Effect of a Single Dose of Deflazacort on Postoperative Pain, Swelling, and Trismus after Impacted Lower Third Molar Surgery: Randomised Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
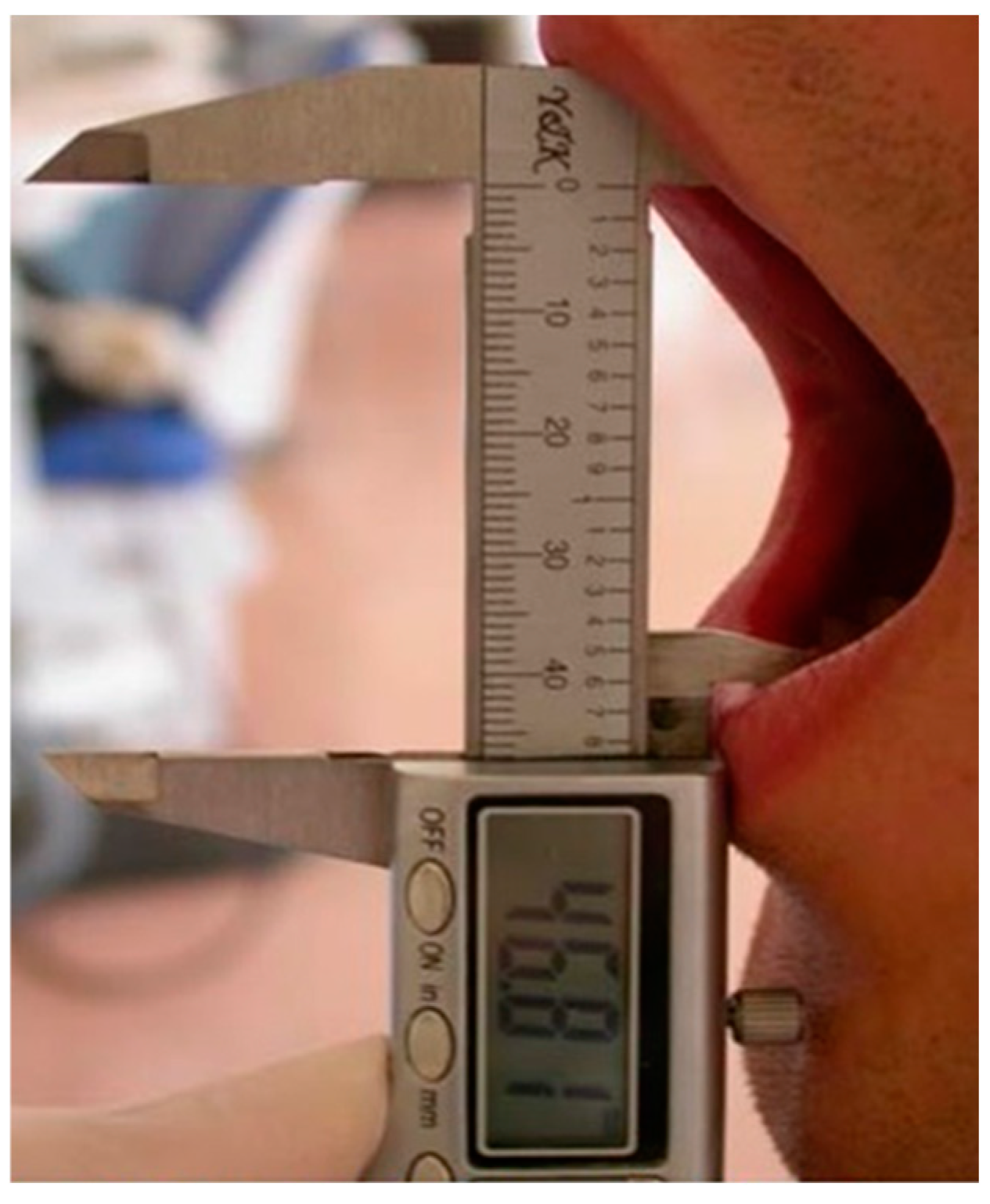
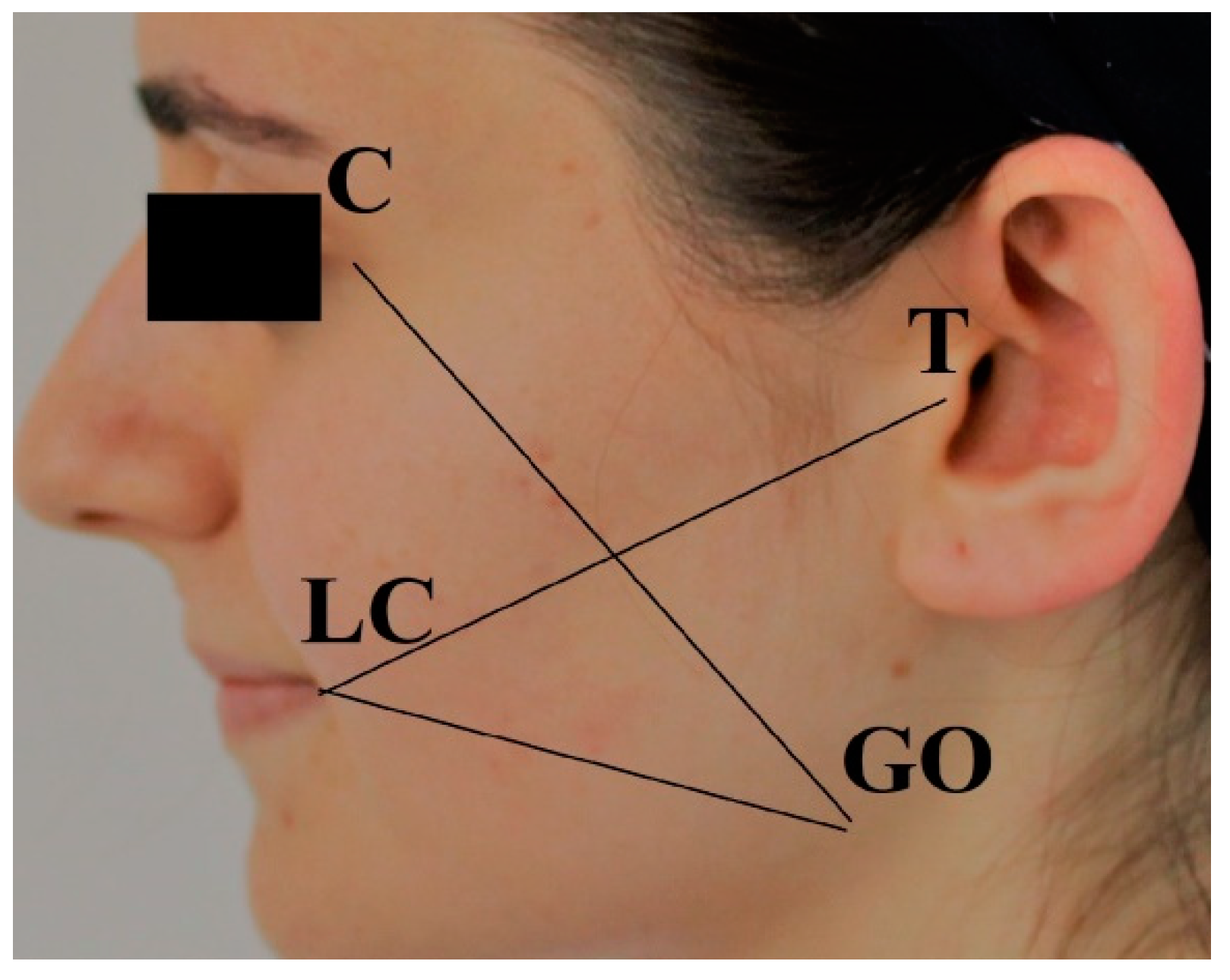
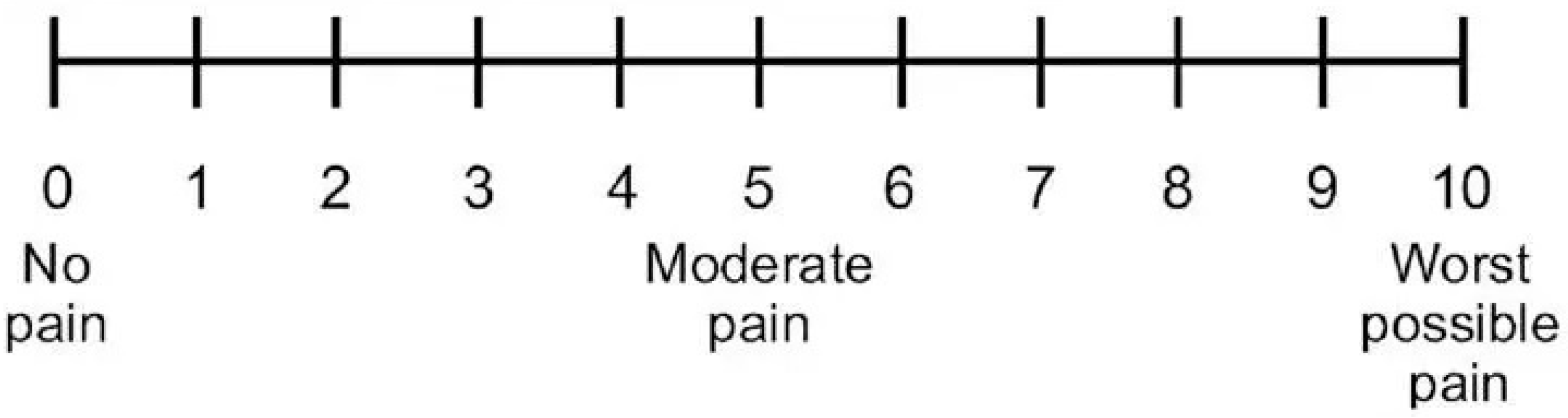
2.2. Outcome Variables
2.3. Patient Allocation
2.4. Surgical Procedure
2.5. Data Collection
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saravanan, K.; Kannan, R.; John, R.R.; Nantha Kumar, C. A Single Pre Operative Dose of Sub Mucosal Dexamethasone Is Effective in Improving Post Operative Quality of Life in the Surgical Management of Impacted Third Molars: A Comparative Randomised Prospective Study. J. Maxillofac. Oral. Surg. 2016, 15, 67–71. [Google Scholar] [CrossRef]
- Dilan, O.Z.; Levent, C.; Volkan, K.; Mehmet, G.; Abdurrahman, G.; Mohammad, A.; Anıl, Ö. Evaluation of the Effects of Silk and Polyethylene Terephthalate Sutures on Postoperative Complications in Impacted Lower Third Molar Surgery. J. Mater. Sci. Mater. Med. 2023, 34, 51. [Google Scholar] [CrossRef]
- Akbulut, N.; Üstüner, E.; Atakan, C.; Çölok, G. Comparison of the Effect of Naproxen, Etodolac and Diclofenac on Postoperative Sequels Following Third Molar Surgery: A Randomised, Double-Blind, Crossover Study. Med. Oral. Patol. Oral. Cirugia Bucal 2014, 19, e149–e156. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamiri, H.M.; Shawky, M.; Hassanein, N. Comparative Assessment of Preoperative versus Postoperative Dexamethasone on Postoperative Complications Following Lower Third Molar Surgical Extraction. Int. J. Dent. 2017, 2017, 1350375. [Google Scholar] [CrossRef]
- Kaplan, V.; Ciğerim, L.; Çınarsoy Ciğerim, S.; Bazyel, Z.D.; Dinç, G. Comparison of Various Measurement Methods in the Evaluation of Swelling After Third Molar Surgery. Van. Med. J. 2021, 28, 412–420. [Google Scholar] [CrossRef]
