Enhancing Comprehensive Assessments in Chronic Heart Failure Caused by Ischemic Heart Disease: The Diagnostic Utility of Holter ECG Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patients, and Investigations
2.2. Statistical Analysis
2.3. Ethics
3. Results
3.1. Baseline Characteristics
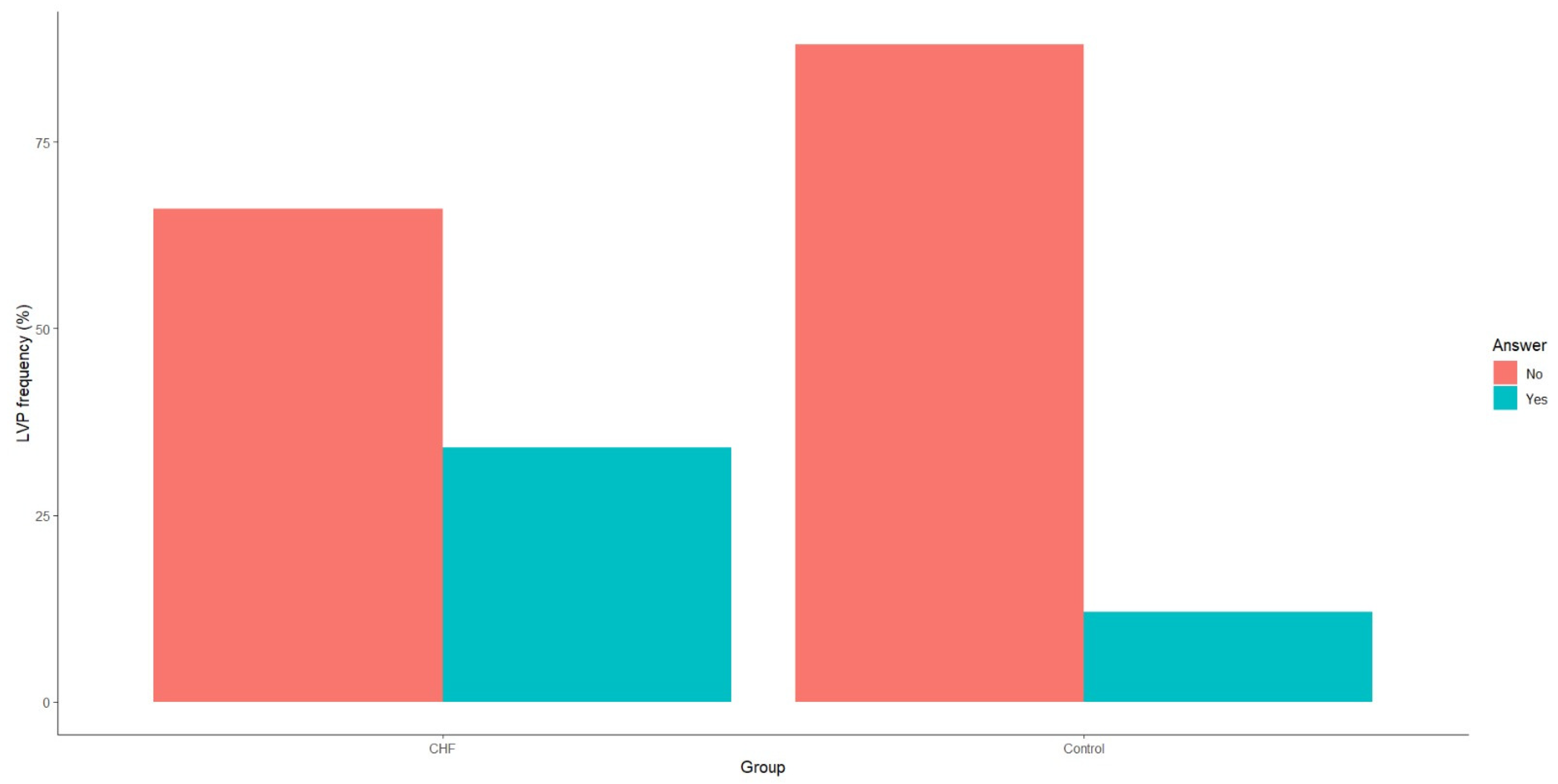
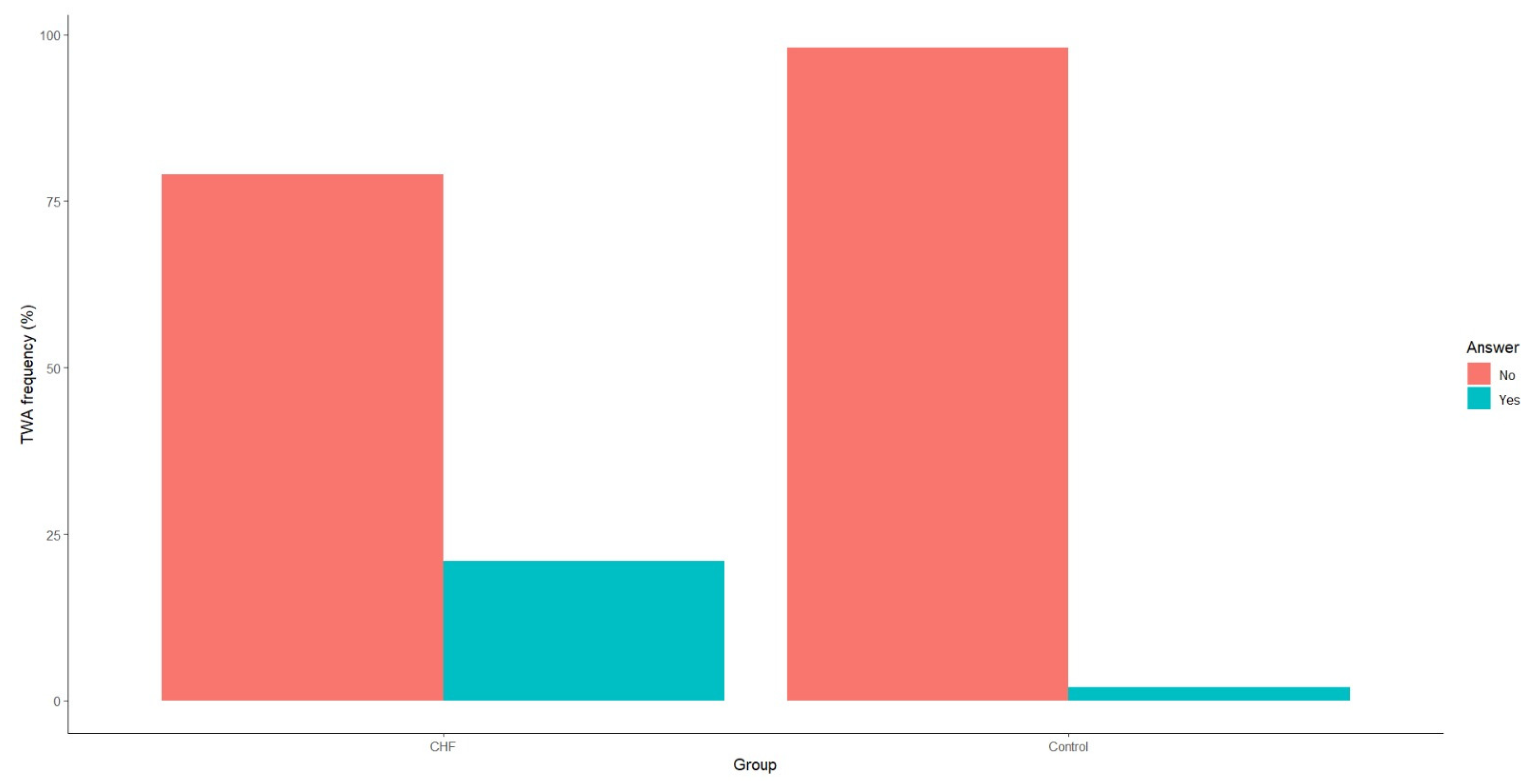
3.2. Diagnostic Performance of Late Ventricular Potentials and T-Wave Alternans in Chronic Heart Failure
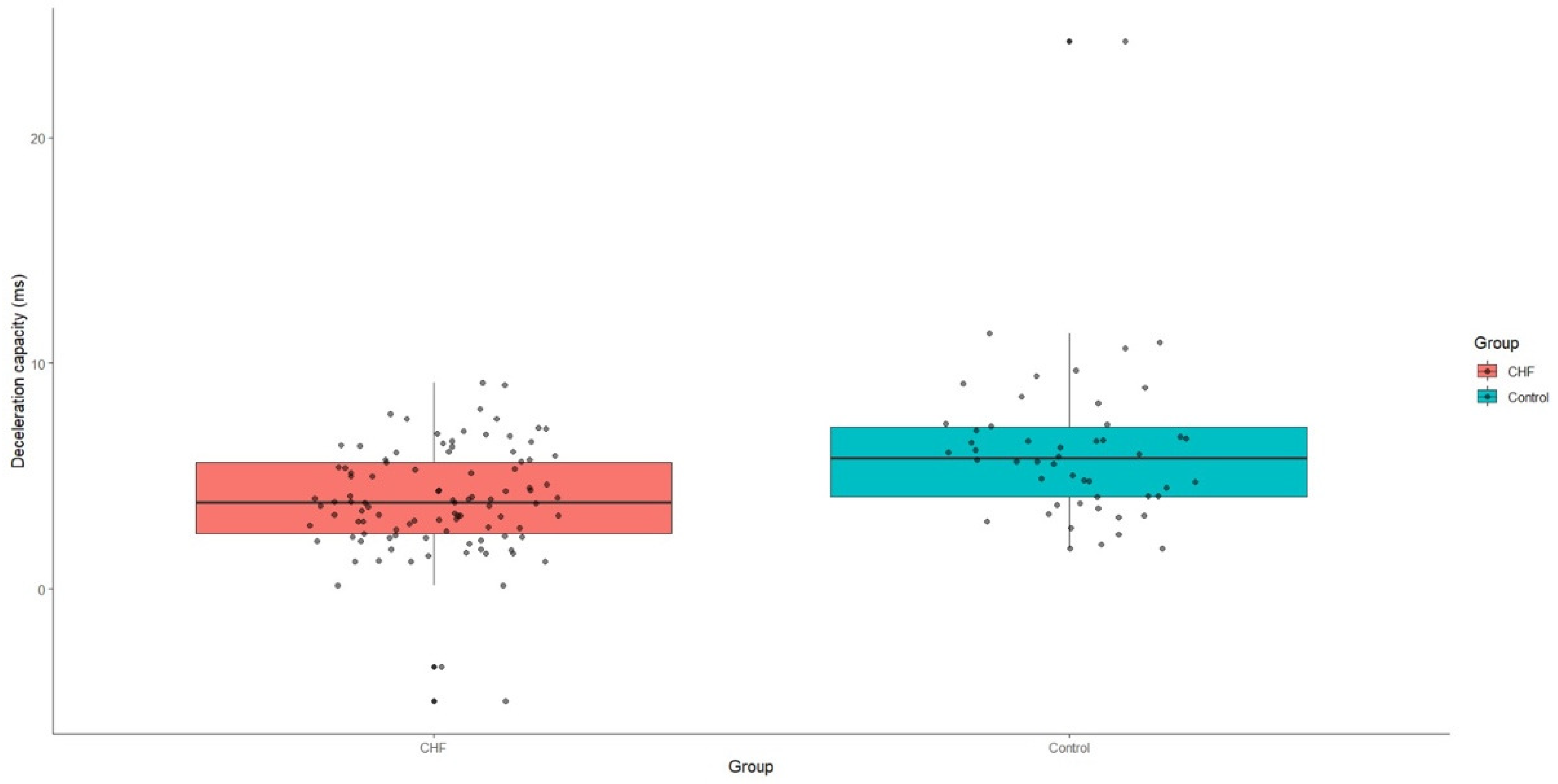
3.3. Diagnostic Performance of Heart Rate Variability in Chronic Heart Failure
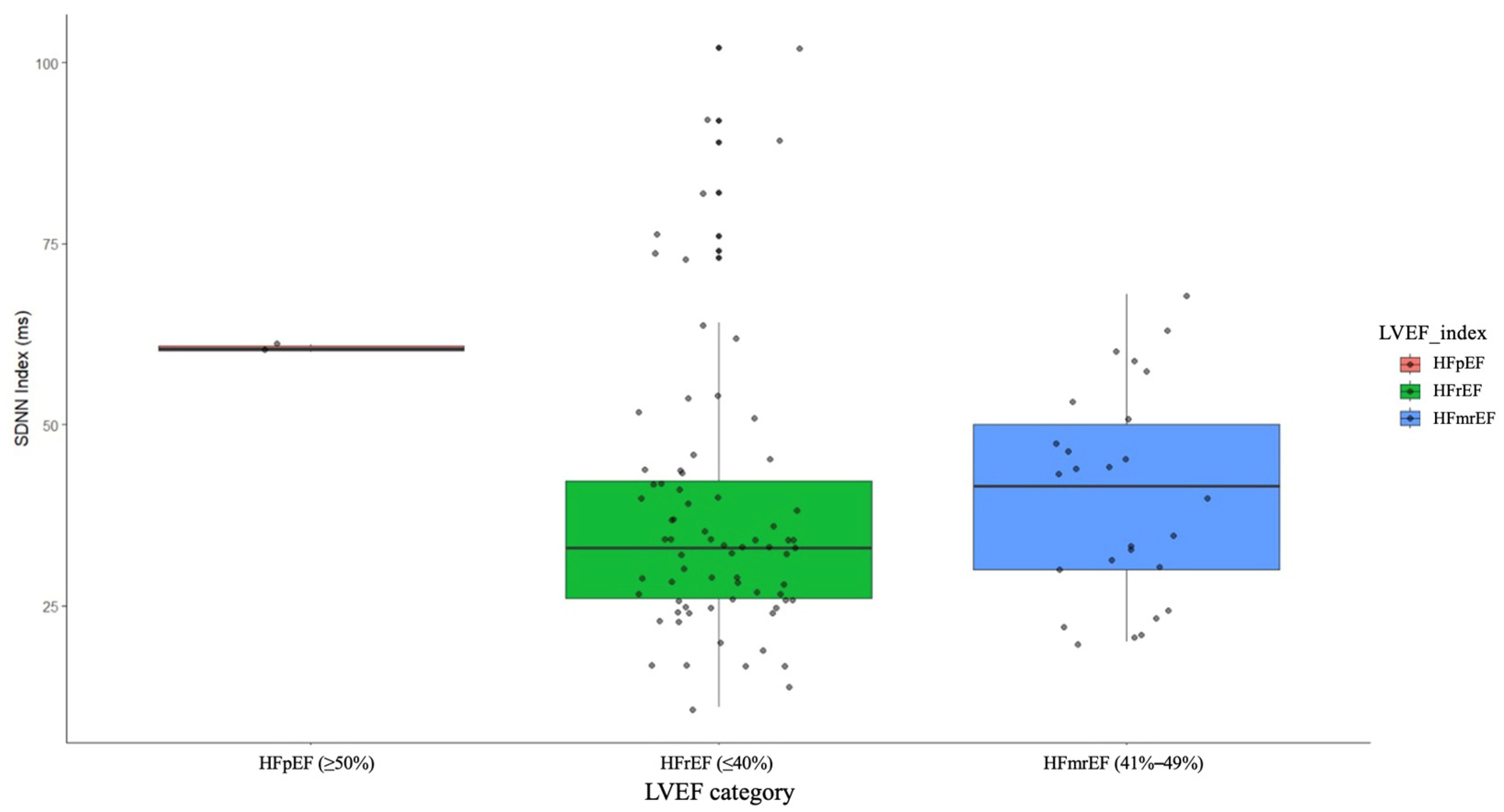
3.4. Can LVEF Influence the HRV Parameters in Patients with Chronic Heart Failure?
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heusch, G. Myocardial ischemia/reperfusion: Translational pathophysiology of ischemic heart disease. Med 2024, 5, 10–31. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.V.; Hjortbak, M.V.; Bøtker, H.E. Ischemic Heart Disease: An Update. Semin. Nucl. Med. 2020, 50, 195–207. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Birtolo, L.I.; Mariani, M.V.; Lavalle, C.; Maestrini, V.; Mancone, M.; Fedele, F. Ischemic Heart Disease and Heart Failure: Role of Coronary Ion Channels. Int. J. Mol. Sci. 2020, 21, 3167. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, B.R.; Cannata, F.; Stefanini, G.G.; Bolognese, L. Myocardial Ischemia and Coronary Disease in Heart Failure. Heart Fail. Rev. 2020, 25, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Silverdal, J.; Sjöland, H.; Bollano, E.; Pivodic, A.; Dahlström, U.; Fu, M. Prognostic Impact Over Time of Ischaemic Heart Disease vs. Non-Ischaemic Heart Disease in Heart Failure. ESC Heart Fail. 2020, 7, 264–273. [Google Scholar] [CrossRef]
- Wang, K.; Tian, J.; Zheng, C.; Yang, H.; Ren, J.; Liu, Y.; Han, Q.; Zhang, Y. Interpretable Prediction of 3-Year All-Cause Mortality in Patients with Heart Failure Caused by Coronary Heart Disease Based on Machine Learning and SHAP. Comput. Biol. Med. 2021, 137, 104813. [Google Scholar] [CrossRef]
- Tymińska, A.; Ozierański, K.; Balsam, P.; Maciejewski, C.; Wancerz, A.; Brociek, E.; Marchel, M.; Crespo-Leiro, M.G.; Maggioni, A.P.; Drożdż, J.; et al. Ischemic Cardiomyopathy versus Non-Ischemic Dilated Cardiomyopathy in Patients with Reduced Ejection Fraction-Clinical Characteristics and Prognosis Depending on Heart Failure Etiology (Data from European Society of Cardiology Heart Failure Registries). Biology 2022, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of Heart Failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaiti, S.S.; Fallavollita, J.A.; Canty, J.M., Jr.; Carey, M.G. Electrocardiographic Predictors of Sudden and Non-Sudden Cardiac Death in Patients with Ischemic Cardiomyopathy. Heart Lung 2014, 43, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.; Cuong, H.X.; Oanh, N.O. The Relationship between NT-ProBNP and Clinical, Paraclinical Characteristics in Stable Ischemic Heart Disease Patients with Heart Failure. Intern. Med. J. Vietnam 2021, 22, 92–97. [Google Scholar]
- Kinoshita, T.; Hashimoto, K.; Yoshioka, K.; Miwa, Y.; Yodogawa, K.; Watanabe, E.; Nakamura, K.; Nakagawa, M.; Nakamura, K.; Watanabe, T.; et al. Risk Stratification for Cardiac Mortality Using Electrocardiographic Markers Based on 24-Hour Holter Recordings: The JANIES-SHD Study. J. Cardiol. 2020, 75, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Freund, O.; Caspi, I.; Alcalay, I.; Brezis, M.R.; Frydman, S.; Bornstein, G. An Old Diagnostic Tool for New Indications: Inpatient Holter ECG for Conditions Other than Syncope or Stroke. Sci. Rep. 2023, 13, 12510. [Google Scholar] [CrossRef] [PubMed]
