [Google Scholar] [CrossRef] [PubMed]

| Study | Country | Arms | N | Treatment | Outcome |
|---|---|---|---|---|---|
| Ao, L., 2024 [29] | China | DK | 34 | Dexmedetomidine 2 μg/kg and ketorolac 3 mg/kg with NS IV PCA: 0.5 mL bolus, 2 mL/h basal, 15 min lockout | 48 h: PONV 2/34 |
| SUF | 32 | Sufentanil 1.5 μg/kg with NS IV PCA: 0.5 mL bolus, 2 mL/h basal, 15 min lockout | 48 h: PONV 8/32 | ||
| Choi, J.W., 2016 [24] | Republic of Korea | FK | 30 | Fentanyl: LD 1.0 μg/kg, continuous infusion 0.4 μg/kg/h Ketorolac: IV single dose 30 mg | 30 min: N 3 (10%), V 3 (10%) |
| RK | 30 | Remifentanil: LD 1.0 μg/kg, continuous infusion 0.08 μg/kg/h Ketorolac: IV single dose 30 mg | 30 min: N 3 (10%), V 2 (6.7%) | ||
| DK | 30 | Dexmedetomidine: LD 1 μg/kg, continuous infusion 0.5 μg/kg/h Ketorolac: IV single dose 30 mg | 30 min: N 0 (0%), V 0 (0%) | ||
| Comelon, M., 2013 [34] | Norway | ON | 40 | Oxycodone/naloxone PR 10 mg/5 mg every 12 h for a total of 3 days | 0–4 h: N 7%, V 0% 4–24 h: N 12%, V 3% |
| O | 45 | Oxycodone PR 10 mg every 12 h for a total of 3 days | 0–4 h: N 7%, V 0% 4–24 h: N 13%, V 3% | ||
| Comelon, M., 2021 [33] | Norway | T | 37 | Oral extended-release tapentadol 50 mg | 1 h: N 16.2%, V 2.7% 2 h: N 10.8%, V 0% 3 h: N 8.1%, V 0% 24 h: N 21.6%, V 18.9% |
| O | 36 | Oral extended-release oxycodone 10 mg | 1 h: N 8.3%, V 0% 2 h: N 8.3%, V 5.6% 3 h: N 19.4%, V 5.6% 24 h: N 44.4%, V 27.8% | ||
| Du, J., 2018 [30] | China | CON | 40 | Butorphanol 10 mg PCIA: 0.5 mL bolus, 2 mL/h basal, 15 min lockout | 24 h: N 12 (30.0%), V 7 (17.1%) |
| DEX | 41 | Butorphanol 10 mg, dexmedetomidine 300 μg PCIA: 0.5 mL bolus, 2 mL/h basal, 15 min lockout | 24 h: N 7 (17.1%), V 2 (4.9%) | ||
| Guo, M., 2022 [31] | China | F | 39 | Fentanyl 8.3 μg/kg IV PCA: 3 mL bolus, 2 mL/h basal, 15 min lockout | 48 h: N 4 (10.3%), V 0 (0%) |
| O | 36 | Oxycodone 0.5 mg/kg IV PCA: 3 mL bolus, 2 mL/h basal, 15 min lockout | 48 h: N 8 (21.6%), V 2 (5.4%) | ||
| B | 37 | Butorphanol 0.16 mg/kg IV PCA: 3 mL bolus, 2 mL/h basal, 15 min lockout | 48 h: N 3 (8.3%), V 0 (0%) | ||
| Iorno, V., 2020 [35] | Italy | OXN | 42 | PR oxycodone/naloxone (10 mg/5 mg), every 12 h up to 48 h postoperatively | Day0: N 7, V 2 Day1: N 3, V 0 Day2: N 1, V 0 Day3: N 1, V 0 * |
| M | 41 | Morphine 0.2~0.4 mg/kg/day continuous infusion 48 h postoperatively Ketorolac 30 mg IV three times a day | Day0: N 10, V 4 Day1: N 7, V 2 Day2: N 3, V 0 Day3: N 0, V 0 * | ||
| Jose, D.E., 2016 [36] | India | A | 30 | Buprenorphine 0.3 mg with 10 mL NS injected via epidural catheter | 12 h: NV 4 (13%) |
| B | 30 | Butorphanol 1 mg with 10 mL NS injected via epidural catheter | 12 h: NV 3 (10%) | ||
| Jung, H.S., 2011 [20] | Republic of Korea | D | 25 | Dexmedetomidine: LD 1 μg/kg, continuous infusion 0.2~0.7 μg/kg/h | 30 min: N 3 (13%), V 2 (8%) |