- Cho, H.; Lynham, A.J.; Hsu, E. Postoperative Interventions to Reduce Inflammatory Complications after Third Molar Surgery: Review of the Current Evidence. Aust. Dent. J. 2017, 62, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Bamgbose, B.O.; Akinwande, J.A.; Adeyemo, W.L.; Ladeinde, A.L.; Arotiba, G.T.; Ogunlewe, M.O. Effects of Co-Administered Dexamethasone and Diclofenac Potassium on Pain, Swelling and Trismus Following Third Molar Surgery. Head. Face Med. 2005, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Au, A.H.Y.; Choi, S.W.; Cheung, C.W.; Leung, Y.Y. The Efficacy and Clinical Safety of Various Analgesic Combinations for Post-Operative Pain after Third Molar Surgery: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0127611. [Google Scholar] [CrossRef] [PubMed]
- Minguez-Serra, M.P.; Salort-Llorca, C.; Silvestre-Donat, F.J. Chlorhexidine in the Prevention of Dry Socket: Effectiveness of Different Dosage Forms and Regimens. Med. Oral. Patol. Oral. Cirugia Bucal 2009, 14, e445–e449. [Google Scholar]
- Gelesko, S.; Long, L.; Faulk, J.; Phillips, C.; Dicus, C.; White, R.P. Cryotherapy and Topical Minocycline as Adjunctive Measures to Control Pain after Third Molar Surgery: An Exploratory Study. J. Oral. Maxillofac. Surg. Off. J. Am. Assoc. Oral. Maxillofac. Surg. 2011, 69, e324–e332. [Google Scholar] [CrossRef][Green Version]
- Albanese, M.; Zangani, A.; Manfrin, F.; Bertossi, D.; De Manzoni, R.; Tomizioli, N.; Faccioni, P.; Pardo, A. Influence of Surgical Technique on Post-Operative Complications in the Extraction of the Lower Third Molar: A Retrospective Study. Dent. J. 2023, 11, 238. [Google Scholar] [CrossRef]
- Orhan, Z.D.; Ciğerim, L. Evaluation of the Effect of Polybutester and Polypropylene Sutures on Complications after Impacted Lower Third Molar Surgery. Appl. Sci. 2024, 14, 1448. [Google Scholar] [CrossRef]
- Blum, I.R. Contemporary Views on Dry Socket (Alveolar Osteitis): A Clinical Appraisal of Standardization, Aetiopathogenesis and Management: A Critical Review. Int. J. Oral. Maxillofac. Surg. 2002, 31, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Cigerim, L.; Orhan, Z.D.; Kaplan, V.; Cigerim, S.C.; Feslihan, E. Evaluation of the Efficacy of Topical Rifamycin Application on Postoperative Complications after Lower Impacted Wisdom Teeth Surgery. J. Stomatol. Oral. Maxillofac. Surg. 2023, 101501. [Google Scholar] [CrossRef]