- Ksela, J.; Rupert, L.; Djordjevic, A.; Antonic, M.; Avbelj, V.; Jug, B. Altered Heart Rate Turbulence and Variability Parameters Predict 1-Year Mortality in Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Dev. Dis. 2022, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Senoo, K.; Makino, M.; Munakata, J.; Tomura, N.; Shimoo, S.; Iwakoshi, H.; Shiraishi, H.; Matoba, S. Prediction Model for the New Onset of Atrial Fibrillation Combining Features of 24-Hour Holter Electrocardiogram with 12-Lead Electrocardiogram. Int. J. Cardiol. Heart Vasc. 2023, 47, 101245. [Google Scholar] [CrossRef] [PubMed]
- Lauder, L.; Scholz, S.S.; Ewen, S.; Lettner, C.; Ukena, C.; Böhm, M.; Mahfoud, F. Accuracy of Pulse Rate Derived from 24-h Ambulatory Blood Pressure Monitoring Compared with Heart Rate from 24-h Holter-ECG. J. Hypertens. 2020, 38, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.K.; Chen, L.C.; Lien, L.M.; Lo, H.M.; Liao, Z.Y.; Chao, S.P.; Chuang, C.Y.; Chiu, C.Z. Comparison of Arrhythmia Detection by 24-Hour Holter and 14-Day Continuous Electrocardiography Patch Monitoring. Acta Cardiol. Sin. 2020, 36, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Lee, S.R.; Choi, E.K.; Ahn, H.J.; Song, H.S.; Lee, Y.S.; Oh, S. Validation of Adhesive Single-Lead ECG Device Compared with Holter Monitoring among Non-Atrial Fibrillation Patients. Sensors 2021, 21, 3122. [Google Scholar] [CrossRef] [PubMed]
- Holkeri, A.; Eranti, A.; Haukilahti, M.A.E.; Kerola, T.; Kenttä, T.V.; Tikkanen, J.T.; Anttonen, O.; Noponen, K.; Seppänen, T.; Rissanen, H.; et al. Predicting Sudden Cardiac Death in a General Population Using an Electrocardiographic Risk Score. Heart 2020, 106, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Vessella, T.; De Lazzari, M.; Cipriani, A.; Menegon, V.; Sarto, G.; Spagnol, R.; Merlo, L.; Pegoraro, C.; Marra, M.P.; et al. Screening Young Athletes for Diseases at Risk of Sudden Cardiac Death: Role of Stress Testing for Ventricular Arrhythmias. Eur. J. Prev. Cardiol. 2020, 27, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.M.; Kim, K.H.; Jeon, K.H.; Lee, S.Y.; Park, J.; Oh, B.H. Artificial Intelligence Algorithm for Predicting Cardiac Arrest Using Electrocardiography. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 98. [Google Scholar] [CrossRef] [PubMed]
- Wachter, R.; Gröschel, K.; Gelbrich, G.; Hamann, G.F.; Kermer, P.; Liman, J.; Seegers, J.; Wasser, K.; Schulte, A.; Jürries, F.; et al. Holter-Electrocardiogram-Monitoring in Patients with Acute Ischaemic Stroke (Find-AFRANDOMISED): An Open-Label Randomised Controlled Trial. Lancet Neurol. 2017, 16, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Dorian, P.; Spring, M.; Panzov, V.; Mamdani, M.; Healey, J.S.; Thorpe, K.E.; EMBRACE Steering Committee and Investigators. Atrial Premature Beats Predict Atrial Fibrillation in Cryptogenic Stroke: Results from the EMBRACE Trial. Stroke 2015, 46, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, P.; Karnad, D.R.; Rohekar, P.; Kerkar, V.; Lokhandwala, Y.Y.; Kothari, S. Arrhythmias Seen in Baseline 24-Hour Holter ECG Recordings in Healthy Normal Volunteers During Phase 1 Clinical Trials. J. Clin. Pharmacol. 2016, 56, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, K.; Shiobara, M.; Nakamura, S.; Yamashiro, K.; Yana, K.; Ono, T. Sudden Cardiac Arrest Risk Stratification Based on 24-Hour Holter ECG Statistics. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 5817–5820. [Google Scholar] [CrossRef]