| R | 25 | Remifentanil: LD 0.8~1.2 μg/kg, continuous infusion 0.05~0.1 μg/kg/h | 30 min: N 0 (0%), V 0 (0%) | ||
| Kim, J.J., 2011 [21] | Republic of Korea | R | 20 | Remifentanil IV PCA: LD 1 μg/kg, 0.375 μg/kg bolus, 0.025 μg/kg/min basal | Score 0 (none): 8 (40%) Score 1 (mild): 7 (35.0%) Score 2 (moderate): 4 (20.0%) Score 3 (severe): 0 (0%) Score 4 (vomiting): 1 (5%) |
| RK1 | 19 | Remifentanil IV PCA: LD 0.6 μg/kg, 0.225 μg/kg bolus, 0.015 μg/kg/min basal Ketorolac: LD 30 mg, 0.01 μg/kg bolus, 0.04 μg/kg/h basal, 15 min lockout | Score 0 (none): 12 (63.2%) Score 1 (mild): 4 (21.1%) Score 2 (moderate): 2 (10.5%) Score 3 (severe): 0 (0%) Score 4 (vomiting): 1 (5.3%) | ||
| RK2 | 20 | Remifentanil: LD 0.3 μg/kg, 0.1125 μg/kg bolus, 0.0075 μg/kg/min basal Ketorolac: LD 30 mg, 0.01 μg/kg bolus, 0.04 μg/kg/h basal, 15 min lockout | Score 0 (none): 8 (40.0%) Score 1 (mild): 4(20.0%) Score 2 (moderate): 5 (25.0%) Score 3 (severe): 3 (15.0%) Score 4 (vomiting): 0 (0%) | ||
| F | 20 | Fentanyl: LD 1 μg/kg, 0.075 μg/kg bolus, 0.3 μg/kg/h basal, 15 min lockout Ketorolac: LD 30 mg, 0.01 μg/kg bolus, 0.04 μg/kg/h basal, 15 min lockout | Score 0 (none): 10 (50.0%) Score 1 (mild): 4 (20.0%) Score 2 (moderate): 4 (20.0%) Score 3 (severe): 1 (5.0%) Score 4 (vomiting): 1 (5.0%) | ||
| Kim. N.S., 2015 [23] | Republic of Korea | F | 30 | Fentanyl 700 μg (LD 100 μg) Ketorolac 150 mg (LD S30 mg) IV PCA: 0.5 mL bolus, 14 μg/h basal, 15 min lockout | 0.5 h: N 1 (3.3%), V 0 (0%) 2 h: N 3 (10%), V 1 (3.3%) 4 h: N 6 (20%), V 1 (3.3%) 8 h: N 6 (20%), V 0 (0%) 24 h: N 4 (13.3%), V 2 (6.7%) 48 h: N 4 (13.3%), V 1 (3.3%) |
| O | 30 | Oxycodone 70 mg (LD 10 mg) Ketorolac 150 mg (LD 30 mg) IV PCA: 0.5 mL bolus, 1.4 mg/h basal, 15 min lockout | 0.5 h: N 1 (3.3%), V 0 (0%) 2 h: N 4 (13.3%), V 0 (0%) 4 h: N 14 (46.7%), V 1 (3.3%) 8 h: N 13 (43.3%), V 2 (6.7%) 24 h: N 12 (40.0%), V 4 (13.3%) 48 h: N 12 (40.0%), V 3 (10.0%) | ||
| Kim, N.S., 2017 [27] | Republic of Korea | F | 63 | Fentanyl 700 μg (LD 100 μg) Ketorolac 150 mg (LD 30 mg) IV PCA: 0.5 mL bolus, 14 μg/h basal, 15 min lockout | 0.5 h: N 2 (3.2%, V 0 (0%) 4 h: N 8 (12.7%), V 3 (4.8%) 8 h: N 9 (14.3%), V 1 (1.6%) 24 h: N 9 (14.3%), V 5 (7.9%) 48 h: N 2 (3.2%), V 3 (4.8%) |
| O | 64 | Oxycodone 52.5 mg (LD 7.5 mg) Ketorolac 150 mg (LD 30 mg) IV PCA: 0.5 mL bolus, 1050 μg/h basal, 15 min lockout | 0.5 h: N 2 (3.1%), V 0 (0%) 4 h: N 21 (32.8%), V 2 (3.1%) 8 h: N 21 (32.8%), V 9 (14.1%) 24 h: N 31 (48.4%), V 8 (12.5%) 48 h: N 18 (28.1%), V 2 (3.1%) | ||
| Liu, J., 2025 [32] | China | O | 28 | Oxycodone 0.5 mg/mL IV PCA: 4 mL bolus, 1 mL/h basal, 15 min lockout | 48 h: N 6/28 (21.4%), V 3/28 (10.7%) |
| C | 28 | Sufentanil 0.5 μg/mL IV PCA: 4 mL bolus, 1 mL/h basal, 15 min lockout | 48 h: N 8/28 (28.6%), V 7/28 (25.0%) | ||