- Moraschini, V.; Hidalgo, R.; Porto Barboza, E. dS Effect of Submucosal Injection of Dexamethasone after Third Molar Surgery: A Meta-Analysis of Randomized Controlled Trials. Int. J. Oral. Maxillofac. Surg. 2016, 45, 232–240. [Google Scholar] [CrossRef]
- Selvaraj, L.; Hanumantha Rao, S.; Lankupalli, A.S. Comparison of Efficacy of Methylprednisolone Injection into Masseter Muscle Versus Gluteal Muscle for Surgical Removal of Impacted Lower Third Molar. J. Maxillofac. Oral. Surg. 2014, 13, 495–498. [Google Scholar] [CrossRef][Green Version]
- Sabhlok, S.; Kenjale, P.; Mony, D.; Khatri, I.; Kumar, P. Randomized Controlled Trial to Evaluate the Efficacy of Oral Dexamethasone and Intramuscular Dexamethasone in Mandibular Third Molar Surgeries. J. Clin. Diagn. Res. JCDR 2015, 9, ZC48–ZC51. [Google Scholar] [CrossRef] [PubMed]
- Kapugi, M.; Cunningham, K. Corticosteroids. Orthop. Nurs. 2019, 38, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Ngeow, W.C.; Lim, D. Do Corticosteroids Still Have a Role in the Management of Third Molar Surgery? Adv. Ther. 2016, 33, 1105–1139. [Google Scholar] [CrossRef]
- Parente, L. Deflazacort: Therapeutic Index, Relative Potency and Equivalent Doses versus Other Corticosteroids. BMC Pharmacol. Toxicol. 2017, 18, 1. [Google Scholar] [CrossRef]
- Bylo, M.; Farewell, R.; Coppenrath, V.A.; Yogaratnam, D. A Review of Deflazacort for Patients with Duchenne Muscular Dystrophy. Ann. Pharmacother. 2020, 54, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Bryson, H.M. Deflazacort. A Review of Its Pharmacological Properties and Therapeutic Efficacy. Drugs 1995, 50, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Acharjya, B. Deflazacort versus Other Glucocorticoids: A Comparison. Indian. J. Dermatol. 2008, 53, 167–170. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Sajeev, G.; Yao, Z.; McDonnell, E.; Elfring, G.; Souza, M.; Peltz, S.W.; Darras, B.T.; Shieh, P.B.; Cox, D.A.; et al. Deflazacort vs Prednisone Treatment for Duchenne Muscular Dystrophy: A Meta-Analysis of Disease Progression Rates in Recent Multicenter Clinical Trials. Muscle Nerve 2020, 61, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Destefanis, P.; Fiori, C.; Fontana, D. Effectiveness of Nifedipine and Deflazacort in the Management of Distal Ureter Stones. Urology 2000, 56, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.; Rehan, H.S.; Mehra, P.; Kakkar, A.K. A Randomized, Double-Blind, Placebo-Controlled Study Comparing the Efficacy and Safety of Paracetamol, Serratiopeptidase, Ibuprofen and Betamethasone Using the Dental Impaction Pain Model. Int. J. Oral. Maxillofac. Surg. 2009, 38, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Kazancioglu, H.O.; Ezirganli, S.; Demirtas, N. Comparison of the Influence of Ozone and Laser Therapies on Pain, Swelling, and Trismus Following Impacted Third-Molar Surgery. Lasers Med. Sci. 2014, 29, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M. Clinical Pharmacology of Corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Phipatanakul, W.; Mauger, D.T.; Sorkness, R.L.; Gaffin, J.M.; Holguin, F.; Woodruff, P.G.; Ly, N.P.; Bacharier, L.B.; Bhakta, N.R.; Moore, W.C.; et al. Effects of Age and Disease Severity on Systemic Corticosteroid Responses in Asthma. Am. J. Respir. Crit. Care Med. 2017, 195, 1439–1448. [Google Scholar] [CrossRef]
- Aaron, S.D.; Vandemheen, K.L.; Hebert, P.; Dales, R.; Stiell, I.G.; Ahuja, J.; Dickinson, G.; Brison, R.; Rowe, B.H.; Dreyer, J.; et al. Outpatient Oral Prednisone after Emergency Treatment of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2003, 348, 2618–2625. [Google Scholar] [CrossRef]
- Waljee, A.K.; Rogers, M.A.M.; Lin, P.; Singal, A.G.; Stein, J.D.; Marks, R.M.; Ayanian, J.Z.; Nallamothu, B.K. Short Term Use of Oral Corticosteroids and Related Harms among Adults in the United States: Population Based Cohort Study. BMJ 2017, 357, j1415. [Google Scholar] [CrossRef]
- Liu, D.; Ahmet, A.; Ward, L.; Krishnamoorthy, P.; Mandelcorn, E.D.; Leigh, R.; Brown, J.P.; Cohen, A.; Kim, H. A Practical Guide to the Monitoring and Management of the Complications of Systemic Corticosteroid Therapy. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2013, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Çayakar, A. Steroid usage in clinical practice. Ulus. Romatoloji Derg. 2021, 13, 73–84. [Google Scholar] [CrossRef]
- Cascio, V. Deflazacort and Bone Mass. Clin. Exp. Rheumatol. 2000, 18, S69–S73. [Google Scholar]