- Cygankiewicz, I.; Zareba, W.; Vazquez, R.; Bayes-Genis, A.; Pascual, D.; Macaya, C.; Almendral, J.; Fiol, M.; Bardaji, A.; Gonzalez-Juanatey, J.R.; et al. Risk Stratification of Mortality in Patients with Heart Failure and Left Ventricular Ejection Fraction >35%. Am. J. Cardiol. 2009, 103, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Xhyheri, B.; Manfrini, O.; Mazzolini, M.; Pizzi, C.; Bugiardini, R. Heart Rate Variability Today. Prog. Cardiovasc. Dis. 2012, 55, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Sibrecht, G.; Piskorski, J.; Krauze, T.; Guzik, P. Heart Rate Asymmetry, Its Compensation, and Heart Rate Variability in Healthy Adults during 48-h Holter ECG Recordings. J. Clin. Med. 2023, 12, 1219. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, M.; Nazeran, H.; Haltiwanger, E. Comparison of Heart Rate Variability Signal Features Derived from Electrocardiography and Photoplethysmography in Healthy Individuals. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 2006, 4289–4294. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaiti, S.S.; Pietrasik, G.; Carey, M.G.; Alhamaydeh, M.; Canty, J.M.; Fallavollita, J.A. The Role of Heart Rate Variability, Heart Rate Turbulence, and Deceleration Capacity in Predicting Cause-Specific Mortality in Chronic Heart Failure. J. Electrocardiol. 2019, 52, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.C.; Wu, Y.L.; Tsai, P.S. Heart Rate Variability and Risk of All-Cause Death and Cardiovascular Events in Patients with Cardiovascular Disease: A Meta-Analysis of Cohort Studies. Biol. Res. Nurs. 2020, 22, 45–56, Erratum in: Biol. Res. Nurs. 2020, 22, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Piskorski, J.; Barthel, P.; Bauer, A.; Müller, A.; Junk, N.; Ulm, K.; Malik, M.; Schmidt, G. Heart Rate Deceleration Runs for Postinfarction Risk Prediction. J. Electrocardiol. 2012, 45, 70–76. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Luo, C.; Zhang, K.; Zhang, H. Electrophysiological Mechanisms Underlying T-Wave Alternans and Their Role in Arrhythmogenesis. Front. Physiol. 2021, 12, 614946. [Google Scholar] [CrossRef]
- Quan, X.Q.; Zhou, H.L.; Ruan, L.; Lv, J.G.; Yao, J.H.; Yao, F.; Huang, K.; Zhang, C.T. Ability of Ambulatory ECG-Based T-Wave Alternans to Modify Risk Assessment of Cardiac Events: A Systematic Review. BMC Cardiovasc. Disord. 2014, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Nishibe, T.; Yamashiro, K.; Yana, K.; Ono, T. T-Wave Alternans Search over 24 Hour Holter ECG Recordings Based on Singular Value Decomposition. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; Volume 2013, pp. 2076–2079. [Google Scholar] [CrossRef]
- Lewek, J.; Ptaszynski, P.; Klingenheben, T.; Cygankiewicz, I. The Clinical Value of T-Wave Alternans Derived from Holter Monitoring. Europace 2017, 19, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Harada, N. Recent Progress of Holter-Based Late Potential for Predicting Serious Cardiac Events and Its Implications and Future Challenges. J. Electrocardiol. 2023, 81, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Amino, M.; Yoshioka, K.; Kasamaki, Y.; Kinoshita, T.; Ikeda, T. Combined Evaluation of Ambulatory-Based Late Potentials and Nonsustained Ventricular Tachycardia to Predict Arrhythmic Events in Patients with Previous Myocardial Infarction: A Japanese Noninvasive Electrocardiographic Risk Stratification of Sudden Cardiac Death (JANIES) Substudy. Ann. Noninvasive Electrocardiol. 2021, 26, e12803. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in: Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jin, X.; Zhang, P.; Yu, Q.; Yin, G.; Lu, Y.; Xiao, H.; Chen, Y.; Zhang, D. Deceleration and Acceleration Capacities of Heart Rate Associated with Heart Failure with High Discriminating Performance. Sci. Rep. 2016, 6, 23617. [Google Scholar] [CrossRef] [PubMed]