| Moon, J.Y., 2016 [25] | Republic of Korea | A | 27 | Fentanyl 1000 μg (LD 20 μg) IV PCA: 1 mL bolus, 5 min lockout, 10 mL/h max, total daily max 60 mL (no basal infusion) | 48 h: NV 17 (59.3%) |
| B | 28 | Fentanyl 500 μg (LD 10 μg) Nefopam 200 mg (LD 4 mg) IV PCA: 1 mL bolus, 5 min lockout, 10 mL/h max, total daily max 60 mL (no basal infusion) | 48 h: NV 18 (64.3%) | ||
| C | 26 | Fentanyl 500 μg (LD 10 μg) Nefopam 400 mg (LD 8 mg) IV PCA: 1 mL bolus, 5 min lockout, 10 mL/h max, total daily max 60 mL (no basal infusion) | 48 h: NV 18 (69.2%) | ||
| Nam, S., 2025 [28] | Republic of Korea | C | 42 | Fentanyl 500 μg + nefopam 80 mg IV PCA IV PCA: 1 mL bolus, 0.5 mL/h basal, 10 min lockout | 24 h: N 15 (35.7%), V 1 (2.4%) |
| T | 41 | Acetaminophen IV 1 g Fentanyl 500 μg + nefopam 80 mg IV PCA IV PCA: 1 mL bolus, 0.5 mL/h basal, 10 min lockout | 24 h: N 15 (26.6%), V 4 (9.8%) | ||
| Park, J.H., 2015 [22] | Republic of Korea | O | 37 | Oxycodone IV PCA: 0.9 mg bolus, 0.9 mg/h basal, 15 min lockout | 48 h: N 11, V 3 |
| F | 32 | Fentanyl IV PCA: 15 μg bolus, 15 μg/h basal, 15 min lockout | 48 h: N 4, V 1 | ||
| Yoon, J.U., 2016 [26] | Republic of Korea | A | 30 | Morphine 60 mg + ketorolac 180 mg IV PCA: 1 mL bolus, 1 mL/h basal, 15 min lockout | PACU: N 7 (23.3%), V 0 (0%) 12 h: N 14 (46.7%), V 3 (10.0%) 24 h: N 12 (40.0%), V 2 (6.7%) 48 h: N 9 (30.0%), V 0 (0%) |
| B | 30 | Nefopam 200 mg IV PCA: 1 mL bolus, 1 mL/h basal, 15 min lockout | PACU: N 6 (20.0%), V 0 (0%) 12 h: N 3 (10.0%), V 1 (3.3%) 24 h: N 3 (10.0%), V 0 (0%) 48 h: N 3 (10.0%), V 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Bang, H.; Shin, S.; Kim, H.; Park, S.; Lee, P.S.; Lee, E.E. Opioid-Associated Postoperative Nausea and Vomiting in Women Undergoing Laparoscopic Hysterectomy: A Network Meta-Analysis. Medicina 2025, 61, 1728. https://doi.org/10.3390/medicina61101728
Cho S, Bang H, Shin S, Kim H, Park S, Lee PS, Lee EE. Opioid-Associated Postoperative Nausea and Vomiting in Women Undergoing Laparoscopic Hysterectomy: A Network Meta-Analysis. Medicina. 2025; 61(10):1728. https://doi.org/10.3390/medicina61101728
Chicago/Turabian StyleCho, Sueyoung, Heesoo Bang, Sangyoon Shin, Hyunjoo Kim, Seohyeon Park, Paul S. Lee, and Eunkyung Euni Lee. 2025. "Opioid-Associated Postoperative Nausea and Vomiting in Women Undergoing Laparoscopic Hysterectomy: A Network Meta-Analysis" Medicina 61, no. 10: 1728. https://doi.org/10.3390/medicina61101728
APA StyleCho, S., Bang, H., Shin, S., Kim, H., Park, S., Lee, P. S., & Lee, E. E. (2025). Opioid-Associated Postoperative Nausea and Vomiting in Women Undergoing Laparoscopic Hysterectomy: A Network Meta-Analysis. Medicina, 61(10), 1728. https://doi.org/10.3390/medicina61101728