- Torrejon-Moya, A.; Saka-Herrán, C.; Izquierdo-Gómez, K.; Marí-Roig, A.; Estrugo-Devesa, A.; López-López, J. Oral Lichen Planus and Dental Implants: Protocol and Systematic Review. J. Clin. Med. 2020, 9, 4127. [Google Scholar] [CrossRef]
- Konagala, R.K.; Mandava, J.; Pabbati, R.K.; Anupreeta, A.; Borugadda, R.; Ravi, R. Effect of pretreatment medication on postendodontic pain: A double-blind, placebo-controlled study. J. Conserv. Dent. 2019, 22, 54–58. [Google Scholar] [CrossRef]
- de Vicente, J.C.; Hernández-Vallejo, G.; Braña-Abascal, P.; Peña, I. Maxillary Sinus Augmentation with Autologous Bone Harvested from the Lateral Maxillary Wall Combined with Bovine-Derived Hydroxyapatite: Clinical and Histologic Observations. Clin. Oral. Implants Res. 2010, 21, 430–438. [Google Scholar] [CrossRef]
- Ramos, J.M.F.; Orozco, S.H.A.; Zaragoza, M.G.O. Betamethasone (sodium phosphate + acetate) prevents inflammation and trismus in retained lower third-molar surgery. Glucocorticoids in third-molar surgery. Rev. ADM Órgano Of. Asoc. Dent. Mex. 2013, 70, 190–196. [Google Scholar]
- Dereci, O.; Tüzüner-Öncül, A.M.; Kocer, G.; Yüce, E.; Askar, M.; Öztürk, A. Efficacy of immediate postoperative intramasseteric dexamethasone injection on postoperative swelling after mandibular impacted third molar surgery: A preliminary split-mouth study. J. Pak. Med. Assoc. 2016, 66, 320–323. [Google Scholar]
- Chakraborty, P.K.; Shai, A.; Tilak, P.B.D.; Kumar, A.; Kamdar, A.; Niranjana, A.; Kisave, P.N. Comparative Analgesic Efficacy of Intramuscular Dexamethasone, Ketorolac, Tramadol, and Butorphanol with Regard to Postoperative Pain After Mandibular Third Molar Surgery. J. Pharm. Bioallied Sci. 2024, 16, 1378–1380. [Google Scholar] [CrossRef]
- dos Santos Canellas, J.V.; Ritto, F.G.; Tiwana, P. Comparative efficacy and safety of different corticosteroids to reduce inflammatory complications after mandibular third molar surgery: A systematic review and network meta-analysis. Br. J. Oral Maxillofac. Surg. 2022, 60, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Barone, S.; Bennardo, F.; Giudice, A. Three-dimensional facial swelling evaluation of pre-operative single-dose of prednisone in third molar surgery: A split-mouth randomized controlled trial. BMC Oral. Health 2023, 23, 614. [Google Scholar] [CrossRef] [PubMed]