- Vozda, M.; Cerny, M. Methods for Derivation of Orthogonal Leads from 12-Lead Electrocardiogram: A Review. Biomed. Signal Process. Control 2015, 19, 23–34. [Google Scholar] [CrossRef]
- Nademanee, K.; Veerakul, G.; Chandanamattha, P.; Chaothawee, L.; Ariyachaipanich, A.; Jirasirirojanakorn, K.; Likittanasombat, K.; Bhuripanyo, K.; Ngarmukos, T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation 2011, 123, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, A.; Yoshioka, K.; Amino, M.; Shima, M.; Hashida, T.; Fujibayashi, D.; Kanda, S.; Kobayashi, Y.; Tanabe, T.; Ikari, Y. Usefulness of Continuous 24-Hour Ventricular Late Potential to Predict Prognosis in Patients with Heart Failure. Tokai J. Exp. Clin. Med. 2014, 39, 128–136. [Google Scholar] [PubMed]
- Pepine, C.J.; Nichols, W.W. The pathophysiology of chronic ischemic heart disease. Clin. Cardiol. 2007, 30 (Suppl. S1), I4–I9. [Google Scholar] [CrossRef]
- El-Sherif, N.; Boutjdir, M.; Turitto, G. Sudden Cardiac Death in Ischemic Heart Disease: Pathophysiology and Risk Stratification. Card. Electrophysiol. Clin. 2017, 9, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.; Appelman, Y.E. Gender differences in coronary heart disease. Neth. Heart J. 2010, 18, 598–602. [Google Scholar] [CrossRef]
- Hansen, S.; Rasmussen, V.; Torp-Pedersen, C.; Jensen, G.B. QT intervals and QT dispersion determined from a 12-lead 24-hour Holter recording in patients with coronary artery disease and patients with heart failure. Ann. Noninvasive Electrocardiol. 2008, 13, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Liew, R. Electrocardiogram-based predictors of sudden cardiac death in patients with coronary artery disease. Clin. Cardiol. 2011, 34, 466–473. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Mortara, A.; Capomolla, S.; Febo, O.; Ferrari, R.; Franchini, M.; Gnemmi, M.; Opasich, C.; et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003, 107, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, L.; Li, K.; Wang, Q.; Liu, G.; Jiang, Q. A Novel and Effective Method for Congestive Heart Failure Detection and Quantification Using Dynamic Heart Rate Variability Measurement. PLoS ONE 2016, 11, e0165304. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Hampel, R.; Ibald-Mulli, A.; Zareba, W.; Schmidt, G.; Schneider, R.; Rückerl, R.; Couderc, J.P.; Mykins, B.; Oberdörster, G.; et al. Changes in deceleration capacity of heart rate and heart rate variability induced by ambient air pollution in individuals with coronary artery disease. Part. Fibre Toxicol. 2010, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Ricca-Mallada, R.; Migliaro, E.R.; Piskorski, J.; Guzik, P. Exercise training slows down heart rate and improves deceleration and acceleration capacity in patients with heart failure. J. Electrocardiol. 2012, 45, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Jin, J.; Zhao, X.; Huang, X.; Zhu, W.; Jiang, S.; Gao, M.; Yuan, J. Heart rate acceleration and deceleration capacities associated with circadian blood pressure variation. Ann. Noninvasive Electrocardiol. 2020, 25, e12748. [Google Scholar] [CrossRef] [PubMed]