- Miroshnychenko, A.; Azab, M.; Ibrahim, S.; Roldan, Y.; Martinez, J.P.D.; Tamilselvan, D.; Carrasco-Labra, A. Corticosteroids for managing acute pain subsequent to surgical extraction of mandibular third molars: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2023, 154, 727–741. [Google Scholar] [CrossRef]
- Bakri, M.M.H.; Alabdali, F.H.; Mahzari, R.H.; Rajhi, T.J.; Gohal, N.M.; Sufyani, R.A.; Hezam, A.A.; Qurishi, A.A.; Bakri, H.M.; Ali, F.M. Comparison of the effects of two different styles of orally prescribing prednisolone on postoperative sequelae of surgical extraction of an impacted mandibular third molar: A single-blind randomized study. J. Korean Assoc. Oral. Maxillofac. Surg. 2024, 50, 27–34. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| * ASA 1, healthy * 18–40 years old * Bilateral impacted lower 3rd molars * Mesioangular (Angulation) * Class II and Class B (level of impaction) | * Systemic disease that interferes with surgical treatment * Smokers * Alcohol drinkers * Substance abusers * Pregnant and breastfeeding mothers * Patients with facial asymmetry * Patients currently receiving anti-inflammatory medication |
| Gender | Male (%) | 12 (46.2) |
| Female (%) | 14 (53.8) | |
| Age (year) | Min–Max (Median) | 18–35 (22.5) |
| Mean ± SD | 23.31 ± 5.01 |
| 6th Hour | 12th Hour | 24th Hour | 2nd Day | 3rd Day | 4th Day | 5th Day | 6th Day | 7th Day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | |
| Group 1 | 5.8 ± 2.1 | 6.00 | 4.81 ± 2.19 | 5.0 | 4.35 ± 3.02 | 5.0 | 3.92 ± 2.64 | 3.0 | 3.04 ± 2.72 | 2.5 | 1.96 ± 2.22 | 1.5 | 1.46 ± 1.90 | 0.5 | 1.04 ± 1.28 | 0.0 | 0.85 ± 1.54 | 0.0 |
| Group 2 | 3.1 ± 1.6 | 3.00 | 3.27 ± 2.49 | 3.0 | 4.27 ± 2.71 | 5.0 | 4.04 ± 2.68 | 4.0 | 2.73 ± 2.41 | 2.0 | 1.77 ± 2.10 | 1.5 | 0.85 ± 1.26 | 0.0 | 0.65 ± 1.02 | 0.0 | 0.50 ± 0.81 | 0.0 |
| p | 1 0.000 * | 1 0.025 * | 1 0.912 | 1 0.861 | 1 0.773 | 1 0.832 | 1 0.276 | 1 0.290 | 1 0.520 | |||||||||
| n | Mean | SD | Median | Minimum | Maximum | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Maximum mouth opening (%) | Day 2 | Group 1 | 26 | −31.93 | 15.25 | −30.59 | −60 | −4.08 | 1 0.701 |
| Group 2 | 26 | −32.91 | 14.28 | −34.52 | −55.56 | 0.00 | |||
| Day 7 | Group 1 | 26 | −13.17 | 11.75 | −9.05 | −43.75 | 0.00 | 1 0.203 | |
| Group 2 | 26 | −17.22 | 11.99 | −15.65 | −41.67 | −2.04 | |||
| n | Mean | SD | Median | Minimum | Maximum | p | |||
| Maximum mouth opening (mm) | Pre-op | Group 1 and Group 2 | 26 | 44.5 | 5.13 | 45 | 31 | 55 | 1 0.999 |
| Day 2 | Group 1 | 26 | 30.15 | 7.17 | 30.00 | 16 | 47 | 1 0.652 | |
| Group 2 | 26 | 29.35 | 4.33 | 29.00 | 20 | 36 | |||
| Day 7 | Group 1 | 26 | 38.54 | 6.56 | 38.00 | 25 | 55 | 1 0.478 | |
| Group 2 | 26 | 36.58 | 5.36 | 38.00 | 28 | 48 |
| Group 1 (%) | Group 2 (%) | Grup 1 (mm) | Grup 2 (mm) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | Mean ± SD | Median | |||
| Day 2 Distance | 4.12 ± 2.13 | 3.67 | 3.07 ± 2.37 | 3.05 | 112.94 ± 9.22 | 112.83 | 111.68 ± 7.34 | 111.33 | 1 0.089 | |
| Day 7 Distance | 1.74 ± 1.80 | 1.12 | 1.44 ± 1.71 | 0.91 | 110.36 ± 8.93 | 109.67 | 109.95 ± 7.70 | 110.17 | 1 0.349 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, V.; Ciğerim, L.; Feslihan, E.; Çınarsoy Ciğerim, S. Effect of a Single Dose of Deflazacort on Postoperative Pain, Swelling, and Trismus after Impacted Lower Third Molar Surgery: Randomised Clinical Trial. Medicina 2024, 60, 1206. https://doi.org/10.3390/medicina60081206
Kaplan V, Ciğerim L, Feslihan E, Çınarsoy Ciğerim S. Effect of a Single Dose of Deflazacort on Postoperative Pain, Swelling, and Trismus after Impacted Lower Third Molar Surgery: Randomised Clinical Trial. Medicina. 2024; 60(8):1206. https://doi.org/10.3390/medicina60081206
Chicago/Turabian StyleKaplan, Volkan, Levent Ciğerim, Erkan Feslihan, and Saadet Çınarsoy Ciğerim. 2024. "Effect of a Single Dose of Deflazacort on Postoperative Pain, Swelling, and Trismus after Impacted Lower Third Molar Surgery: Randomised Clinical Trial" Medicina 60, no. 8: 1206. https://doi.org/10.3390/medicina60081206
APA StyleKaplan, V., Ciğerim, L., Feslihan, E., & Çınarsoy Ciğerim, S. (2024). Effect of a Single Dose of Deflazacort on Postoperative Pain, Swelling, and Trismus after Impacted Lower Third Molar Surgery: Randomised Clinical Trial. Medicina, 60(8), 1206. https://doi.org/10.3390/medicina60081206