- Hautala, A.J.; Karjalainen, J.; Kiviniemi, A.M.; Kinnunen, H.; Mäkikallio, T.H.; Huikuri, H.V.; Tulppo, M.P. Physical activity and heart rate variability measured simultaneously during waking hours. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H874–H880. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Stein, P.K. Origin of heart rate variability and turbulence: An appraisal of autonomic modulation of cardiovascular function. Front. Physiol. 2011, 2, 95. [Google Scholar] [CrossRef] [PubMed]
- Fogt, D.L.; Cooper, P.J.; Freeman, C.N.; Kalns, J.E.; Cooke, W.H. Heart rate variability to assess combat readiness. Mil. Med. 2009, 174, 491–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, H.; Zhan, P.; Shi, J.; Wang, G.; Wang, B.; Wang, W. A refined method of quantifying deceleration capacity index for heart rate variability analysis. Biomed. Eng. Online 2018, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- d’Unienville, N.M.A.; Nelson, M.J.; Bellenger, C.R.; Blake, H.T.; Buckley, J.D. Heart-Rate Acceleration Is Linearly Related to Anaerobic Exercise Performance. Int. J. Sports Physiol. Perform. 2022, 17, 78–82. [Google Scholar] [CrossRef]
- Schneider, C.; Wiewelhove, T.; Raeder, C.; Flatt, A.A.; Hoos, O.; Hottenrott, L.; Schumbera, O.; Kellmann, M.; Meyer, T.; Pfeiffer, M.; et al. Heart Rate Variability Monitoring During Strength and High-Intensity Interval Training Overload Microcycles. Front. Physiol. 2019, 10, 582. [Google Scholar] [CrossRef]
- Arsenos, P.; Manis, G.; Gatzoulis, K.A.; Dilaveris, P.; Gialernios, T.; Angelis, A.; Papadopoulos, A.; Venieri, E.; Trikas, A.; Tousoulis, D. Deceleration Capacity of Heart Rate Predicts Arrhythmic and Total Mortality in Heart Failure Patients. Ann. Noninvasive Electrocardiol. 2016, 21, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Giunta, S.; Xia, S.; Pelliccioni, G.; Olivieri, F. Autonomic nervous system imbalance during aging contributes to impair endogenous anti-inflammaging strategies. Geroscience 2024, 46, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wang, J.; Yu, F.; Song, L.; Liu, C.; Sun, J.; Deng, Q.; Wang, Y.; Zhou, Z.; Guo, F.; et al. Enrichment of the Postdischarge GRACE Score with Deceleration Capacity Enhances the Prediction Accuracy of the Long-Term Prognosis after Acute Coronary Syndrome. Front. Cardiovasc. Med. 2022, 9, 888753. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Zhao, H.M.; Wang, J. Metabolism and Chronic Inflammation: The Links between Chronic Heart Failure and Comorbidities. Front. Cardiovasc. Med. 2021, 8, 650278. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Samman Tahhan, A.; Vaduganathan, M.; Greene, S.J.; Alrohaibani, A.; Anker, S.D.; Vardeny, O.; Fonarow, G.C.; Butler, J. Trends in prevalence of comorbidities in heart failure clinical trials. Eur. J. Heart Fail. 2020, 22, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.K.; Skoularigis, J. Prevalence and importance of comorbidities in patients with heart failure. Curr. Heart Fail. Rep. 2012, 9, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Taçoy, G.; Açikgöz, K.; Kocaman, S.A.; Ozdemir, M.; Cengel, A. Is there a relationship between obesity, heart rate variability, and inflammatory parameters in heart failure? J. Cardiovasc. Med. 2010, 11, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef]
- Yadav, R.L.; Yadav, P.K.; Yadav, L.K.; Agrawal, K.; Sah, S.K.; Islam, M.N. Association between obesity and heart rate variability indices: An intuition toward cardiac autonomic alteration—A risk of CVD. Diabetes Metab. Syndr. Obes. 2017, 10, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; Paolillo, S.; Mercurio, V.; Ruocco, G.; Tocchetti, C.G.; Palazzuoli, A. Non-cardiovascular comorbidities in heart failure patients and their impact on prognosis. Kardiol. Pol. 2021, 79, 493–502. [Google Scholar] [CrossRef]
- Ren, L.; Fang, X.; Wang, Y.; Qi, G. T-wave alternans and heart rate variability: A comparison in patients with myocardial infarction with or without diabetes mellitus. Ann. Noninvasive Electrocardiol. 2011, 16, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Riaz, B.; Khan, M.A.; Ali, H.; Majeed, S.M.I. Correlation of Signal Averaged ECG Parameters with Left Ventricular Mass Index in Patients with Systemic Arterial Hypertension. Pak. J. Physiol. 2018, 14, 19–22. [Google Scholar]
- Stoyell-Conti, F.F.; Santos, F.; Machi, J.F.; Hernandez, D.R.; Barboza, C.A.; Irigoyen, M.C.; De Angelis, K.; Morris, M. Measurement of Mouse Heart Rate Variability using Echocardiographic System. J. Cardiovasc. Echogr. 2018, 28, 90–94. [Google Scholar] [PubMed]
- Petelczyc, M.; Zebrowski, J.J.; Baranowski, R.; Chojnowska, L. Stochastic analysis of heart rate variability and its relation to echocardiography parameters in hypertrophic cardiomyopathy patients. Physiol. Meas. 2010, 31, 1635–1649. [Google Scholar] [CrossRef] [PubMed]
- Mele, D.; Andrade, A.; Bettencourt, P.; Moura, B.; Pestelli, G.; Ferrari, R. From left ventricular ejection fraction to cardiac hemodynamics: Role of echocardiography in evaluating patients with heart failure. Heart Fail. Rev. 2020, 25, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.C.; Mandoli, G.E.; Aboumarie, H.S.; Santoro, C.; Bandera, F.; D’Andrea, A.; Benfari, G.; Esposito, R.; Evola, V.; Sorrentino, R.; et al. Basic and advanced echocardiography in advanced heart failure: An overview. Heart Fail. Rev. 2020, 25, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Krummerman, A.; Vijayaraman, P.; Rosengarten, M.; Suryadevara, V.; Lejemtel, T.; Ferrick, K.J. Heart rate variability and diastolic heart failure. Pacing Clin. Electrophysiol. 2004, 27, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Monasterio, V.; Laguna, P.; Cygankiewicz, I.; Vázquez, R.; Bayés-Genís, A.; de Luna, A.B.; Martínez, J.P. Average T-wave alternans activity in ambulatory ECG records predicts sudden cardiac death in patients with chronic heart failure. Heart Rhythm 2012, 9, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, P.; Infusino, F.; Sgueglia, G.A.; Sestito, A.; Lanza, G.A. Ventricular late potentials: A critical overview and current applications. J. Electrocardiol. 2008, 41, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Perkiömäki, J.; Exner, D.V.; Piira, O.P.; Kavanagh, K.; Lepojärvi, S.; Talajic, M.; Karvonen, J.; Philippon, F.; Junttila, J.; Coutu, B.; et al. Heart Rate Turbulence and T-Wave Alternans in Patients with Coronary Artery Disease: The Influence of Diabetes. Ann. Noninvasive Electrocardiol. 2015, 20, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Lutfi, M.F. Ventricular late potential in cardiac syndrome X compared to coronary artery disease. BMC Cardiovasc. Disord. 2017, 17, 35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bangalore, S.; Sawhney, S.; Messerli, F.H. Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J. Am. Coll. Cardiol. 2008, 52, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Goupil, R.; Dupuis, D.; Troyanov, S.; Madore, F.; Agharazii, M. Heart rate dependent and independent effects of beta-blockers on central hemodynamic parameters: A propensity score analysis. J. Hypertens. 2016, 34, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Elghozi, J.L.; Julien, C. Sympathetic control of short-term heart rate variability and its pharmacological modulation. Fundam. Clin. Pharmacol. 2007, 21, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Muthalaly, R.G.; Evans, R.M. Applications of Machine Learning in Cardiac Electrophysiology. Arrhythm. Electrophysiol. Rev. 2020, 9, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Agliari, E.; Barra, A.; Barra, O.A.; Fachechi, A.; Franceschi Vento, L.; Moretti, L. Detecting cardiac pathologies via machine learning on heart-rate variability time series and related markers. Sci. Rep. 2020, 10, 8845. [Google Scholar] [CrossRef] [PubMed]
- Ishaque, S.; Khan, N.; Krishnan, S. Trends in Heart-Rate Variability Signal Analysis. Front. Digit. Health 2021, 3, 639444. [Google Scholar] [CrossRef]
- Freund, O.; Caspi, I.; Shacham, Y.; Frydman, S.; Biran, R.; Abu Katash, H.; Zornitzki, L.; Bornstein, G. Holter ECG for Syncope Evaluation in the Internal Medicine Department—Choosing the Right Patients. J Clin Med. 2022, 11, 4781. [Google Scholar] [CrossRef] [PubMed]
- Uppoor, R.B.; Patel, K. Syncope: Diagnostic Yield of Various Clinical Investigations. Cureus. 2022, 14, 3. [Google Scholar] [CrossRef]
- Canepa, M.; Fonseca, C.; Chioncel, O.; Laroche, C.; Crespo-Leiro, M.G.; Coats, A.J.S.; Mebazaa, A.; Piepoli, M.F.; Tavazzi, L.; Maggioni, A.P.; et al. Performance of Prognostic Risk Scores in Chronic Heart Failure Patients Enrolled in the European Society of Cardiology Heart Failure Long-Term Registry. JACC Heart Fail. 2018, 6, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients with Implanted Devices: Results from the MultiSENSE Study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.; Severino, P.; Mancone, M.; Mariani, M.V.; Prosperi, S.; Colombo, L.; Myftari, V.; Cestiè, C.; Labbro Francia, A.; Germanò, R.; et al. Prognostic Assessment of HLM Score in Heart Failure Due to Ischemic Heart Disease: A Pilot Study. J. Clin. Med. 2024, 13, 3322. [Google Scholar] [CrossRef] [PubMed]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of Survival in Heart Failure. Circulation 2006, 113, 424–433. [Google Scholar] [CrossRef] [PubMed]

| Chronic Heart Failure (n = 100) | Control (n = 50) | p-Value | |
|---|---|---|---|
| Sex (M/F) | F—32 (32%) M—68 (68%) | F—20 (40%) M—30 (60%) | 0.33 |
| Age (years) | 68 ± 11 | 63 ± 12 | 0.04 |
| Place of residence (urban/rural) | Urban—42 (42%) Rural—58 (58%) | Urban—29 (58%) Rural—21 (42%) | 0.06 |
| Smoking (pack-year) | 13.2 (IQR: 0.0–27.5) | 6.76 (IQR: 0.0–11.0) | 0.02 |
| Alcohol consumption (Yes/No) | Yes—16 (16%) No—84 (84%) | Yes—11 (22%) No—39 (78%) | 0.37 |
| Diabetes (Yes/No) | Yes—30 (30%) No—70 (70%) | Yes—10 (20%) No—40 (80%) | 0.19 |
| Chronic kidney disease (Yes/No) | Yes—21 (21%) No—79 (79%) | Yes—7 (14%) No—43 (86%) | 0.47 |
| Body mass index (kg/m2) | 27.4 (IQR: 24.1–30.8) | 26.9 (IQR: 23.7–32.5) | 0.75 |
| NT-proBNP (pg/mL) | 2798.0 (IQR: 697.0–7589.0) | 55.6 (IQR: 22.0–105.0) | <0.001 |
| NYHA classification (I/II/III/IV) | I—6 (6%) II—50 (50%) III—40 (40%) IV—4 (4%) | N/A | N/A |
| Bundle branch block | LBBB—12 (12%) RBBB—8 (8%) No—80 (80%) | N/A | N/A |
| Beta-blockers | Carvedilol—64 (64%) Bisoprolol—27 (27%) Metoprolol—9 (9%) | N/A | N/A |
| Chronic Heart Failure (n = 100) | Control (n = 50) | p-Value | |
|---|---|---|---|
| LVEF (%) | 0.32 ± 0.10 | 0.55 ± 0.04 | <0.001 |
| LVEDV (mL) | 195.0 (IQR: 153.0–238.0) | 149.0 (IQR: 125.0–175.0) | <0.001 |
| LVESV (mL) | 129.0 (IQR: 96.0–166.0) | 65.0 (IQR: 53.8–81.0) | <0.001 |
| E/A | 1.0 (IQR: 0.7–1.8) | 0.9 (IQR: 0.7–1.2) | 0.07 |
| Average E/E’ | 11.9 (IQR: 8.1–15.6) | 6.80 (IQR: 5.9–9.2) | <0.001 |
| E/E’ lateral | 9.5 (IQR: 7.1–12.6) | 6.1 (IQR: 4.9–8.3) | <0.001 |
| E/E’ septal | 12.8 (IQR: 8.7–18.5) | 7.6 (IQR: 6.6–10.1) | <0.001 |
| S’ lateral (mm/s) | 0.07 (IQR: 0.05–0.08) | 0.09 (IQR: 0.07–0.10) | <0.001 |
| S’ septal (mm/s) | 0.06 (IQR: 0.05–0.08) | 0.09 (IQR: 0.07–0.10) | <0.001 |
| MV Dec T (ms) | 178.0 (IQR: 141–215) | 201.0 (IQR: 163.0–246.0) | 0.03 |
| LAVI (mL/m2) | 22.1 (IQR: 18.4–26.8) | 18.1 (IQR: 15.4–20.1) | <0.001 |
| MAPSE (mm) | 12.0 (IQR: 10.0–14.0) | 14.0 (IQR: 12.0–16.0) | <0.001 |
| RVEDD (mm) | 34.0 (IQR: 30.0–38.0) | 33.0 (IQR: 30.0–37.0) | 0.31 |
| LVEDD (mm) | 55.0 (IQR: 50.0–62.3) | 48.0 (IQR: 44.0–54.0) | <0.001 |
| ePAPS | 26.5 (IQR: 20.0–37.0) | 20.5 (IQR: 17.3–24.8) | <0.001 |
| Cardiac output (L/min) | 4.60 ± 1.40 | 5.54 ± 1.40 | <0.001 |
| Aortic Vmax (m/s) | 1.3 (IQR: 1.2–1.8) | 1.2 (IQR: 1.1–1.4) | <0.001 |
| Chronic Heart Failure (n = 100) | Control (n = 50) | p-Value | |
|---|---|---|---|
| LVPs (Yes/No) | Yes—34 (34%) No—66 (66%) | Yes—6 (12%) No—44 (88%) | <0.01 |
| TWA (Yes/No) | Yes—21 (21%) No—79 (79%) | Yes—1 (2%) No—49 (98%) | <0.01 |
| Chronic Heart Failure (n = 100) | Control (n = 50) | p-Value | |
|---|---|---|---|
| SDNN (ms) | 74.0 (IQR: 56.0–96.0) | 105.0 (IQR: 74.3–132.0) | <0.001 |
| SDANN (ms) | 67.3 ± 27.0 | 81.0 ± 32.7 | <0.01 |
| SDNN Index (ms) | 34.0 (IQR: (26.0–45.3) | 48.0 (IQR: 35.3–63.8) | <0.001 |
| RMSSD | 23.0 (IQR: 16.0–34.5) | 30.5 (IQR: 21.0–43.0) | 0.03 |
| PNN50 (%) | 3.0 (IQR: 0.0–8.0) | 5.0 (IQR: 2.0–11.0) | 0.06 |
| Triangular index (ms) | 17.6 (IQR: 12.8–22.9) | 27.0 (IQR: 16.4–34.5) | <0.001 |
| VLF (Hz) | 845.0 (IQR: 544.0–1591.0) | 1450.0 (IQR: 680.0–2159.0) | 0.02 |
| LF (Hz) | 179.0 (IQR: 92.2–359.0) | 353.0 (IQR: 169.0–633.0) | <0.001 |
| HF (Hz) | 76.8 (IQR: 35.9–181.0) | 104.0 (IQR: 58.5–241.0) | 0.11 |
| Deceleration capacity (ms) | 3.8 (IQR: 2.4–5.6) | 5.8 (IQR: 4.1–7.2) | <0.001 |
| Acceleration capacity (ms) | −4.3 (IQR: −6.3–−2.9) | −6.5 (IQR:−7.8–−4.2) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duca, Ș.-T.; Tudorancea, I.; Haba, M.Ș.C.; Costache, A.-D.; Șerban, I.-L.; Pavăl, D.R.; Loghin, C.; Costache-Enache, I.-I. Enhancing Comprehensive Assessments in Chronic Heart Failure Caused by Ischemic Heart Disease: The Diagnostic Utility of Holter ECG Parameters. Medicina 2024, 60, 1315. https://doi.org/10.3390/medicina60081315
Duca Ș-T, Tudorancea I, Haba MȘC, Costache A-D, Șerban I-L, Pavăl DR, Loghin C, Costache-Enache I-I. Enhancing Comprehensive Assessments in Chronic Heart Failure Caused by Ischemic Heart Disease: The Diagnostic Utility of Holter ECG Parameters. Medicina. 2024; 60(8):1315. https://doi.org/10.3390/medicina60081315
Chicago/Turabian StyleDuca, Ștefania-Teodora, Ionuț Tudorancea, Mihai Ștefan Cristian Haba, Alexandru-Dan Costache, Ionela-Lăcrămioara Șerban, D. Robert Pavăl, Cătălin Loghin, and Irina-Iuliana Costache-Enache. 2024. "Enhancing Comprehensive Assessments in Chronic Heart Failure Caused by Ischemic Heart Disease: The Diagnostic Utility of Holter ECG Parameters" Medicina 60, no. 8: 1315. https://doi.org/10.3390/medicina60081315
APA StyleDuca, Ș.-T., Tudorancea, I., Haba, M. Ș. C., Costache, A.-D., Șerban, I.-L., Pavăl, D. R., Loghin, C., & Costache-Enache, I.-I. (2024). Enhancing Comprehensive Assessments in Chronic Heart Failure Caused by Ischemic Heart Disease: The Diagnostic Utility of Holter ECG Parameters. Medicina, 60(8), 1315. https://doi.org/10.3390/medicina60081315








